Introduction
Exposure to traumatic life events can have severe effects on the functioning of physiological systems, including the autonomic nervous system (ANS) (Orr & Roth, Reference Orr and Roth2000). The cardiovascular system, digestive tract, respiratory system and the regulation of the metabolism are controlled and regulated by the ANS (Jänig, Reference Jänig2008). The ANS consists of two branches, the sympathetic nervous system (SNS), and the parasympathetic nervous system (PNS). Both branches are interconnected and work mostly unconsciously (Johnson, Reference Johnson, Farag, Argalious, Tetzlaff and Sharma2018). The SNS can boost body performance during increased activity or stress, which is indicated by heightened blood pressure, heart rate and respiration (Murison, Reference Murison, Al'Absi and Flaten2016). The PNS is responsible for storing and building up energy during periods of rest or recovery. During PNS activity, heart rate slows down and blood pressure lowers (Pichon & Chapelot, Reference Pichon and Chapelot2010). A balanced interaction of both systems is thought to enable an individual's adaptive adjustment to changing environments (Elliott & Lawrenson, Reference Elliott and Lawrenson2009).
Dysregulations in the ANS are thought to characterize a variety of psychological disorders, including posttraumatic stress disorder (PTSD) (Minassian et al., Reference Minassian, Maihofer, Baker, Nievergelt, Geyer and Risbrough2015). Symptoms of intrusion, avoidance, negative cognitions and mood, and alterations in arousal and reactivity, characterize PTSD (American Psychiatric Association, 2013). Alterations in arousal and reactivity suggest ANS abnormalities in PTSD patients. Yehuda, Southwick, Giller, Ma, and Mason (Reference Yehuda, Southwick, Giller, Ma and Mason1992) reported higher levels of dopamine and norepinephrine concentrations in PTSD patients, which might contribute to changes in ANS activity. Furthermore, a meta-analysis by Pole (Reference Pole2007) revealed that resting heart rate is increased in patients with PTSD as compared to healthy controls. Alterations in arousal may also change cardiovascular stress activity and reactivity. This can lead to either increased or blunted responsiveness of individuals with PTSD to challenging and stressful tasks (Cohen et al., Reference Cohen, Benjamin, Geva, Matar, Kaplan and Kotler2000; Dennis et al., Reference Dennis, Dedert, Van Voorhees, Watkins, Hayano, Calhoun and Beckham2016; Jovanovic, Norrholm, Sakoman, Esterajher, & Kozarić-Kovačić, Reference Jovanovic, Norrholm, Sakoman, Esterajher and Kozarić-Kovačić2009).
Analysis of heart rate variability (HRV) allows to gain more insight into the functioning of the ANS (for an overview, see Schwerdtfeger et al., Reference Schwerdtfeger, Schwarz, Pfurtscheller, Thayer, Jarczok and Pfurtscheller2020). HRV is based on the beat to beat variations in the heart rate, which are caused by varying influences of the SNS and the PNS. HRV parameters are derived from electrocardiogram signal analysis, and can be quantified using time-domain, frequency-domain and non-linear methods. Root mean square of successive differences (RMSSD) and standard deviation of NN intervals (SDNN) are common time-domain parameters. High-frequency HRV (HF-HRV; 0.15–0.40 Hz), low-frequency HRV (LF-HRV; 0.04–0.15 Hz) and the LF/HF ratio are commonly examined frequency domain-parameters. Mechanisms of the cardiovascular regulation may also interact in a non-linear manner. Approximate entropy and detrended fluctuation analysis quantify short-term HRV (Camm et al., Reference Camm, Malik, Bigger, Breithardt, Cerutti, Cohen and Lombardi1996). Non-linear analysis of HRV, however, has been less often conducted, and its benefits relative to the traditional time and frequency domain measures are under debate (de Godoy, Reference de Godoy2016; Voss, Schulz, Schroeder, Baumert, & Caminal, Reference Voss, Schulz, Schroeder, Baumert and Caminal2008).
RMSSD and HF-HRV are highly intercorrelated and associated with parasympathetic (i.e. vagal) activity (Thayer & Lane, Reference Thayer and Lane2007). Both, SNS and PNS activities influence SDNN, which displays overall flexibility of the ANS (Shaffer & Ginsberg, Reference Shaffer and Ginsberg2017). Influence of the SNS and PNS on LF-HRV is discussed controversially. Recent research showed that LF-HRV might be associated with baroreceptor activity, suggesting that LF-HRV might primarily reflect parasympathetic activity (Goldstein, Bentho, Park, & Sharabi, Reference Goldstein, Bentho, Park and Sharabi2011). High HRV is a sign of good adaptability of the cardiovascular system, which enables an individual to adapt to inner and outer changes (McCraty & Shaffer, Reference McCraty and Shaffer2015; Schwerdtfeger et al., Reference Schwerdtfeger, Schwarz, Pfurtscheller, Thayer, Jarczok and Pfurtscheller2020). Overactivation of the SNS and decreased PNS activity may cause low HRV. Low HRV is a maker of impaired health (Thayer & Lane, Reference Thayer and Lane2007), and a risk factor for the onset of cardiovascular disease (Hillebrand et al., Reference Hillebrand, Gast, de Mutsert, Swenne, Jukema, Middeldorp and Dekkers2013). Therefore, lower HRV might impose risk for the development of secondary cardiovascular diseases in patients with PTSD.
