Chronic obstructive pulmonary disease (COPD) is a common, preventable and treatable clinical condition, characterised by persistent respiratory symptoms and progressive airflow limitation due to airway and/or alveolar abnormalities usually resulting from significant exposure to harmful particles or gases(1). At a global level, COPD prevalence is about 12·2 %(Reference Varmaghani, Dehghani and Heidari2), and according to WHO(3), the disease was the third leading mortality cause in 2020, responsible for approximately 6 % of all deaths. Recent epidemiological findings indicate an increase in COPD prevalence, associated with the world population ageing(Reference Varmaghani, Dehghani and Heidari2).
In addition to pulmonary involvement, the disease has an extra-pulmonary component evidenced by its systemic effects, which affect the nutritional status of patients(1). The nutritional abnormalities manifested mainly as reduced muscle mass, strength and/or function and involuntary weight loss – regardless of BMI values(Reference Machado, Spruit and Groenen4), low body weight (BW) and nutrient deficiencies(Reference Schols, Ferreira and Franssen5) tend to coexist and predict worse outcomes, including poor pulmonary function(Reference Alea, Mateo and De Guia6), impaired exercise capacity(Reference Machado, Schneider and Fonseca7), health-related quality of life(Reference Shoup, Dalsky and Warner8), higher hospital readmissions(Reference Girón, Matesanz and García-Río9), length of hospital stay(Reference Teixeira, Kowalski and Valduga10,Reference Attaway, Welch and Hatipoğlu11) , health costs(Reference Attaway, Welch and Hatipoğlu11,Reference Jerng, Tang and Cheng12) and mortality rates(Reference Attaway, Welch and Hatipoğlu11,Reference Schols, Slangen and Olovics13,Reference Schols, Broekhuizen and Weling-Scheepers14) .
Compromised nutritional status prevalence among COPD patients has been reported to be as high as 45 %(Reference Dávalos-Yerovi, Marco and Sánchez-Rodríguez15), depending on assessment method, diagnostic criteria and cutoffs applied, as well the studied population. The pathogenesis of nutritional abnormalities in COPD is complex and involves multiple factors interaction, including increased systemic inflammation and oxidative stress, hypoxia, acute exacerbations, corticosteroids use(Reference Rawal and Yadav16,Reference Gea, Sancho-Muñoz and Chalela17) , COPD symptoms and patient-related factors such as age, genetics, lifestyle and psychological aspects(Reference Gea, Sancho-Muñoz and Chalela17).
The impact of nutritional status on the general condition of COPD patients manifests itself mainly through weight loss and muscle wasting. Unintentional weight loss occurs in almost 50 % of the patients with severe COPD and about 15 % of patients with mild-to-moderate COPD(Reference Schols, Soeters and Dingemans18). The decline in pulmonary function(Reference Bhakare, Godbole and Khismatrao19,Reference Chaudhary, Rao and Sawlani20) , acceleration in disease progression(Reference Rawal and Yadav16) and impaired resistance to infections can be expected in malnourished COPD patients(Reference Gea, Sancho-Muñoz and Chalela17). In addition, COPD patients present peripheral muscle dysfunction and atrophy, expressed as muscle strength and endurance reduction(Reference Maltais, Decramer and Casaburi21). Dynamometric parameters are negatively associated with the disease stage(Reference Kovarik, Joskova and Patkova22) and with peak inspiratory flow rate generation(Reference Samarghandi, Ioachimescu and Qayyum23).
In clinical practice, oral nutritional supplements (ONS) or fortified food (FF) prescription is the therapeutic first choice for patients with or at risk of malnutrition(1,Reference Anker, Laviano and Filippatos24,Reference Bauer, Biolo and Cederholm25) . However, the first meta-analysis(Reference Ferreira, Brooks and Lacasse26) investigating the ONS (energetic supplementation for at least 2 weeks) influence in stable COPD patients outcomes, involving nine trials (n 277 subjects), failed to show consistent benefits on anthropometric measurements, lung function and exercise capacity in patients with COPD. There is a growing literature on the effect of energy and protein-based supplementation or FF on nutritional and clinical outcomes in the COPD population. The latest published meta-analyses of randomised controlled trials (RCT) evaluating the effect of nutritional support in COPD patients concluded that ONS(Reference Collins, Stratton and Elia27–Reference Ferreira, Brooks and White29), mainly in depleted patients, resulted in statistically significant increases in BW, midarm muscle circumference, skinfold thickness and in respiratory muscle strength. However, these reviews have emphasised the need for more high-quality RCT to confirm the role of nutritional support in COPD.
Two of the meta-analyses cited above had several analytical limitations(Reference Collins, Stratton and Elia27,Reference Collins, Elia and Stratton28) . The authors did not report whether a dose–response gradient was performed and did not make the subgroups analysis considering features, including patients’ clinical status and energy and/or protein quantities prescribed(Reference Collins, Stratton and Elia27,Reference Collins, Elia and Stratton28) . In addition, since 2012, more than forty references about this topic were published. Furthermore, a more recent systematic review(Reference Aldhahir, Rajeh and Aldabayan30) of twenty-two studies (observational and intervention design) investigated the current evidence supporting the use of any nutritional supplementation (vitamins, PUFA, protein, carbohydrates, etc.) to improve outcomes during PR in stable COPD patients, concluded that the results are controversial and pointed to the need for further studies in this area. However, the authors performed only a narrative synthesis of the evidence. The current systematic review aimed to overcome the limitations of previously published systematic reviews addressing the effects of energy and/or protein ONS or FF on anthropometry, body composition and muscle strength of COPD patients and to provide an up-to-date evidence synthesis.
Methods
Study design
This systematic review of RCT was conducted according to the recommendations of Cochrane Handbook for Systematic Reviews of Interventions Version 6·11(Reference Higgins, Thomas and Chandler31) and reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines(Reference Page, McKenzie and Bossuyt32). We registered its protocol in the International Prospective Register of Systematic Reviews (PROSPERO) under number CRD42020207577.
Research question
The objective of this study was to evaluate the available evidence from RCT for the following clinical question: ‘What is the effect of dietary interventions with energy and/ or protein ONS and/or FF on nutritional status parameters in COPD patients?’.
We defined the intervention as any nutritional therapy based on energy and/or protein (e.g. creatine or whey protein or amino acid metabolites) and/or amino acids (e.g. isolated or in combination form) ONS or FF added to the diet for one or more week. And we defined the control group as a usual diet or placebo or dietary advice, as reported by authors in the primary studies.
Eligibility criteria
We developed inclusion and exclusion criteria using the Patient, Intervention, Comparators, Outcome, and Study Design (PICOS) method (Table 1). We included all RCT (parallel or crossover design) that assessed the effects of different ONS or FF reporting at least one of the outcomes of interest: (a) BW; (b) lean body mass (LBM); (c) fat mass (FM) and (d) peripheral muscle strength. The studies should be performed in adults over 40 years of age with a COPD diagnosis.
Table 1. PICO strategy for inclusion and exclusion criteria
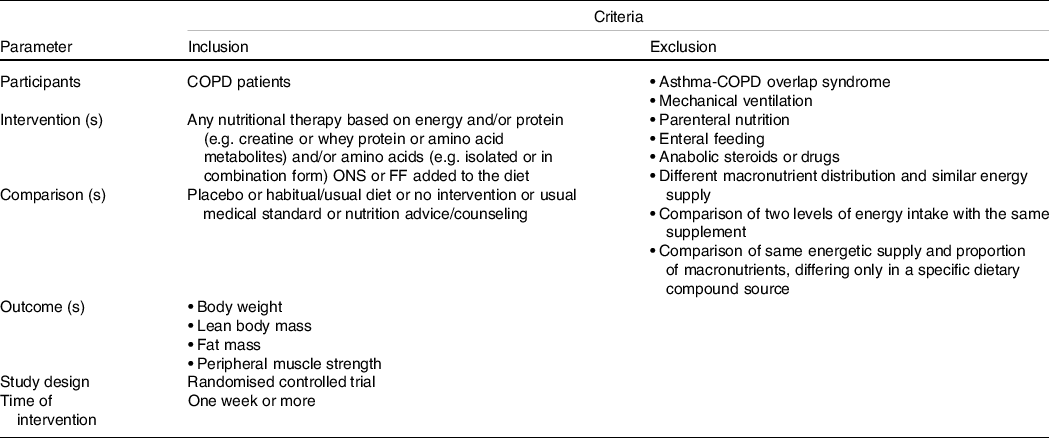
We excluded studies that used the following interventions: 1. Enteral or parenteral nutrition. 2. ONS with different macronutrient distribution between the groups. 3. Comparison between two active ONS, but with distinct energy proposes. 4. Comparison between two protein sources ONS. 5. ONS of antioxidants or nitrate. Moreover, we excluded studies with COPD patients under mechanical ventilation, observational studies, literature reviews, opinion papers, non-randomised trials and abstracts with irretrievable full-text after two attempts to contact the authors.