Importantly, previous meta-analyses on HRV and PTSD suggest that HRV is lower in PTSD patients compared to controls (Campbell, Wisco, Silvia, & Gay, Reference Campbell, Wisco, Silvia and Gay2019; Nagpal, Gleichauf, & Ginsberg, Reference Nagpal, Gleichauf and Ginsberg2013). Campbell et al. (Reference Campbell, Wisco, Silvia and Gay2019) focused on differences in HRV parameters, which mainly reflect parasympathetic activity. Several vagally-mediated HRV parameters were combined, leading to one average effect size per study. Results revealed lower vagally-mediated HRV in individuals with PTSD compared to controls (Hedges' g = −0.26). Nagpal et al. (Reference Nagpal, Gleichauf and Ginsberg2013) analyzed HRV parameters separately. The aggregated effect sizes for HF-HRV, LF-HRV, LF/HF, RMSSD and SDNN were quite large (range Hedges' g: −0.33 to −2.94), probably resulting from a lower number of studies included in the meta-analyses and the exclusion of unpublished studies. HRV parameters were lower in PTSD patients compared to controls, indicated by medium to large effect sizes. The largest effect was found for HF-HRV (Hedges' g = −2.27). Both studies reported moderate to high levels of heterogeneity though.
The aim of the current meta-analysis was to examine differences in HRV parameters between individuals with PTSD and controls, both at rest and during stress tasks. In contrast to the meta-analysis of Campbell et al. (Reference Campbell, Wisco, Silvia and Gay2019), which combined interrelated measures of HRV, the current meta-analysis will provide separate results for specific HRV parameters. This approach was chosen, since there is no consensus regarding treating interrelated HRV measures equivalent (Shaffer & Ginsberg, Reference Shaffer and Ginsberg2017). Separate analysis of HRV parameters should lead to a differentiated understanding of alterations in HRV in PTSD. Furthermore, a more complete picture of the relation between PTSD and HRV will be provided by including unpublished studies and LF-HRV. Additionally, moderator variables will be examined that may influence differences in HRV alterations in PTSD and controls. Finally, a new method to verify and control for publication bias will be applied, which allows for correction of effect size estimates of publication bias in the presence of heterogeneity in true effect sizes. Analyzing effect sizes for different HRV parameters both at rest and during stress will contribute to a deeper understanding of the adaptive capacity in individuals suffering from PTSD.
Methods
The current meta-analysis was conducted in accordance to the ‘Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA)’ guidelines (Moher et al., Reference Moher, Shamseer, Clarke, Ghersi, Liberati, Petticrew and Stewart2015).
Literature search and inclusion criteria
A systematic literature search was performed up to March 2019. Databases used to identify relevant articles were: PubMed, PsycInfo, Cinahl, Web of Science and Google Scholar. The following search terms were used: ((post traumatic stress disorder) OR (post traumatic stress) OR (posttraumatic stress disorder) OR (PTSD)) AND ((heart rate variability) OR (HRV) OR (respiratory sinus arrhythmia) OR (RS) OR (heart period variability) OR (RR variability) OR (cycle length variability) OR (vagal nerve activity) OR (sinus arrhythmias) OR (autonomic nervous system) OR (parasympathetic nervous system) OR (sympathetic nervous system) OR (vagus nerve)). Reference lists of articles included in the meta-analysis were browsed for additional studies. Unpublished data were retrieved by contacting researchers, who had already published data on PTSD and HRV. Additionally, effect sizes of unpublished studies were collected through supplemental material of Campbell et al.'s (Reference Campbell, Wisco, Silvia and Gay2019) meta-analysis. Time period restrictions or language restrictions were not set. Details on study search and selection process are shown in Fig. 1. Studies were included in the meta-analysis if they (1) reported a measure of PTSD, (2) reported a measure of HRV and (3) included only adult samples (⩾18 years). Studies were excluded if they did not report quantitative data necessary for effect size calculation. Case studies and systematic reviews were excluded as well.
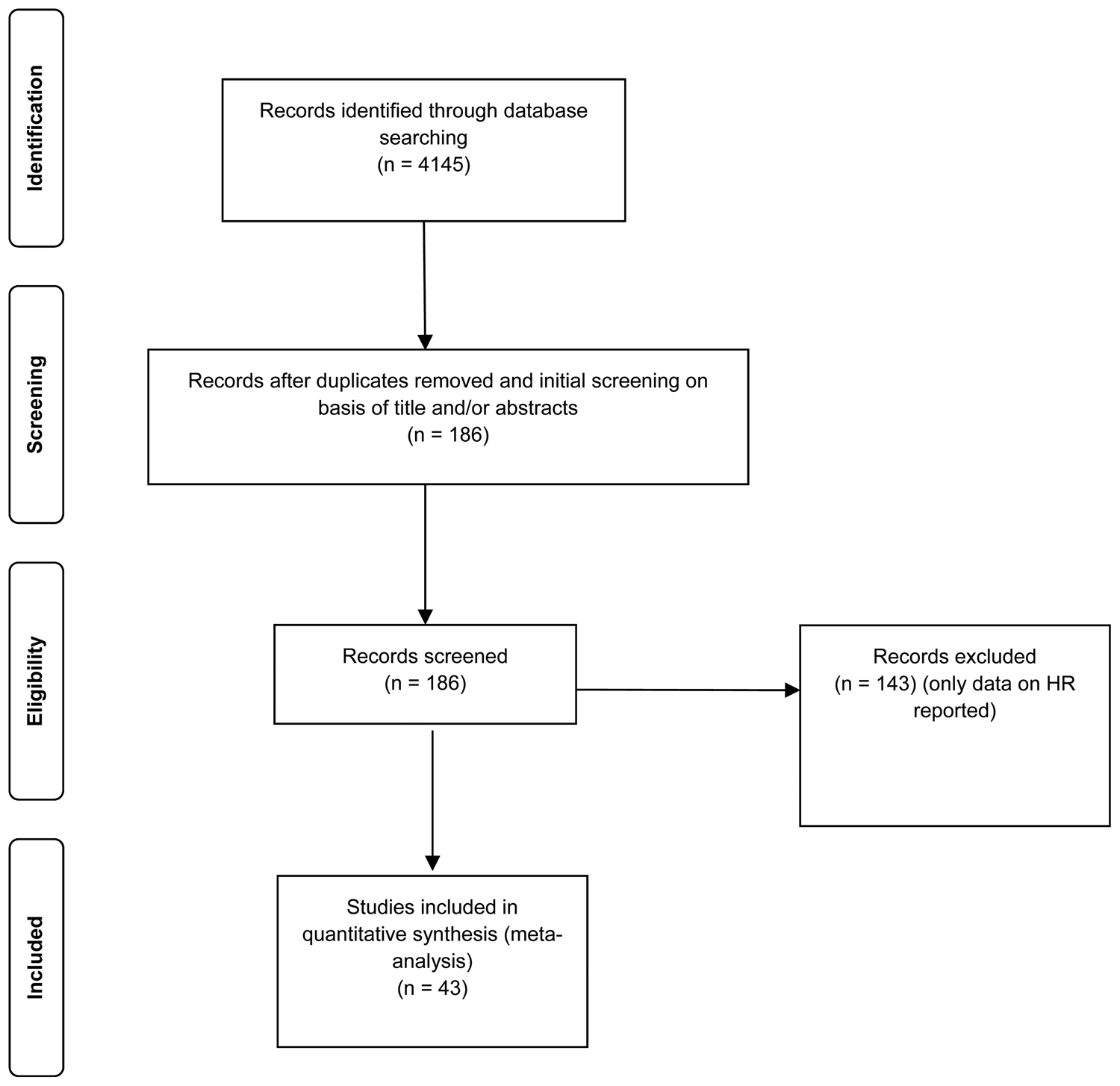
Fig. 1. PRISMA flow diagram.