Search methods for identification of studies
We identified studies through a comprehensive search strategy developed and conducted by S.B. and F.M.S. in the following electronic databases on 2 September 2020: MEDLINE (PubMed), EMBASE, Cochrane Library and Scopus. There were no restrictions to language or date of publication. We identified appropriate controlled vocabulary (e.g. MeSH terms and Emtree terms) and free-text terms (considering, for example, spelling variants, synonyms, acronyms) in all databases: COPD (population); nutrition therapy, dietary supplements, food supplements and FF (interventions). Supplementary Table S1 presents the full search strategy performed in PubMed. We updated the search on 18 May 2021.
Selection of studies
We screened records by title and abstract in a reference manager software (EndNote X9.3.1, Clarivate Analytics) based on the eligibility criteria by two independent investigators (S.B. and F.M.S.) after the automatic exclusion of duplicates. Bibliographic references of all studies included in this systematic review were hand-searched to identify additional RCT not identified through electronic searching. Also, we used the ‘cited by’ link function in PubMed for each article included in this review to identify potential eligible RCT. To explore the grey literature, we searched the USA National Library of Medicine (ClinicalTrials.gov). After exclusion of irrelevant records, we assessed full-text articles for eligibility through a standardised electronic form in Google Forms® by each investigator in an independent manner (S.B. and V.K./S.B. and C.F.B.). A third researcher (F.M.S.) resolved any disagreement between reviewers through discussion before inclusion.
Data collection
Data were also independently extracted by three reviewers grouped in pairs (S.B., V.K. and C.F.B.) through a standardised electronic form in Google Forms®, and subsequently cross-checked in conjunction with F.M.S. before computing entries in structured tables in Microsoft Office Excel®. We extracted the following data from each study: first author; year of publication; study design; country of study; intervention time; measured endpoints; inclusion and exclusion criteria; sample size; male percentage of sample; sample mean age; sample mean baseline values of BMI or ideal BW percentage and sample mean baseline forced expiratory volume in the first second in liters and/or predicted%. We also collected information about the setting of nutritional intervention, considering a Pulmonary Rehabilitation setting when the study referred to it as such or if the trial applied an exercise resistance training systematic, regardless of its period and frequency. Detailed descriptions of each intervention were also extracted, as well as baseline and at the end of the intervention measurements of the outcomes values – as mean and standard deviation (sd) in each intervention. When studies report only median values, standard error, CI, interquartile intervals and minimum and maximum, we transformed these values, obtained by the calculations presented in the Cochrane Handbook(Reference Higgins, Thomas and Chandler31).
Assessment of risk of bias in included studies
The risk of bias within individual trials was assessed by two independent investigators (S.B. and F.M.S.) using the revised Cochrane risk-of-bias tool for randomised trials (RoB 2)(Reference Sterne, Savović and Page33), and final judgements were established by consensus. Each study was evaluated with regard to the five following domains: (1) bias arising from the randomisation process; (2) bias due to deviations from intended interventions; (3) bias due to missing outcome data; (4) bias in the measurement of the outcome and (5) bias in the selection of the reported result. The overall risk of bias judgement was (a) low risk of bias, if the trial judgement was at low risk of bias for all domains; (b) some concerns, if the trial judgement raised some concerns in at least one domain for this result, but not was at high risk of bias for any domain and (c) high risk of bias, if the trial judgement was at high risk of bias in at least one domain or some concerns for multiple domains in a way that substantially lowered confidence in the results.
Grading of Recommendations, Assessment, Development and Evaluations assessment of the certainty of the evidence
We assessed the overall certainty of the evidence for each outcome across studies following the Grading of Recommendations, Assessment, Development and Evaluations approach(Reference Schünemann, Brożek and Guyatt34). Certainty of evidence was considered ‘high’ by default and thereafter downgraded to ‘moderate’, ‘low’ or ‘very low’ depending on the seriousness of the limitations in five criteria: within-study risk of bias, the directness of evidence, inconsistency, the precision of effect estimates and risk of publication bias.
Data synthesis and analysis
We collected the mean change of the data values between post- and pre-treatment of the study (delta) and the sd of this value (delta sd) in each arm of the study for the outcome of interest. If the primary studies did not report mean change in each group, we obtained it by subtracting the post-intervention mean from the baseline. While in delta sd absence, we estimated it assuming a correlation of 0·5 between the baseline and final measures within each group, according to the formula of Follmann et al. (Reference Follmann, Elliott and Suh35), as proposed in the Cochrane guidelines(Reference Higgins, Thomas and Chandler31). Equal variance was assumed among trials and between intervention and controls.
We performed our meta-analyses using DerSimonian and Laird random-effects model regardless of statistical heterogeneity, assuming that there is not one single true effect size across studies due to clinical and methodological diversity between studies. Statistical tests of the significance of τ2 to choose between fixed and random-effects models are not recommended by the Cochrane Handbook(Reference Higgins, Thomas and Chandler31) because these tests have undesirable statistical properties and fundamentally should not determine the most appropriate meta-analytic model. Instead, we used our desired inference to guide which model to use. The choice of random effects allows for unconditional inferences that are not restricted to the observed studies(Reference Hedges and Vevea36). In addition, the meta-analytic model was adjusted using the Hartung–Knapp–Sidik–Jonkman method(Reference IntHout, Ioannidis and Borm37) to produce more robust estimates with more conservative results.
We calculated continuous outcomes and presented them as weighted mean differences (WMD) of changes from baseline with means and sd for BW, midarm muscle circumference and triceps skinfold when studies measured outcomes in the same way and as standardised mean difference (SMD) and 95 % CI for LBM and FM, and handgrip and quadriceps strength presented as Hedges’ g(Reference Higgins, Thomas and Chandler31) to combine trials that measure the same outcome but used different units of measurement (e.g. for fat-free mass in kg, percent and kg/m2). As a rough guide, effect sizes in SMD are interpreted as suggested by Cohen(Reference Cohen38). Small effects ‘that cannot be discerned by the naked eye’ are around 0·2; medium effects are around 0·5 and large effects ‘that can be seen by the naked eye’ are around 0·8. For outcomes reported in SMD, we also performed stratified analyses based on the original measurement scale presented as WMD to enhance the interpretability of our results. All results are presented with point estimates along with 95 % CI.
Assessment of heterogeneity, exploratory analyses and publication bias
We assessed the magnitude of statistical heterogeneity using the I 2 , accordingly the following interpretation(Reference Higgins, Thomas and Chandler31): values from 0 % to 40 % might not be important; 30 % to 60 % may represent moderate heterogeneity; 50 % to 90 % may represent substantial heterogeneity and 75 % to 100 % considerable heterogeneity. Aiming to identify potential sources of heterogeneity and effect mediators, we planned to perform several exploratory subgroup analyses based on: (a) within-study risk of bias, (b) whether the intervention consisted of an energy supplement, protein supplement or energy-protein supplement, (c) clinical status (stable v. unstable patients) and (d) baseline overall sample BMI (with 22 kg/m2 as the cutoff point). Furthermore, we performed univariate meta-regression analyses to further investigate the statistical heterogeneity and to identify potential associations between our primary outcomes and the following study-level covariates as continuous variables: (a) baseline overall sample BMI; (b) intervention duration (in weeks); (c) total amount of prescribed supplement energy content in the intervention arm and (d) total amount of prescribed supplement protein content in the control group. In the presence of crossover RCT design, we temporarily excluded these studies to determine whether its removal altered the results of the meta-analysis. Where there were at least ten studies (BW, LBM, HGS and QS), we used funnel plots to visually assess the risk for publication bias and small-study effects, along with Egger’s regression test. The trim-and-fill method was used to adjust for publication bias.
Changes in the review protocol
During the review process, we opted to perform two modifications in the study protocol, due to the high amount of data extracted. First, we restricted the intervention to oral nutritional supplements or food fortification adding energy and/or protein. Second, we opted by presenting the results in two independent publications: the first one is the current and will answer the research question ‘effect of nutrition therapy in anthropometric parameters, body composition and peripheral muscle strength in COPD patients’, while the second one will answer the research question ‘effect of nutrition therapy in clinical and functional outcomes in COPD patients’. It was included in the PROSPERO.