Data extraction
A data extraction sheet was generated to collect data based on inclusion criteria and common study characteristics. Throughout the process of study extraction, the sheet was continuously adapted. The data extraction sheet included basic study information (author, publication year and published v. unpublished data), details on PTSD (sample size, DSM measure and PTSD measure), details on HRV (measure of HRV, laboratory or ambulatory assessment, recording position, length of measurement and sampling rate) and information on moderators. HRV parameters for baseline and – if available – for stress conditions were extracted. Data on heart rate (HR) were extracted as a secondary measure of HRV. Data on HR were only extracted in case the primary study included measures on HRV. In case of a stressor task, baseline was defined as the HRV recording time before the stressor occurred. Reported standard errors were transformed to standard deviations for effect size estimation, using the following formula: s.d. = s.e. × √n (Higgins & Green, Reference Higgins and Green2011). In case of missing standard errors for reported effect size from categorical studies, the following approach was used: to convert from Cohens' d to Hedges' g a correction factor was used, called J. An approximation was used for J (J = 1 − (3/4v − 1). Hedges' g was transformed to Cohens' d (g = J × d). The variance for Cohens' d was calculated (V d = n 1 + n 2/n 1 × n 2 + d 2/2(n 1 + n 2)). Sampling variance of g was derived from back converting (V g = J 2V d). Standard error was derived via square root. Effect size estimation was based on absolute values and log-transformed values. Indexes of HRV in normalized units were not included.
Data analysis
Meta-analytical procedure
Statistical analyses were performed with R, version 3.5.2 (R Core Team, 2018) using the following packages: meta (Schwarzer, Reference Schwarzer2007), metafor (Viechtbauer, Reference Viechtbauer2010), esc (Lüdecke, Reference Lüdecke2018) and dplyr (Wickham, Francois, Henry, & Müller, Reference Wickham, Francois, Henry and Müller2015). Hedges' g was used as a measure of effect size, since it provides a better estimation of the standardized mean differences in small sample sizes than Cohen's d (Borenstein, Hedges, Higgins, & Rothstein, Reference Borenstein, Hedges, Higgins and Rothstein2009). Quantification of the effect size magnitude for Hedge's g is equal to the thresholds defined for Cohen's d (Cohen, Reference Cohen1992): small (0.2), medium (0.5) and large (0.8), respectively. Individuals suffering from PTSD represent a heterogeneous population, differing in several aspects such as trauma type or duration of trauma exposure (Brewin, Andrews, & Valentine, Reference Brewin, Andrews and Valentine2000). Therefore, random-effects models were chosen, which assume that the true effects in the examined studies are derived from a distribution of true effects (Borenstein et al., Reference Borenstein, Hedges, Higgins and Rothstein2009).
Heterogeneity analysis
Cochran Q test was used to examine significance of observed heterogeneity. I 2 index (Higgins & Thompson, Reference Higgins and Thompson2002) was used to assess degree of heterogeneity. I 2 refers to the amount of variation between studies that is based on true variation in effect size and is interpreted according to benchmarks set by Higgins and Thompsons (2002Reference Higgins and Thompson2002): ≈25% low, ≈50% moderate and ≈75% high level of heterogeneity. Prediction intervals (PI) for each effect size were reported, thus allowing to estimate the range of effect sizes of future studies, based on current meta-analytical evidence (IntHout, Ioannidis, Rovers, & Goeman, Reference IntHout, Ioannidis, Rovers and Goeman2016). Robustness of estimated effects was also examined through outlier detection. In case the confidence interval (CI) of a single study did not overlap with the CI of the aggregated effect size, the study was judged as an outlier. In case of outliers, meta-analysis was rerun without the corresponding outliers. Meta-regressions and subgroup analyses were conducted in case of significant heterogeneity.
Moderator variables
A number of variables impact HRV and PTSD, thus calling for the analysis of moderator variables. For example, individuals with PTSD show higher rates of smoking (Kearns et al., Reference Kearns, Carl, Stein, Vujanovic, Zvolensky, Smits and Powers2018) and obesity (Smith, Tyzik, Neylan, & Cohen, Reference Smith, Tyzik, Neylan and Cohen2015) and both these variables influence HRV (Ralevski, Petrakis, & Altemus, Reference Ralevski, Petrakis and Altemus2018; Triggiani et al., Reference Triggiani, Valenzano, Ciliberti, Moscatelli, Villani, Monda and Cibelli2017). Therefore, these variables were considered as moderators. Gender might be important as well, since women have a higher risk to develop lower HRV after trauma (Insulander & Vallin, Reference Insulander and Vallin2005; Keary, Hughes, & Palmieri, Reference Keary, Hughes and Palmieri2009). Another moderator to consider is age, since HRV declines with aging (Umetani, Singer, McCraty, & Atkionson, Reference Umetani, Singer, McCraty and Atkionson1998). Experience of trauma may not always lead to the development of PTSD (Sahar, Shalev, & Porges, Reference Sahar, Shalev and Porges2001). Hence, type of control group (healthy controls or trauma-exposed without PTSD) as well as type of trauma (interpersonal v. non-interpersonal) were investigated. Differences in autonomic arousal might also stem from the presence or absence of dissociative symptoms in PTSD (Seligowski et al., Reference Seligowski, Lebois, Hill, Kahhale, Wolff, Jovanovic and Ressler2019). Therefore, dissociation was included as a moderator. Moreover, PTSD measure (clinical interview v. self-report) was examined. PTSD symptom severity might be connected to diminished LF-HRV and HF-HRV in acute distress (Dennis et al., Reference Dennis, Dedert, Van Voorhees, Watkins, Hayano, Calhoun and Beckham2016), thus constituting potential moderators. Furthermore, medication use and comorbidities were examined as moderators. Other exploratory moderators were the presence of a stress task, study design (laboratory or ambulatory assessment), sample type, sleep measures, recording position, length of measurement and sampling rate of the ECG. Moderator variables were considered for all examined HRV indices. Moderator analyses were conducted if there were sufficient studies available for subgroup-comparisons and meta-regressions. In order to control for inflation of Type I error due to multiple testing, the Benjamini–Hochberg procedure (Benjamini & Hochberg, Reference Benjamini and Hochberg1996) was applied to control for false positive findings among significant moderatos (p < 0.05). Moderators that survived this procedure are reported.
Publication bias
Publication bias was assessed via different approaches. Visual inspection of funnel plots was used to estimate evidence of publication bias. Asymmetric patterns in the funnel plot indicate publication bias. Regression test by Egger, Smith, Schneider, and Minder (Reference Egger, Smith, Schneider and Minder1997) was used to test for funnel plot asymmetry. Regression intercept is supposed to be zero in the absence of publication bias. The trim and fill method (Duval & Tweedie, Reference Duval and Tweedie2000) was used to adjust for publication bias. Trim and fill describes an iterative approach, where the most extreme effect sizes on the positive side of the funnel plot are trimmed and missing effect sizes are filled into the funnel plot until funnel plot symmetry is reached. The goal is to reach an unbiased estimate of the effect size. P-uniform* is relatively new and constitutes a robust test of publication bias and considers significant and non-significant effect sizes when estimating publication bias (van Aert & van Assen, Reference van Aert and van Assen2018). P-uniform* is a selection approach model. According to the underlying selection model, probability of publishing statistically significant and non-significant effects sizes is constant, though the probabilities for these two assumptions may differ from each other.
Results
Study selection and study characteristics
The studies included in the meta-analysis span two decades (published between 2000 and 2019). Electronic database search on HRV and PTSD revealed 4145 studies. Title and abstract of these studies were screened to evaluate their suitability for full-text review. Non-matching studies (according to predefined inclusion and exclusion criteria, see ‘Methods’ section) and duplicates (the same article found across multiple search engines) were removed, leading to 186 studies suitable for full-text review. In total, 143 studies were excluded due to missing data on HRV. In total, 43 studies were included in the meta-analysis. Four studies came from unpublished datasets. Correlational and categorical data were included in the meta-analyses. Study characteristics of all primary studies included in the meta-analyses are shown in Tables 1–9.
Table 1. Description of moderators and inclusion rate
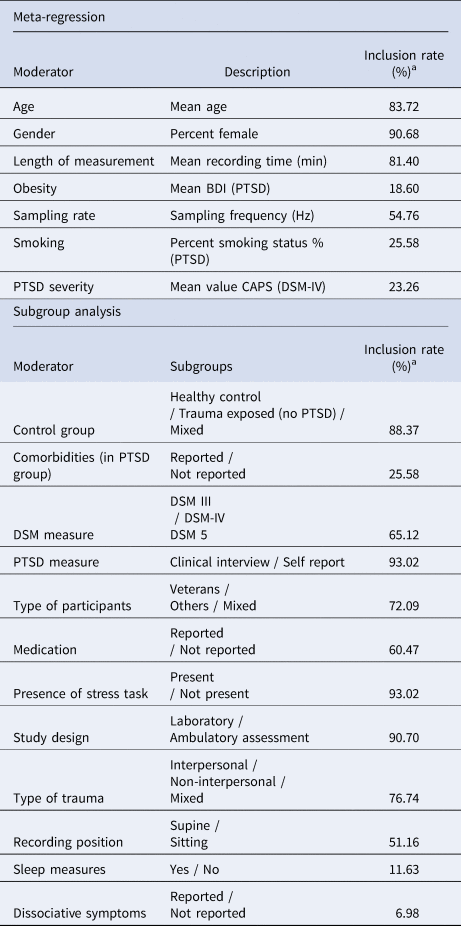
a Inclusion rate = percent of studies reporting information on the moderator.