Results
Selection and general characteristics of included studies
We initially identified a total of 3807 articles through database searches, of which 490 were duplicates. We assessed the full text of forty-eight studies for eligibility and included thirty-two(Reference Lewis, Belman and Dorr-Uyemura39–Reference Saudny-Unterberger, Martin and Gray-Donald70) of them in the current systematic review (Fig. 1). The number of studies included in the meta-analysis varied according to the outcomes. Supplementary Table S2 presents the list of excluded studies after inspection of the full report and a justification for this exclusion(Reference Angelillo, Bedi and Durfee71–Reference Zongxing86).

Fig. 1. Flow diagram of study selection.
Table 2 presents general features of eligible studies for this systematic review. Trials were conducted in a variety of countries including Canada(Reference Knowles, Fairbarn and Wiggs51,Reference Saudny-Unterberger, Martin and Gray-Donald70) , Denmark(Reference Ahnfeldt-Mollerup, Hey and Johansen57,Reference Otte, Ahlburg and D’Amore62) , India(Reference Khan, Kumar and Daga58), Iran(Reference Ahmadi, Eftekhari and Mazloom64), Italy(Reference Baldi, Aquilani and Pinna48,Reference Dal Negro, Aquilani and Bertacco49,Reference Dal Negro, Testa and Aquilani52,Reference Marinari, Manigrasso and De Benedetto56,Reference De Benedetto, Pastorelli and Ferrario60) , Japan(Reference Sugawara, Takahashi and Kasai50,Reference Sugawara, Takahashi and Kashiwagura53) , Netherlands(Reference Goris, Vermeeren and Wouters41,Reference Vermeeren, Wouters and Geraerts-Keeris43,Reference van Beers, Rutten-van Mölken and van de Bool63,Reference Schols, Soeters and Mostert69) , Spain(Reference Entrenas Costa, Domínguez Platas and Checa Pinilla66), Sweden(Reference Faager, Söderlund and Sköld45,Reference van de Bool, Rutten and van Helvoort59) , Switzerland(Reference Ganzoni, Heilig and Schönenberger68), Turkey(Reference Gurgun, Deniz and Argin55,Reference Degirmenci, Şahin and Soylu61) , England(Reference Efthimiou, Fleming and Gomes40,Reference Steiner, Barton and Singh42,Reference Fuld, Kilduff and Neder44,Reference Deacon, Vincent and Greenhaff46,Reference Weekes, Emery and Elia47,Reference Constantin, Menon and Houchen-Wolloff54) and USA(Reference Lewis, Belman and Dorr-Uyemura39,Reference Fuenzalida, Petty and Jones65,Reference Rogers, Donahoe and Costantino67) . The studies were published between 1987 39 and 2020(Reference van Beers, Rutten-van Mölken and van de Bool63,Reference Ahmadi, Eftekhari and Mazloom64) , most of which (n 21; 1252 participants)(Reference Goris, Vermeeren and Wouters41–Reference Sugawara, Takahashi and Kasai50,Reference Constantin, Menon and Houchen-Wolloff54–Reference Degirmenci, Şahin and Soylu61,Reference van Beers, Rutten-van Mölken and van de Bool63,Reference Ahmadi, Eftekhari and Mazloom64) from the year 2003. Almost all manuscripts were available in the English language, except for one in German(Reference Ganzoni, Heilig and Schönenberger68) and another in Spanish(Reference Entrenas Costa, Domínguez Platas and Checa Pinilla66). Only one study applied a crossover design (twenty-five participants), with eight weeks of the intervention period (no washout period reported)(Reference Knowles, Fairbarn and Wiggs51). The remaining thirty-one studies were parallel-group design, with a mean intervention duration of about 12 weeks (ranging from nine days(Reference Vermeeren, Wouters and Geraerts-Keeris43) to 12 months(Reference van Beers, Rutten-van Mölken and van de Bool63,Reference Ganzoni, Heilig and Schönenberger68) ).
Table 2. General characteristics of included randomised controlled trial (RCT) (n 32) investigating the effect of oral nutrition therapy on nutritional parameters of chronic obstructive pulmonary disease (COPD) patients
(Mean values and standard deviations)

NR, not reported; FEV1, forced expiratory volume in 1 s; FVC, forced vital Capacity; CRC, Clinical Research Centre; CRU, Clinical Research Unit; PRP, pulmonary rehabilitation program.
* There is one other group in this study not included in our analysis that was not considered for us since the intervention in this study arm was anabolic steroid-based.
The thirty-two studies included in this review randomised a total of 1680 participants. Of these, we included 1414 in the statistical analysis because most studies reported only an analysis per protocol, excluding participants who dropped out of the study or did not complete the protocol sufficiently. The mean sample size was equal to 52·5 participants (ranging from nine(Reference Fuenzalida, Petty and Jones65) to 233(Reference Schols, Soeters and Mostert69)), with a mean age of 67·0 years (ranging from 54·2(Reference Khan, Kumar and Daga58) to 77·7(Reference Sugawara, Takahashi and Kasai50)), of which approximately 70 % were male (although in six studies(Reference Sugawara, Takahashi and Kasai50,Reference Marinari, Manigrasso and De Benedetto56,Reference Entrenas Costa, Domínguez Platas and Checa Pinilla66–Reference Schols, Soeters and Mostert69) gender information was unavailable). The mean of forced expiratory volume in one second was equal to 42·1 % of predicted values (ranging from 31·4(Reference Efthimiou, Fleming and Gomes40) to 56 %(Reference De Benedetto, Pastorelli and Ferrario60)) – data not reported in eight studies(Reference Lewis, Belman and Dorr-Uyemura39,Reference Dal Negro, Aquilani and Bertacco49,Reference Dal Negro, Testa and Aquilani52,Reference Ahnfeldt-Mollerup, Hey and Johansen57,Reference Fuenzalida, Petty and Jones65,Reference Rogers, Donahoe and Costantino67–Reference Schols, Soeters and Mostert69) – and mean of BMI was equal 22·3 kg/m2 (ranging from 17·2(Reference Degirmenci, Şahin and Soylu61) to 30·9 kg/m2(Reference De Benedetto, Pastorelli and Ferrario60)) (data not reported in eleven studies(Reference Lewis, Belman and Dorr-Uyemura39,Reference Efthimiou, Fleming and Gomes40,Reference Sugawara, Takahashi and Kashiwagura53,Reference Otte, Ahlburg and D’Amore62,Reference Fuenzalida, Petty and Jones65,Reference Entrenas Costa, Domínguez Platas and Checa Pinilla66,Reference Ganzoni, Heilig and Schönenberger68–Reference Saudny-Unterberger, Martin and Gray-Donald70) ).
Twenty-nine studies included COPD patients in stable clinical condition (1554)(Reference Lewis, Belman and Dorr-Uyemura39–Reference Goris, Vermeeren and Wouters41,Reference Fuld, Kilduff and Neder44–Reference Weekes, Emery and Elia47,Reference Dal Negro, Aquilani and Bertacco49–Reference Dal Negro, Testa and Aquilani52,Reference Constantin, Menon and Houchen-Wolloff54–Reference Khan, Kumar and Daga58,Reference De Benedetto, Pastorelli and Ferrario60–Reference Ahmadi, Eftekhari and Mazloom64,Reference Ganzoni, Heilig and Schönenberger68) , and the majority of them were outpatient based (1256 participants). In twelve studies, the patients participated in a pulmonary rehabilitation program (798 participants)(Reference Steiner, Barton and Singh42,Reference Fuld, Kilduff and Neder44–Reference Deacon, Vincent and Greenhaff46,Reference Baldi, Aquilani and Pinna48,Reference Sugawara, Takahashi and Kasai50,Reference Sugawara, Takahashi and Kashiwagura53–Reference Gurgun, Deniz and Argin55,Reference Ahnfeldt-Mollerup, Hey and Johansen57,Reference van de Bool, Rutten and van Helvoort59,Reference Schols, Soeters and Mostert69) ; in three studies, the nutritional intervention started in inpatient base and continued in an outpatient modality after discharge (sixty-five participants),(Reference Baldi, Aquilani and Pinna48,Reference Fuenzalida, Petty and Jones65,Reference Rogers, Donahoe and Costantino67) of which one study (twenty-eight participants) took place in pulmonary rehabilitation in the outpatient part,(Reference Baldi, Aquilani and Pinna48) and in one study (233 participants) the intervention was entirely in an inpatient pulmonary rehabilitation(Reference Schols, Soeters and Mostert69). On the other hand, three studies included only COPD patients admitted to the hospital for an acute exacerbation (126 participants)(Reference Vermeeren, Wouters and Geraerts-Keeris43,Reference Entrenas Costa, Domínguez Platas and Checa Pinilla66,Reference Saudny-Unterberger, Martin and Gray-Donald70) .
Treatment groups of the included studies received the following interventions, stratified according to ONS composition (online Supplementary Table S3):
-
(1) Supplement of energy and high protein (≥ 20 % of total energy from protein): it was the intervention in fourteen RCT (659 participants)(Reference Efthimiou, Fleming and Gomes40,Reference Vermeeren, Wouters and Geraerts-Keeris43,Reference Knowles, Fairbarn and Wiggs51,Reference Sugawara, Takahashi and Kashiwagura53,Reference Constantin, Menon and Houchen-Wolloff54,Reference Ahnfeldt-Mollerup, Hey and Johansen57,Reference van de Bool, Rutten and van Helvoort59,Reference Degirmenci, Şahin and Soylu61,Reference van Beers, Rutten-van Mölken and van de Bool63,Reference Rogers, Donahoe and Costantino67,Reference Ganzoni, Heilig and Schönenberger68) , the mean energy density was 1·3 kcal/ml (ranging from 0·54 to 1·5) and it provided a mean of 528·6 kcal (ranging from 312·5(Reference van de Bool, Rutten and van Helvoort59,Reference van Beers, Rutten-van Mölken and van de Bool63) to 960(Reference Efthimiou, Fleming and Gomes40) kcal) and 27·2 grams of protein (ranging from 10·4(Reference van Beers, Rutten-van Mölken and van de Bool63) – 54(Reference Efthimiou, Fleming and Gomes40) g protein);
-
(2) Protein-based and amino acids supplements: it was the intervention in ten studies (560 participants)(Reference Fuld, Kilduff and Neder44–Reference Deacon, Vincent and Greenhaff46,Reference Baldi, Aquilani and Pinna48,Reference Dal Negro, Aquilani and Bertacco49,Reference Dal Negro, Testa and Aquilani52,Reference Marinari, Manigrasso and De Benedetto56,Reference Khan, Kumar and Daga58,Reference De Benedetto, Pastorelli and Ferrario60,Reference Ahmadi, Eftekhari and Mazloom64) , of which five used creatine (306 participants)(Reference Fuld, Kilduff and Neder44–Reference Deacon, Vincent and Greenhaff46,Reference Marinari, Manigrasso and De Benedetto56,Reference De Benedetto, Pastorelli and Ferrario60) , three prescribed essential amino acids mixture (148 participants)(Reference Baldi, Aquilani and Pinna48,Reference Dal Negro, Aquilani and Bertacco49,Reference Dal Negro, Testa and Aquilani52) , one used whey protein (forty-six participants)(Reference Ahmadi, Eftekhari and Mazloom64), one prescribed a ‘protein powder’, but did not specify its sources (sixty participants)(Reference Khan, Kumar and Daga58);
-
(3) Energy supplement (< 20 % of total energy from protein): it was the intervention in eight studies (461 participants)(Reference Lewis, Belman and Dorr-Uyemura39,Reference Weekes, Emery and Elia47,Reference Sugawara, Takahashi and Kasai50,Reference Gurgun, Deniz and Argin55,Reference Fuenzalida, Petty and Jones65,Reference Entrenas Costa, Domínguez Platas and Checa Pinilla66,Reference Schols, Soeters and Mostert69,Reference Saudny-Unterberger, Martin and Gray-Donald70) , the mean of ONS energy density was 1·6 kcal/ml (ranging from 1·0(Reference Sugawara, Takahashi and Kasai50) to 2·1(Reference Schols, Soeters and Mostert69)) and it provided mean daily energy of 565 kcal (ranging from 400(Reference Sugawara, Takahashi and Kasai50) to 720(Reference Lewis, Belman and Dorr-Uyemura39,Reference Fuenzalida, Petty and Jones65) ) and 18·9 grams of protein (ranging from 9·0(Reference Weekes, Emery and Elia47) to 28·8(Reference Fuenzalida, Petty and Jones65)).
Most comparisons involved ONS v. placebo(Reference Steiner, Barton and Singh42–Reference Deacon, Vincent and Greenhaff46,Reference Dal Negro, Aquilani and Bertacco49,Reference Constantin, Menon and Houchen-Wolloff54,Reference Marinari, Manigrasso and De Benedetto56,Reference van de Bool, Rutten and van Helvoort59,Reference De Benedetto, Pastorelli and Ferrario60,Reference Otte, Ahlburg and D’Amore62,Reference van Beers, Rutten-van Mölken and van de Bool63,Reference Schols, Soeters and Mostert69) , but we found a variety of other comparisons including usual diet(Reference Lewis, Belman and Dorr-Uyemura39,Reference Efthimiou, Fleming and Gomes40,Reference Knowles, Fairbarn and Wiggs51,Reference Khan, Kumar and Daga58,Reference Rogers, Donahoe and Costantino67) hospital diet(Reference Fuenzalida, Petty and Jones65,Reference Entrenas Costa, Domínguez Platas and Checa Pinilla66,Reference Saudny-Unterberger, Martin and Gray-Donald70) , nutritional advice(Reference Goris, Vermeeren and Wouters41,Reference Ahmadi, Eftekhari and Mazloom64) , leaflet providing advice (content was never discussed)(Reference Weekes, Emery and Elia47), monthly general education program(Reference Sugawara, Takahashi and Kasai50), normal energy diet(Reference Ganzoni, Heilig and Schönenberger68), normal meals alone with dietary instruction(Reference Sugawara, Takahashi and Kashiwagura53) and the remaining three trials not informed the comparators(Reference Baldi, Aquilani and Pinna48,Reference Ahnfeldt-Mollerup, Hey and Johansen57,Reference Degirmenci, Şahin and Soylu61) (online Supplementary Table S3).
Eighteen studies assessed the compliance with the study protocol(Reference Steiner, Barton and Singh42,Reference Vermeeren, Wouters and Geraerts-Keeris43,Reference Faager, Söderlund and Sköld45–Reference Baldi, Aquilani and Pinna48,Reference Sugawara, Takahashi and Kashiwagura53–Reference Gurgun, Deniz and Argin55,Reference Ahnfeldt-Mollerup, Hey and Johansen57–Reference van de Bool, Rutten and van Helvoort59,Reference Otte, Ahlburg and D’Amore62–Reference Ahmadi, Eftekhari and Mazloom64,Reference Ganzoni, Heilig and Schönenberger68,Reference Schols, Soeters and Mostert69) , of which half of them reported results(Reference Steiner, Barton and Singh42,Reference Vermeeren, Wouters and Geraerts-Keeris43,Reference Deacon, Vincent and Greenhaff46–Reference Baldi, Aquilani and Pinna48,Reference van de Bool, Rutten and van Helvoort59,Reference Degirmenci, Şahin and Soylu61,Reference Ahmadi, Eftekhari and Mazloom64,Reference Fuenzalida, Petty and Jones65) (online Supplementary Table S3) with inconsistent definitions and measurement units, which made it impossible to estimate the general rates of patient compliance among the studies that report it. Nineteen papers did not inform regarding funding.
Risk of bias in primary studies
Supplementary Fig. S1 summarises the bias risk assessment results for the studies included in this systematic review accordingly RoB 2 tool. Our judgement of the overall risk of bias did not result in any study ranked as ‘low’, while we judge fourteen studies as ‘some concerns’(Reference Lewis, Belman and Dorr-Uyemura39,Reference Goris, Vermeeren and Wouters41–Reference Vermeeren, Wouters and Geraerts-Keeris43,Reference Baldi, Aquilani and Pinna48–Reference Sugawara, Takahashi and Kasai50,Reference Dal Negro, Testa and Aquilani52,Reference Marinari, Manigrasso and De Benedetto56,Reference Khan, Kumar and Daga58–Reference De Benedetto, Pastorelli and Ferrario60,Reference Ahmadi, Eftekhari and Mazloom64,Reference Schols, Soeters and Mostert69) and eighteen studies as ‘high’ risk of bias(Reference Efthimiou, Fleming and Gomes40,Reference Fuld, Kilduff and Neder44–Reference Weekes, Emery and Elia47,Reference Knowles, Fairbarn and Wiggs51,Reference Sugawara, Takahashi and Kashiwagura53–Reference Gurgun, Deniz and Argin55,Reference Ahnfeldt-Mollerup, Hey and Johansen57,Reference Degirmenci, Şahin and Soylu61–Reference van Beers, Rutten-van Mölken and van de Bool63,Reference Fuenzalida, Petty and Jones65–Reference Ganzoni, Heilig and Schönenberger68,Reference Saudny-Unterberger, Martin and Gray-Donald70) .