Table 2. Characteristics of studies included in meta-analysis 1 (RMSSD)
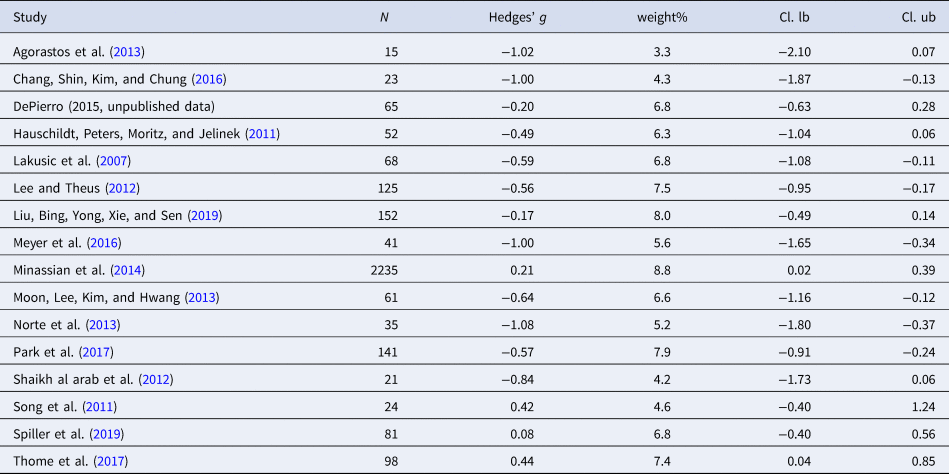
Table 3. Characteristics of studies included in meta-analysis 2 (SDNN)
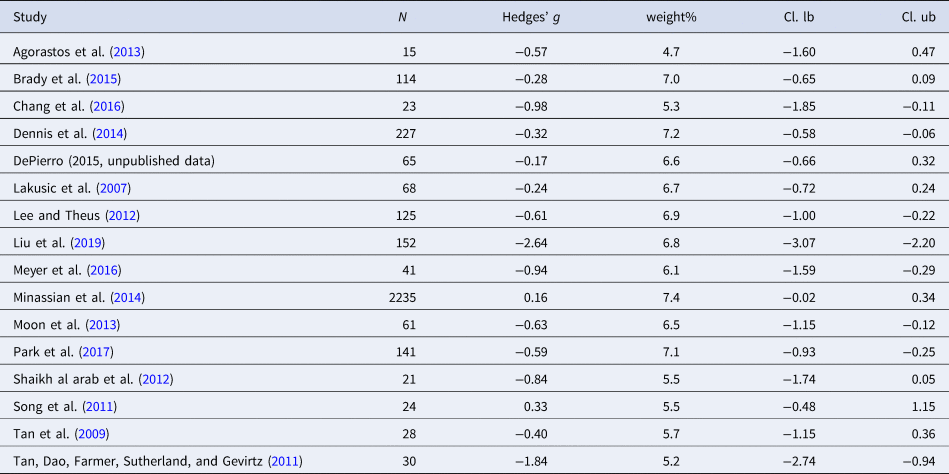
Table 4. Characteristics of studies included in meta-analysis 3 (HF-HRV)
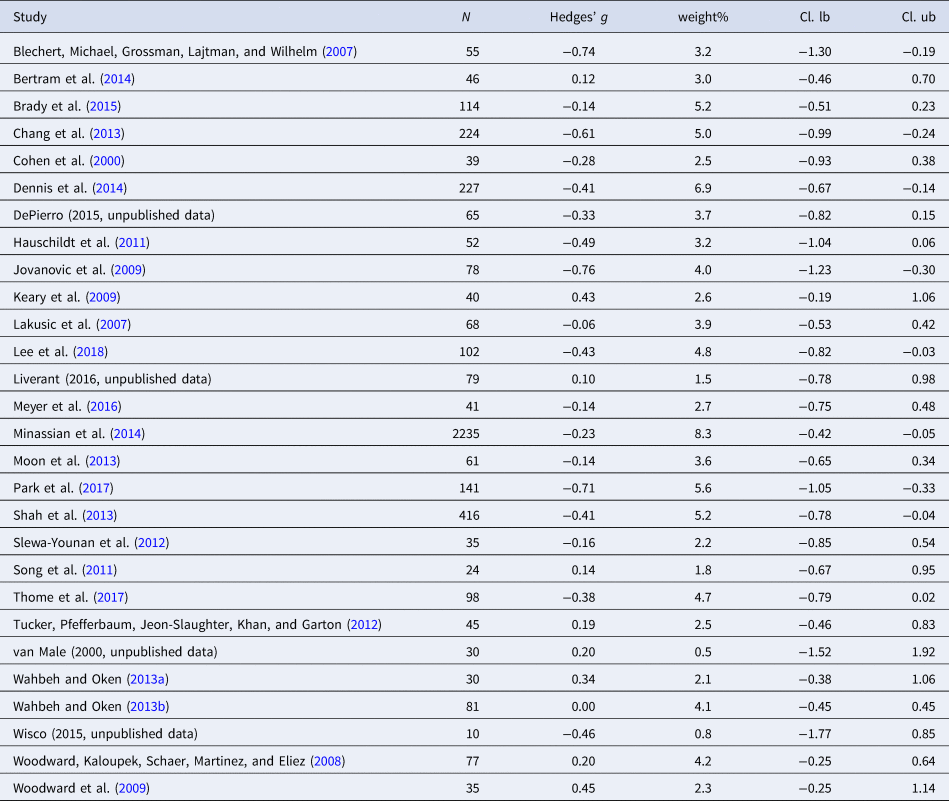
Table 5. Characteristics of studies included in meta-analysis 4 (LF-HRV)
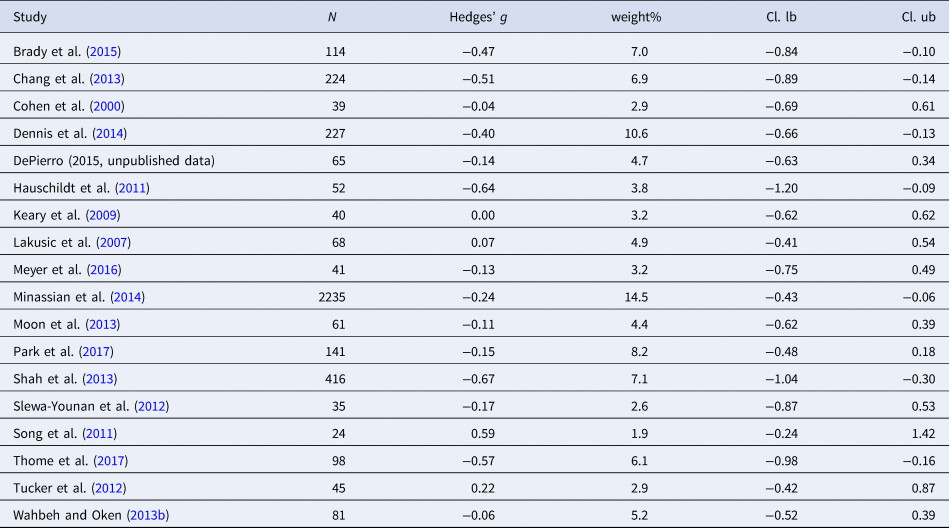
Table 6. Characteristics of studies included in meta-analysis 5 (LF/HF)
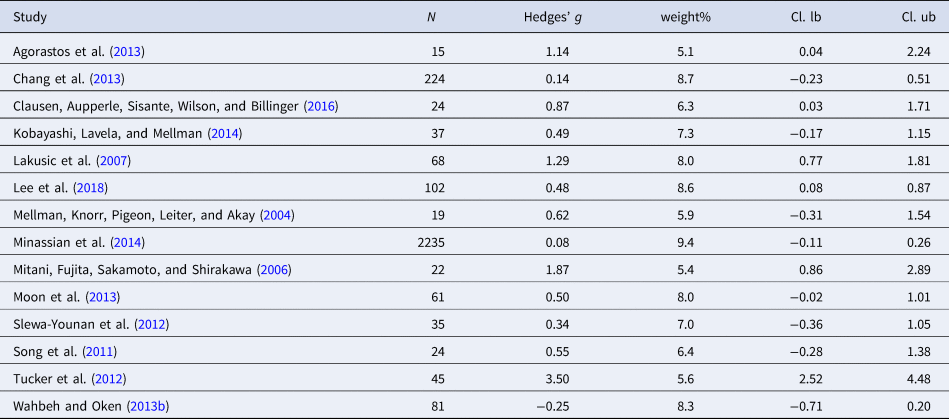
Table 7. Characteristics of studies included in meta-analysis 6 (HR)
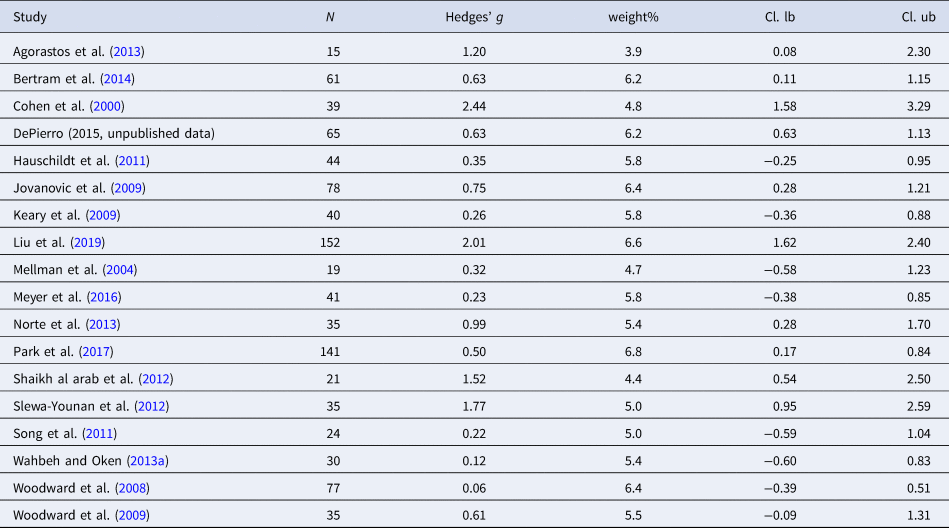
Table 8. Characteristics of studies included in meta-analysis 7 (HR stress activity)
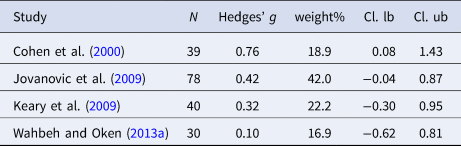
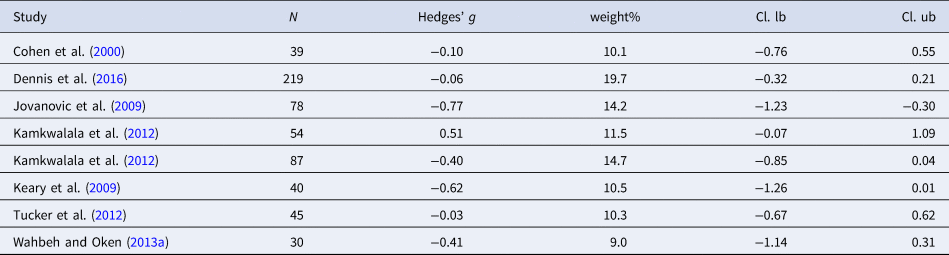
Table 9. Characteristics of studies included in meta-analysis 8 (HF-HRV stress activity)
Average age of the examined samples was 38.81 years. Thirty studies were laboratory studies, nine studies were ambulatory studies and in four studies information about study design was not available. Thirty-three studies reported the type of trauma, with interpersonal trauma being the most frequently reported trauma type. In the majority of studies, PTSD diagnosis was based on the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV, American Psychiatric Association, 1994). One study used the Minnesota Multiphasic Personality Inventory (MMPI-PTSD; Keane, Malloy, and Fairbank, Reference Keane, Malloy and Fairbank1984), one study used the Chinese Version of the Modified Schedule of Affective disorder and Schizophrenia-Lifetime (SADSL, Endicott & Spitzer, Reference Endicott and Spitzer1978) and one study used the International Statistical Classification of Diseases and Related Health Problems (ICD-9). One study utilized the Impact of Event Scale-revised (IES-R, Asukai et al., Reference Asukai, Kato, Kawamura, Kim, Yamamoto, Kishimoto and Nishizono-Maher2002) for PTSD diagnosis, using a cut-off score of 18 points. PTSD symptom severity was analyzed through the symptom severity score of the Clinical Administrated PTSD scale for DSM-IV (CAPS; Blake et al., Reference Blake, Weathers, Nagy, Kaloupek, Charney and Keane1998). Symptom severity scores ranged from 42.0 to 74.59 points, indicating moderate to severe PTSD symptomatology (Weathers, Keane, & Davidson, Reference Weathers, Keane and Davidson2001). Duration of time passed since the last traumatic event ranged from 2 months to 27.8 years. Fourteen studies reported recording HRV in a sitting position and seven studies in a supine position. Shortest measurement length of HRV was half a minute. Sampling rate ranged from 200 to 1440 Hz. Reported diagnosed psychiatric comorbidities were reported in 11 studies, comprising the following classes of mental disorders: depressive and anxiety disorders, eating disorders, personality disorders, substance-related and addictive disorders and somatic symptom disorders.