In the first domain of RoB 2 (bias arising from the randomisation process) all studies were judged as ‘some concerns’, except one study judged as ‘high’ risk of bias(Reference Entrenas Costa, Domínguez Platas and Checa Pinilla66); while in the second domain (deviations from intended interventions), twelve studies were judged as ‘low’(Reference Steiner, Barton and Singh42–Reference Weekes, Emery and Elia47,Reference Dal Negro, Aquilani and Bertacco49,Reference Constantin, Menon and Houchen-Wolloff54,Reference Marinari, Manigrasso and De Benedetto56,Reference van de Bool, Rutten and van Helvoort59,Reference De Benedetto, Pastorelli and Ferrario60) , fifteen as ‘some concerns’(Reference Lewis, Belman and Dorr-Uyemura39,Reference Goris, Vermeeren and Wouters41,Reference Baldi, Aquilani and Pinna48,Reference Sugawara, Takahashi and Kasai50,Reference Sugawara, Takahashi and Kashiwagura53,Reference Gurgun, Deniz and Argin55,Reference Ahnfeldt-Mollerup, Hey and Johansen57,Reference Degirmenci, Şahin and Soylu61,Reference van Beers, Rutten-van Mölken and van de Bool63,Reference Ahmadi, Eftekhari and Mazloom64,Reference Rogers, Donahoe and Costantino67–Reference Saudny-Unterberger, Martin and Gray-Donald70) and the five remaining studies as ‘high’(Reference Efthimiou, Fleming and Gomes40,Reference Knowles, Fairbarn and Wiggs51,Reference Otte, Ahlburg and D’Amore62,Reference Fuenzalida, Petty and Jones65,Reference Entrenas Costa, Domínguez Platas and Checa Pinilla66) . Regarding to missing outcome data (third domain), nine studies were judged as ‘low’(Reference Lewis, Belman and Dorr-Uyemura39,Reference Goris, Vermeeren and Wouters41,Reference Vermeeren, Wouters and Geraerts-Keeris43,Reference Baldi, Aquilani and Pinna48,Reference Sugawara, Takahashi and Kasai50,Reference Khan, Kumar and Daga58,Reference Otte, Ahlburg and D’Amore62,Reference Ahmadi, Eftekhari and Mazloom64,Reference Schols, Soeters and Mostert69) , fifteen as ‘some concerns’(Reference Efthimiou, Fleming and Gomes40,Reference Steiner, Barton and Singh42,Reference Dal Negro, Aquilani and Bertacco49,Reference Knowles, Fairbarn and Wiggs51–Reference Ahnfeldt-Mollerup, Hey and Johansen57,Reference Fuenzalida, Petty and Jones65–Reference Rogers, Donahoe and Costantino67) and eight as ‘high’(Reference Fuld, Kilduff and Neder44–Reference Weekes, Emery and Elia47,Reference Degirmenci, Şahin and Soylu61,Reference van Beers, Rutten-van Mölken and van de Bool63,Reference Ganzoni, Heilig and Schönenberger68,Reference Saudny-Unterberger, Martin and Gray-Donald70) . In addition, all studies were considered as having low risk for the domain measurement of the outcome, while twenty-eight studies were assessed as ‘low’(Reference Lewis, Belman and Dorr-Uyemura39–Reference Sugawara, Takahashi and Kasai50,Reference Dal Negro, Testa and Aquilani52,Reference Gurgun, Deniz and Argin55–Reference Rogers, Donahoe and Costantino67,Reference Schols, Soeters and Mostert69,Reference Saudny-Unterberger, Martin and Gray-Donald70) and four studies as ‘some concerns’(Reference Knowles, Fairbarn and Wiggs51,Reference Sugawara, Takahashi and Kashiwagura53,Reference Constantin, Menon and Houchen-Wolloff54,Reference Ganzoni, Heilig and Schönenberger68) in fifth domain (selection of the reported results). Supplementary Table S4 presents the reasons for these judgments.
Effect of intervention on body weight
Twenty-seven RCT (1164 participants)(Reference Lewis, Belman and Dorr-Uyemura39–Reference Sugawara, Takahashi and Kashiwagura53,Reference Gurgun, Deniz and Argin55,Reference Khan, Kumar and Daga58,Reference van de Bool, Rutten and van Helvoort59,Reference Otte, Ahlburg and D’Amore62–Reference Saudny-Unterberger, Martin and Gray-Donald70) measured BW as an outcome, of which twenty-six(Reference Lewis, Belman and Dorr-Uyemura39–Reference Sugawara, Takahashi and Kashiwagura53,Reference Gurgun, Deniz and Argin55,Reference Khan, Kumar and Daga58,Reference van de Bool, Rutten and van Helvoort59,Reference Otte, Ahlburg and D’Amore62–Reference Rogers, Donahoe and Costantino67,Reference Schols, Soeters and Mostert69,Reference Saudny-Unterberger, Martin and Gray-Donald70) of them reported data that could be pooled. We found a statistically significant benefit of nutritional intervention on BW using the random-effects model (MD = 1·44 kg, 95 % CI 0·81 to 2·08, P < 0·01, I2 = 73 %, (1144 participants)) (Fig. 2). We excluded Ganzoni et al.’s study(Reference Ganzoni, Heilig and Schönenberger68) from the meta-analysis because it did not report sufficient information to impute the measures of dispersion of the treatment effect. In this study, the intervention group (n 15) received advice to follow a high-energy diet (energy supply corresponding to 1·8 times the basal metabolic rate, i.e. ∼ 2840 kcal/d) supplemented with the ONS (Fresubin®, OP 200 ml), once or twice a day, while the control group (n 15) followed a normal energy diet. Although the intervention group obtained more weight gain (7·0 kg), the difference relative to the control group (weight gain of 2·3 kg) was not statistically significant (P = 0·08).

Fig. 2. Forest plot diagrams for body weight.
In sensitivity analysis, the exclusion of the only crossover RCT did not materially change the results (MD = 1·18 kg, 95 % CI 0·66 to 1·70, P < 0·01, I2 = 55·1 %, (1094 participants)). Table 3 describes the results of the subgroup analysis for the BW outcome. The magnitude of BW gain was significantly higher in BMI < 22 kg/m2 subgroup compared with BMI ≥ 22 kg/m2 subgroup, and it was also significantly higher in studies conducted with stable COPD patients compared with unstable COPD patients. On the other hand, we did not observe significant differences for BW between the three intervention subgroups, neither between the studies grouped by the risk of bias of primary studies. In univariate meta-regression analyses (online Supplementary Table S5) for between-group differences in BW, the amount of energy and protein prescribed, as well as the length of intervention, were able to partially explain the observed statistical heterogeneity (R² = 16·66 to 45·95 %), but were not statistically significantly associated with between-group changes in BW. Univariate meta-regression analysis using baseline BMI as the predictor variable did not explain the heterogeneity and was not significantly associated with between-group differences in BW.
Table 3. Subgroup analysis for randomised controlled trials on body weight, lean mass, handgrip strength and quadriceps strength
(Numbers and percentages; odd ratios and 95 % confidence intervals)

MD, mean difference; SMD, standard mean difference.
* Analyses for lean body mass did not perform since none study that reported this outcome was conducted with unstable patients.
† Analyses for lean body mass did not perform since none study that reported this outcome was a crossover randomised controlled trial.
Visual inspection of the funnel plot (online Supplementary Fig. S2) suggests no substantial asymmetry, with non-significant Egger’s test (intercept: 0·34, (CI 95 % −0·83, 1·52); P = 0·57).