Effect sizes at baseline
Meta-analysis 1 – time-domain: root mean square of successive heart beat (RMSSD)
Sixteen studies were included in the meta-analysis (n = 3237). RMSSD was reduced in individuals with PTSD as compared to controls [Hedges' g = −0.38 (95% CI −0.62 to −0.13; PI = −1.30 to 0.55), p = 0.003], indicating a small effect size. Two outliers were identified. Exclusion of the outliers led to a slight increase in the effect size [Hedges' g = −0.49 (95% CI −0.68 to −0.29; PI = −1.05 to 0.08), p < 0.001]. Significant Q statistic (Q = 64.91, p < 0.001) indicated heterogeneity. Heterogeneity of the effect size was high (I 2 = 76.9%). Evidence for publication bias was revealed through Eggers regression test (p = 0.01). Trim and fill test indicated five missing studies. Adding the missing studies to the right part of the funnel plot changed the effect size substantially [Hedges' g = −0.16 (95% CI −0.40 to 0.08; PI = −1.17 to 0.85), p = 0.19], leading to a non-significant effect. Moderator analysis did not find significant moderators.
Meta-analysis 2 – time-domain: standard deviation of NN intervals (SDNN)
Sixteen studies were included in the meta-analysis (n = 3370). The aggregated effect size for SDNN was medium [Hedges' g = −0.64 (95% CI −1.01 to −0.27; PI = −2.17 to 0.88), p < 0.001], indicating that individuals with PTSD had smaller SDNN as compared to controls. Two outliers were found. Exclusion of the outliers lead to a decrease in the effect size [Hedges' g = −0.50 (95% CI −0.69 to −0.31; PI = −1.02 to 0.02), p < 0.001]. High levels of heterogeneity were detected (I 2 = 90.6%). Tests for publication bias did not indicate evidence for publication bias. Moderator analysis revealed sampling rate as a possible moderator variable (p = 0.01). Sampling rate ranged from 250 to 1024 Hz. A higher sampling rate was associated with a smaller effect size.
Meta-analysis 3 – frequency-domain: high-frequency HRV (HF-HRV)
Twenty-eight studies were included in the meta-analysis (n = 4548). Group differences were also evident in the HF-HRV parameter [Hedges' g = −0.23 (95% CI −0.34 to −0.11; PI = −0.65 to 0.20), p < 0.001]. The aggregated effect size was small. No outliers were detected and heterogeneity was low (I 2 = 44.6%). There was no evidence for publication bias. Significant moderators were not detected.
Meta-analysis 4 – frequency-domain: low-frequency HRV (LF-HRV)
Eighteen studies were included in the meta-analysis (n = 4006). LF-HRV was smaller in individuals with PTSD as compared to controls. The aggregated effect size was small [Hedges' g = −0.27 (95% CI −0.39 to −0.14; PI = −0.57 to 0.03), p ≤ 0.001]. No outliers were observed. The level of heterogeneity was low (I 2 = 27.6%). Evidence for publication bias was not present.
Meta-analysis 5 – frequency-domain: low-frequency/high-frequency ratio (LF/HF)
Fourteen studies were included in the meta-analysis (n = 2992). A medium effect size was detected for LF/HF ratio [Hedges' g = 0.72 (95% CI 0.36 to 1.08; PI = −0.69 to 2.08), p < 0.001]. Three outliers were detected. Exclusion of this outlier led to a decrease in the effect size [Hedges' g = 0.66 (95% CI 0.38–0.94; PI = −0.14 to 1.47), p < 0.001]. The level of heterogeneity was high (I 2 = 84.2%). Egger's regression test (p < 0.001) revealed evidence of publication bias. Trim and fill test indicated six missing studies. Adding the missing studies to the left part of the funnel plot changed the effect size substantially [Hedges' g = 0.22 (95% CI −0.17 to 0.60; PI = −1.50 to 1.94), p = 0.27], leading to a non-significant effect. Moderator analyses did not lead to a reduction in heterogeneity.
Meta-analysis 6 – heart rate (HR)
Eighteen studies were included in the meta-analysis (n = 952). HR was significantly higher in individuals with PTSD compared to controls [Hedges' g = 0.78 (95% CI 0.46 to 1.11; PI = −0.56 to 2.13), p < 0.001]. Two outliers were detected. Exclusion of the outliers led to a slight decrease in the effect size [Hedges' g = 0.56 (95% CI 0.36 to 0.76; PI = −0.01 to 1.14), p < 0.001]. No evidence for publication bias was found. High level of heterogeneity was observed (I 2 = 80.8%). Significant moderators were not detected.
Effect sizes during stress
Meta-analyses 7 and 8 – HR and HRV stress activity
Four studies were included in the analysis of HR during stress (n = 187). Individuals with PTSD evidenced higher HR during a stress task as compared to controls [Hedges' g = 0.41 (95% CI 0.11–0.70; PI = −0.24 to 1.05), p < 0.001]. No heterogeneity was observed (I 2 = 0.0%). Eight studies were included in the analysis of HF-HRV (n = 592). Individuals with PTSD tended to show lower HF-HRV during the stress task compared to controls [Hedges' g = −0.24 (95% CI −0.50 to −0.03; PI = −1.00 to 0.54), p = 0.089]; however, effect size did not reach significance. Moderate heterogeneity was observed (I 2 = 55.3%). Due to the low numbers of included studies in the meta-analyses on stress activity, no moderator analyses were carried out.