We judged the certainty of evidence regarding this outcome as low, due to within-study risk of bias that was classified as some concerns in twelve studies(Reference Lewis, Belman and Dorr-Uyemura39,Reference Goris, Vermeeren and Wouters41–Reference Vermeeren, Wouters and Geraerts-Keeris43,Reference Baldi, Aquilani and Pinna48–Reference Sugawara, Takahashi and Kasai50,Reference Dal Negro, Testa and Aquilani52,Reference van de Bool, Rutten and van Helvoort59,Reference Ahmadi, Eftekhari and Mazloom64,Reference Schols, Soeters and Mostert69) and high in fourteen studies(Reference Efthimiou, Fleming and Gomes40,Reference Fuld, Kilduff and Neder44–Reference Weekes, Emery and Elia47,Reference Knowles, Fairbarn and Wiggs51,Reference Sugawara, Takahashi and Kashiwagura53,Reference Gurgun, Deniz and Argin55,Reference Otte, Ahlburg and D’Amore62,Reference van Beers, Rutten-van Mölken and van de Bool63,Reference Fuenzalida, Petty and Jones65–Reference Rogers, Donahoe and Costantino67,Reference Saudny-Unterberger, Martin and Gray-Donald70) , the persistent inconsistency that could not be explained in subgroup analyses or meta-regression and the low precision of effect estimates (Table 4).
Table 4. Summary of findings: nutritional supplementation or food fortification compared with placebo or usual diet or no intervention effect on nutritional parameters of chronic obstructive pulmonary disease (COPD) patients

MD, mean difference; SMD, standard mean difference; GRADE, Grading of Recommendations Assessment, Development and Evaluation Working Group grades of evidence(Reference Steiner, Barton and Singh42).
* Parameters and units of measurement applied in the studies for lean body mass: free-fat mass (FFM) in kilogram (kg) (n 8 studies)(Reference Goris, Vermeeren and Wouters41,Reference Steiner, Barton and Singh42,Reference Fuld, Kilduff and Neder44,Reference Deacon, Vincent and Greenhaff46,Reference Weekes, Emery and Elia47,Reference Sugawara, Takahashi and Kasai50,Reference Knowles, Fairbarn and Wiggs51,Reference Otte, Ahlburg and D’Amore62) , FFM index in kilogram per square meter (kg/m2) (n 3 studies)(Reference Baldi, Aquilani and Pinna48,Reference Sugawara, Takahashi and Kashiwagura53,Reference Constantin, Menon and Houchen-Wolloff54) , FFM (%) (n 1 study)(Reference Khan, Kumar and Daga58) and appendicular skeletal muscle mass in kg (n 1 study)(Reference Degirmenci, Şahin and Soylu61).
† Units of measurement applied in the studies for handgrip strength: kilogram-force (kgf) (n 5 studies)(Reference Teixeira, Kowalski and Valduga10,Reference Rawal and Yadav16,Reference Maltais, Decramer and Casaburi21,Reference Samarghandi, Ioachimescu and Qayyum23,Reference Anker, Laviano and Filippatos24) and kilogram (kg) (n 7 studies)(Reference Attaway, Welch and Hatipoğlu11,Reference Bhakare, Godbole and Khismatrao19,Reference Kovarik, Joskova and Patkova22,Reference Ferreira, Brooks and Lacasse26,Reference Aldhahir, Rajeh and Aldabayan30,Reference Lewis, Belman and Dorr-Uyemura39,Reference Goris, Vermeeren and Wouters41) .
‡ Units of measurement applied in the studies for quadriceps strength: kgf (n 1 study)(Reference Maltais, Decramer and Casaburi21), kg (n 1 study)(Reference Ferreira, Brooks and White29), Newton-meter (n 7 studies)(Reference Kovarik, Joskova and Patkova22–Reference Bauer, Biolo and Cederholm25,Reference Page, McKenzie and Bossuyt32,Reference IntHout, Ioannidis and Borm37,Reference Efthimiou, Fleming and Gomes40) and Newtons per kg (n 1 study)(Reference Follmann, Elliott and Suh35).
§ Due to within-study risk of bias that was classified as some concerns in twelve studies(Reference Lewis, Belman and Dorr-Uyemura39,Reference Goris, Vermeeren and Wouters41–Reference Vermeeren, Wouters and Geraerts-Keeris43,Reference Baldi, Aquilani and Pinna48–Reference Sugawara, Takahashi and Kasai50,Reference Dal Negro, Testa and Aquilani52,Reference Khan, Kumar and Daga58,Reference van de Bool, Rutten and van Helvoort59,Reference Ahmadi, Eftekhari and Mazloom64,Reference Schols, Soeters and Mostert69), high in fourteen studies(Reference Efthimiou, Fleming and Gomes40,Reference Fuld, Kilduff and Neder44–Reference Weekes, Emery and Elia47,Reference Knowles, Fairbarn and Wiggs51,Reference Sugawara, Takahashi and Kashiwagura53,Reference Gurgun, Deniz and Argin55,Reference Otte, Ahlburg and D’Amore62,Reference van Beers, Rutten-van Mölken and van de Bool63,Reference Fuenzalida, Petty and Jones65–Reference Rogers, Donahoe and Costantino67,Reference Saudny-Unterberger, Martin and Gray-Donald70) and the inconsistency that could not be explained in the subgroup analysis and meta-regression and the imprecision of effect estimates.
|| Due to within-study risk of bias that was classified as some concerns in two studies (Reference Lewis, Belman and Dorr-Uyemura39,Reference Schols, Soeters and Mostert69), high in five studies(Reference Efthimiou, Fleming and Gomes40,Reference Weekes, Emery and Elia47,Reference Knowles, Fairbarn and Wiggs51,Reference Otte, Ahlburg and D’Amore62,Reference Entrenas Costa, Domínguez Platas and Checa Pinilla66) and the unfeasibility of investigate risk of publication bias because of the reduced number of studies.
¶ Due to within-study risk of bias that was classified as some concerns in one study(Reference Lewis, Belman and Dorr-Uyemura39), high in five studies(Reference Efthimiou, Fleming and Gomes40,Reference Knowles, Fairbarn and Wiggs51,Reference Fuenzalida, Petty and Jones65–Reference Rogers, Donahoe and Costantino67) and the imprecision of effect estimates and the unfeasibility of investigate risk of publication bias because of the reduced number of studies.
** Due to within-study risk of bias that was classified as some concerns on eight(Reference Vermeeren, Wouters and Geraerts-Keeris43,Reference Baldi, Aquilani and Pinna48–Reference Sugawara, Takahashi and Kasai50,Reference Dal Negro, Testa and Aquilani52,Reference Marinari, Manigrasso and De Benedetto56,Reference De Benedetto, Pastorelli and Ferrario60,Reference Ahmadi, Eftekhari and Mazloom64) , studies and high in five studies(Reference Fuld, Kilduff and Neder44,Reference Deacon, Vincent and Greenhaff46,Reference Sugawara, Takahashi and Kashiwagura53,Reference Gurgun, Deniz and Argin55,Reference van Beers, Rutten-van Mölken and van de Bool63) and the moderate heterogeneity that could not be explained in subgroup analysis and meta-regression, and publication bias.
†† Due to within-study risk of bias that was classified as some concerns in five studies(Reference Steiner, Barton and Singh42,Reference Vermeeren, Wouters and Geraerts-Keeris43,Reference Sugawara, Takahashi and Kasai50,Reference van de Bool, Rutten and van Helvoort59,Reference De Benedetto, Pastorelli and Ferrario60), high in four studies(Reference Fuld, Kilduff and Neder44,Reference Deacon, Vincent and Greenhaff46,Reference Sugawara, Takahashi and Kashiwagura53,Reference van Beers, Rutten-van Mölken and van de Bool63) and the imprecision of effect estimates, the moderate heterogeneity and the unfeasibility of investigate risk of publication bias because of the reduced number of studies.
‡‡ Due to within-study risk of bias that was classified as some concerns in five studies(Reference Lewis, Belman and Dorr-Uyemura39,Reference Steiner, Barton and Singh42,Reference Vermeeren, Wouters and Geraerts-Keeris43,Reference Dal Negro, Aquilani and Bertacco49,Reference Ahmadi, Eftekhari and Mazloom64) , high in seven studies(Reference Efthimiou, Fleming and Gomes40,Reference Fuld, Kilduff and Neder44,Reference Faager, Söderlund and Sköld45,Reference Weekes, Emery and Elia47,Reference Degirmenci, Şahin and Soylu61,Reference Rogers, Donahoe and Costantino67,Reference Saudny-Unterberger, Martin and Gray-Donald70) and the inconsistency that could not be explained in the subgroup analysis and meta-regression and the imprecision of effect estimates.
§§ Due to within-study risk of bias that was classified as some concerns in four studies(Reference Steiner, Barton and Singh42,Reference Vermeeren, Wouters and Geraerts-Keeris43,Reference Sugawara, Takahashi and Kasai50,Reference van de Bool, Rutten and van Helvoort59) , high in six studies(Reference Fuld, Kilduff and Neder44–Reference Deacon, Vincent and Greenhaff46,Reference Constantin, Menon and Houchen-Wolloff54,Reference Ahnfeldt-Mollerup, Hey and Johansen57,Reference van Beers, Rutten-van Mölken and van de Bool63) and the imprecision of effect estimates.
Effect of nutritional interventions in lean body mass
Sixteen RCT (933 participants)(Reference Steiner, Barton and Singh42–Reference Fuld, Kilduff and Neder44,Reference Deacon, Vincent and Greenhaff46,Reference Baldi, Aquilani and Pinna48–Reference Sugawara, Takahashi and Kasai50,Reference Dal Negro, Testa and Aquilani52,Reference Sugawara, Takahashi and Kashiwagura53,Reference Gurgun, Deniz and Argin55,Reference Marinari, Manigrasso and De Benedetto56,Reference van de Bool, Rutten and van Helvoort59,Reference De Benedetto, Pastorelli and Ferrario60,Reference van Beers, Rutten-van Mölken and van de Bool63,Reference Ahmadi, Eftekhari and Mazloom64,Reference Schols, Soeters and Mostert69) measured LBM as an outcome; however, only thirteen (n 657)(Reference Vermeeren, Wouters and Geraerts-Keeris43,Reference Fuld, Kilduff and Neder44,Reference Deacon, Vincent and Greenhaff46,Reference Baldi, Aquilani and Pinna48–Reference Sugawara, Takahashi and Kasai50,Reference Dal Negro, Testa and Aquilani52,Reference Sugawara, Takahashi and Kashiwagura53,Reference Gurgun, Deniz and Argin55,Reference Marinari, Manigrasso and De Benedetto56,Reference De Benedetto, Pastorelli and Ferrario60,Reference van Beers, Rutten-van Mölken and van de Bool63,Reference Ahmadi, Eftekhari and Mazloom64) studies included data that could be pooled of a statistically significant increase in LBM with the nutritional intervention (SMD = 0·37; 95 % CI, 0·15, 0·59, P < 0·01, I2 = 46 %) (Fig. 3). LBM was measured using bioelectrical impedance (BIA) in eight trials(Reference Vermeeren, Wouters and Geraerts-Keeris43,Reference Deacon, Vincent and Greenhaff46,Reference Dal Negro, Aquilani and Bertacco49,Reference Dal Negro, Testa and Aquilani52,Reference Gurgun, Deniz and Argin55,Reference Marinari, Manigrasso and De Benedetto56,Reference De Benedetto, Pastorelli and Ferrario60,Reference Ahmadi, Eftekhari and Mazloom64) , while three trials used dual-energy x-ray absorptiometry (DEXA)(Reference Fuld, Kilduff and Neder44,Reference Baldi, Aquilani and Pinna48,Reference van Beers, Rutten-van Mölken and van de Bool63) , and two trials(Reference Sugawara, Takahashi and Kasai50,Reference Sugawara, Takahashi and Kashiwagura53) did not report body composition assessment methods. Of the three studies that could not be included in the meta-analysis because they used different units to report LBM, two studies(Reference van de Bool, Rutten and van Helvoort59,Reference Schols, Soeters and Mostert69) found no significant improvement in the proportion of LBM ( presented as fat-free mass/kg(Reference Schols, Soeters and Mostert69) and skeletal muscle mass(Reference van de Bool, Rutten and van Helvoort59)) for the ONS group, while one study(Reference Steiner, Barton and Singh42) observed clinically relevant LBM gains only in the placebo group.

Fig. 3. Forest plot diagrams for lean body mass.
In subgroup analysis, we investigated whether the method of LBM measurement was associated with the observed results, which were not statistically significantly different between subgroups (P for interaction = 0·48). We also performed subgroup analysis for LBM according to BMI, intervention and risk of bias. No clinically relevant differences in intervention effects between these three subgroups were found, with non-significant tests for subgroup differences (Table 3).
Visual inspection of the funnel plot and Egger’s test (intercept: −3·63, (CI 95 % 1·39, 5·86); P = 0·0087) were compatible with publication bias (online Supplementary Fig. S3). There was very-low quality evidence (risk of bias in primary studies, heterogeneity and publication bias) for LBM outcome (Table 4). The results of the trim-and-fill results suggest that four trials might have been missing such that their addition would change the overall effect on LBM to (number of studies combined: k = 17 (with added four studies); SMD = 0·17 (95 % CI, −0·0782, 0·4267, P = 0·1762)).
Regarding midarm muscle circumference, the pooled differences in change from baseline values of seven studies (325 participants)(Reference Lewis, Belman and Dorr-Uyemura39,Reference Efthimiou, Fleming and Gomes40,Reference Weekes, Emery and Elia47,Reference Knowles, Fairbarn and Wiggs51,Reference Otte, Ahlburg and D’Amore62,Reference Entrenas Costa, Domínguez Platas and Checa Pinilla66,Reference Schols, Soeters and Mostert69) resulting in a small, but statistically significant increment circumference (MD 0·29 mm2; 95 % CI 0·02 to 0·57, P = 0·03, I2 = 0 %) in the intervention group as compared with the control group (Fig. 4). There was low-quality evidence (due to risk of bias and unfeasibility to estimate the risk of publication bias) (Table 4) for this outcome.

Fig. 4. Forest plot diagrams for midarm muscle circumference.
Effect of intervention in fat mass
Nine RCT (n 523)(Reference Steiner, Barton and Singh42–Reference Fuld, Kilduff and Neder44,Reference Deacon, Vincent and Greenhaff46,Reference Sugawara, Takahashi and Kasai50,Reference Sugawara, Takahashi and Kashiwagura53,Reference van de Bool, Rutten and van Helvoort59,Reference De Benedetto, Pastorelli and Ferrario60,Reference van Beers, Rutten-van Mölken and van de Bool63) reported data on FM. Pooled results showed no statistically significant differences for this outcome (SMD = 0·16; 95 % CI, –0·07, 0·40, P = 0·18, I2 = 43 %) (Fig. 5). The certainty of evidence for FM was very low (due to risk of bias, imprecision, heterogeneity and the unfeasibility to investigate risk of publication bias) (Table 4).

Fig. 5. Forest plot diagrams for fat mass.
We included six trials (158 participants)(Reference Lewis, Belman and Dorr-Uyemura39,Reference Efthimiou, Fleming and Gomes40,Reference Knowles, Fairbarn and Wiggs51,Reference Fuenzalida, Petty and Jones65–Reference Rogers, Donahoe and Costantino67) in the analysis of nutritional intervention effects on triceps skinfold. The pooled MD was 1·09 mm (95 % CI, 0·01 to 2·16, P = 0·05, I2 = 0 %) (Fig. 6), though from low-quality evidence concerning for FM (due to risk of bias, imprecision, heterogeneity and the unfeasibility to investigate risk of publication bias) (Table 4).

Fig. 6. Forest plot diagrams for triceps skinfold.
Effect of intervention in handgrip and quadriceps strength
Twelve trials (448 participants)(Reference Lewis, Belman and Dorr-Uyemura39,Reference Efthimiou, Fleming and Gomes40,Reference Steiner, Barton and Singh42–Reference Faager, Söderlund and Sköld45,Reference Weekes, Emery and Elia47,Reference Dal Negro, Aquilani and Bertacco49,Reference Degirmenci, Şahin and Soylu61,Reference Ahmadi, Eftekhari and Mazloom64,Reference Rogers, Donahoe and Costantino67,Reference Saudny-Unterberger, Martin and Gray-Donald70) assessed peripheral muscle strength by HGS, resulting in a pooled SMD of 0·39 (95 % CI, 0·07, 0·71, P = 0·02, I2 = 62 %) (Fig. 7).

Fig. 7. Forest plot diagrams for handgrip strength.