Discussion
The aim of this study was to examine differences in HRV parameters between individuals with PTSD and healthy controls at rest and during stress. Results indicate that individuals with PTSD have lower HRV, as compared to healthy controls, both at rest and during stress. Small negative effect sizes in RMSSD, HF-HRV and LF-HRV suggest reduced parasympathetic (i.e., vagal) activity in individuals with PTSD, as compared to controls. The moderate negative effect in SDNN highlights diminished total variability in PTSD. The positive effect size in the LF/HF ratio possibly suggests changes in sympatho-vagal balance in PTSD, and increased HR in PTSD at baseline and during stress may indicate higher SNS activity. Results suggest that changes in the ANS in individuals with PTSD are not restricted to pure vagally-mediated HRV parameters, but may rather indicate a general ANS dysregulation.
Importantly, the performed meta-analyses show predominantly high levels of heterogeneity, which may be due to relatively small sample sizes in the primary studies (median N = 46). Moderator variables such as physical health and physical activity, as well as physical and psychiatric comorbidities might also contribute to high heterogeneity. Moderator analysis did not reveal significant moderatos, with one exception being SDNN, where a higher sampling rate seems to be accompanied by smaller effect sizes. It is particularly surprising that moderator analyses on gender did not reveal significant results, since there is evidence for sex differences in PTSD (Tolin & Foa, Reference Tolin and Foa2006) and HRV (Koenig & Thayer, 2016). One possible reason for not being able to reveal significant moderators might be the relatively limited number of studies that provided sufficient information on moderators, hence questioning the robustness of the moderator analyses. Publication bias was evident in two meta-analyses, namely LF/HF ratio and RMSSD. Of note, a homogeneous pattern of physiological aberrations in PTSD might be questioned, since individuals suffering from PTSD show diverse symptoms, an example being some individuals who do not develop alterations in arousal.
Our findings generally support the results of the previous meta-analysis on PTSD and HRV by Nagpal et al. (Reference Nagpal, Gleichauf and Ginsberg2013) and extend the meta-analysis performed by Campbell et al. (Reference Campbell, Wisco, Silvia and Gay2019). Although the direction of effects is identical in all performed meta-analyses, they diverge in magnitude. Beside our study, only Nagpal et al. (Reference Nagpal, Gleichauf and Ginsberg2013) meta-analyzed differences in LF-HRV in individuals with PTSD and controls. Due to exclusion of unpublished data and a smaller number of studies included, they reported a comparably large effect for LF-HRV (Hedges' g = −1.72), which might overestimate the true effect. In the current meta-analysis, differences in LF-HRV between individuals with PTSD and controls were indicated by a small effect size. Publication bias was not evident, suggesting a more robust effect size estimation. LF-HRV has been related to different ANS mechanisms, reflecting a mixture of parasympathetic and sympathetic activity in long-term ambulatory studies, and primarily parasympathetic activity in resting state assessments in the laboratory (e.g. Shaffer, McCraty, and Zerr, Reference Shaffer, McCraty and Zerr2014). Hence, the findings suggest a general reduction of HRV in PTSD individuals as compared to controls.
One remaining question is, if lower HRV constitutes a risk factor for developing PTSD (vulnerability marker), or if lower HRV is a factor that develops during the course of PTSD (scar marker). Rombold-Bruehl et al. (Reference Rombold-Bruehl, Otte, Renneberg, Schmied, Zimmermann-Viehoff, Wingenfeld and Roepke2019) revealed that low HRV might impose risk for the frequency and recovery from intrusive memories, thus suggesting enhanced vulnerability. To examine the role of HRV as a scar marker, longitudinal studies are needed, which examine changes in HRV before a traumatic event, and within the development of PTSD. So far, mainly cross-sectional studies examined the relationship between HRV and PTSD. Hence, no causal relationship can be derived from the calculated effect sizes. Certainly, more research is needed to elucidate the role of HRV in the development of PTSD.
The findings of this meta-analysis are limited by the shortcomings of the primary studies included, and the general availability of studies within this field. First, due to high levels of heterogeneity interpretations of effects should be made with caution. Publication bias was evident in the meta-analyses on RMSSD and LF/HF ratio, thus suggesting rather fragile effects. Second, lower HRV in PTSD might be related to specific features of PTSD. Dennis et al. (Reference Dennis, Watkins, Calhoun, Oddone, Sherwood, Dennis and Beckham2014) showed that sleep disturbances in individuals with PTSD mediated the association between PTSD and HRV. Differences in PTSD symptom expressions could not be examined in the current meta-analyses. Third, due to missing data in primary studies, data on stress reactivity (relative to baseline), as well as recovery, could not be included. Impaired recovery seems to be a main aspect of PTSD symptoms (Pole, Reference Pole2007), which calls for further research.
In conclusion, the current meta-analysis supports previous reviews suggesting that PTSD is associated with lower HRV, thus highlighting the need for a stronger focus on examining HRV changes within the development and course of PTSD. The results highlight that not a single HRV parameter is particularly indicative for PTSD. Rather, changes in HRV occurred in vagally-mediated HRV parameters, as well as in more complex measures, thus indicating a general pattern of ANS dysregulation. Of note, alterations in ANS functioning in individuals with PTSD seem to be evident during both rest and stress. High levels of heterogeneity indicate substantial variance between studies included in the analyses. The majority of the examined moderator variables could not explain this heterogeneity. Therefore, the focus on identifying moderator variables that influence the relationship between PTSD and low HRV seems crucial for gaining a better understanding of the psychophysiological connections between HRV and PTSD.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S003329172000207X.
Conflict of interest
All authors declare that they have no conflict of interest.