Our subgroup analysis showed that the nutritional intervention for COPD patients at stable clinical conditions resulted in a higher effect on handgrip strength than in their unstable counterparts (Table 3). The univariate meta-regression analysis performed, considering the length of intervention and BMI as predictors (online Supplementary Table S6), was unable to satisfactorily explain the substantial heterogeneity for handgrip strength. No evidence of publication bias was found based on the funnel plot visual inspection (online Supplementary Fig. S4), and the Egger’s test was not statistically significant (intercept: −1·81 (CI 95 %, −5·72, –2·10); P = 0·39). There was very low-quality evidence (due to risk of bias, inconsistency and imprecision (Table 4)).
Ten trials (n 510)(Reference Steiner, Barton and Singh42–Reference Deacon, Vincent and Greenhaff46,Reference Sugawara, Takahashi and Kasai50,Reference Constantin, Menon and Houchen-Wolloff54,Reference Ahnfeldt-Mollerup, Hey and Johansen57,Reference van de Bool, Rutten and van Helvoort59,Reference van Beers, Rutten-van Mölken and van de Bool63) reported the effect of intervention on QS (SMD, 0·15, 95 % CI –0·04, 0·35, P = 0·12, I2 = 15 %) (Fig. 8). Seven studies measured QS by isometric strength(Reference Steiner, Barton and Singh42,Reference Vermeeren, Wouters and Geraerts-Keeris43,Reference Deacon, Vincent and Greenhaff46,Reference Constantin, Menon and Houchen-Wolloff54,Reference Ahnfeldt-Mollerup, Hey and Johansen57,Reference van de Bool, Rutten and van Helvoort59,Reference van Beers, Rutten-van Mölken and van de Bool63) , two studies by isokinetic strength(Reference Fuld, Kilduff and Neder44,Reference Faager, Söderlund and Sköld45) , while one study did not report the methodology applied(Reference Sugawara, Takahashi and Kasai50). In subgroup analysis investigating whether units of measurement was associated with the observed results, we found that only for the studies reporting QS in Newton-meters unit(Reference Vermeeren, Wouters and Geraerts-Keeris43–Reference Deacon, Vincent and Greenhaff46,Reference Constantin, Menon and Houchen-Wolloff54,Reference van de Bool, Rutten and van Helvoort59,Reference van Beers, Rutten-van Mölken and van de Bool63) (seven reports, 383 participants) there was a significant effect in QS (MD = 2·53; 95 % CI 0·44, 4·63, P = 0·02, I2 = 0 %), with a significant test for subgroup differences (P = 0·04). Subgroup analyses revealed no statistically significant associations between explanatory variables (clinical status, type of intervention, BMI and overall risk of bias of primary studies) on QS outcomes, and univariate meta-regression analysis with BMI as the predictor variable only partially (R² = 25·93 %) explained the observed statistical heterogeneity with no significant association with the outcome (P = 0·23) (online Supplementary Table S6).

Fig. 8. Forest plot diagrams for quadriceps strength.
No evidence of publication bias was found based on the funnel plot inspection and a non-significant Egger’s test (intercept: 0·83, (CI 95 % −2·00, –3·65); P = 0·58) (online Supplementary Fig. S5). There was low-quality evidence (risk of bias and imprecision) (Table 4).
Discussion
In this systematic review with meta-analysis of RCT, we evaluated the effect of energy and/or protein ONS or food fortification (FF) on nutritional outcomes of COPD patients. The review identified thirty-two studies (1680 participants) and showed that these nutritional interventions compared with control (placebo or usual care or dietary instruction) resulted in a significantly positive impact in several nutritional parameters. It is necessary to highlight that we identified a high risk of bias in most of the primary studies, and the certainty of the evidence was very low/low for all outcomes.
Our findings are consistent with most of the results from three meta-analyses(Reference Collins, Stratton and Elia27–Reference Ferreira, Brooks and White29) published between 2012 and 2013, limited to stable COPD patients, in which nutritional support, mainly in the form of ONS (for more than two weeks) compared with placebo or usual diet also revealed significant improvements in BW(Reference Collins, Stratton and Elia27–Reference Ferreira, Brooks and White29), midarm muscle circumference(Reference Collins, Stratton and Elia27,Reference Ferreira, Brooks and White29) , skinfold thickness(Reference Collins, Stratton and Elia27,Reference Ferreira, Brooks and White29) (especially in malnourished patients) and handgrip strength(Reference Collins, Elia and Stratton28), as well as a lack of benefit from nutritional interventions for free-fat mass(Reference Collins, Elia and Stratton28) and quadriceps strength(Reference Collins, Elia and Stratton28,Reference Ferreira, Brooks and White29) . Notwithstanding, we observed methodological differences between the present meta-analysis and the previous ones(Reference Collins, Stratton and Elia27–Reference Ferreira, Brooks and White29), including intervention selection criteria (administration route, nutritional composition and duration) and the absence of information about protocol study registration.
The current review also provides a comprehensive analysis of the body of evidence by applying proper methodological safeguards to several limitations, compared with the previous ones(Reference Collins, Stratton and Elia27,Reference Collins, Elia and Stratton28) , which used a tool (Jadad scale) to assess the risk of bias explicitly discouraged by the Cochrane Handbook for Systematic Reviews of Interventions(Reference Higgins, Thomas and Chandler31). Furthermore, such reviews did not judge the certainty of the evidence, according to the GRADE system(Reference Schünemann, Brożek and Guyatt34), and limited the selected studies to English only(Reference Collins, Stratton and Elia27,Reference Collins, Elia and Stratton28) . In this review, we also included an additional ten studies published since these meta-analyses publications(Reference Collins, Stratton and Elia27–Reference Ferreira, Brooks and White29).
The present meta-analyses detected substantial statistical heterogeneity for BW and handgrip strength and a moderate statistical heterogeneity for fat-free mass. The meta-regression and the subgroup analysis were unable to explain the statistical heterogeneity, with nonsignificant interaction tests. However, the subgroup analyses for BW revealed a significant increase in the magnitude of effect from nutritional intervention in patients with lower BMI and clinically stable. Likewise, although the test for subgroup differences indicates that there was no statistically significant subgroup effect between the three categories of nutritional interventions, we observed greater effect size for BW with protein-based supplementation, which, according to the individual evaluation of these studies, suggests that the benefits come from the supplementation of creatine and essential amino acids. Caution is necessary in the interpretation of results on fat-free mass, since a publication bias was identified and the adjustment for funnel asymmetry by trim-and-fill method pointed to the lack of four studies and an important change in the pooled effect of the intervention. It is expected that in the presence of publication bias the summary measure shows a higher effect than the real effect, as evidenced in our results. Maybe, studies with negative results were not published and we did not identify them in our broad literature search.
The better outcomes of nutritional interventions for BW in lower BMI patients can be explained by the potential for improving baseline food intake and for weight gain, compared with those with normal or higher BMI. The demonstration of benefit in clinically stable patients compared with a subgroup of exacerbated patients for BW could be explained by the fact that in the last there is a marked increase in local and systemic inflammation, along with the presence of limiting factors to food intake, that negatively impact the nutritional status(Reference Gea, Sancho-Muñoz and Chalela17). The purported mechanism for the effect of creatine supplementation may be associated with its orexigenic activity (as observed in animal studies)(Reference Sakkas, Schambelan and Mulligan87) and increased water retention, a consequent stimulus to increase in myofibrillar mRNA and protein content(Reference Brose, Parise and Tarnopolsky88), promoting gains in free-fat mass. With anabolic effects in the same direction, the essential amino acids supplementation promotes protein synthesis and the resulting hypertrophy by activating translation and retarding proteolysis and expression of various atrogenes(Reference Dillon, Sheffield-Moore and Paddon-Jones89).
Regarding nutritional interventions, the trial protocols generated some uncertainty about the overall contribution of additional energy or proteins from nutritional supplementation to the usual food intake during interventions. About 88 % of studies did not tailor the nutritional intervention based on individual estimative of the energy-protein requirements of the patients. Of the 56 % of the studies that reported total energy and protein intake during the experiment, in forty percent of them, food intake was lower in the intervention group compared with the control group at the end of the follow-up. Almost 60 % of the studies reported the methodology applied to assess adherence to the protocol, but only 21 % of these revealed the referred level of patient adherence (with heterogeneous reporting this information). These limitations on the guarantee of the intended intervention make it impossible to recognise the true magnitude of the effect of energy and protein supplementation on the outcomes of interest. However, this brings USA pragmatic character and the need to identify strategies to facilitate patient adherence to nutritional interventions in clinical practice, resulting in an overall net benefit for outcomes relevant to the patient.
We conducted the present systematic review with meta-analysis under the latest Cochrane recommendations(Reference Higgins, Thomas and Chandler31), following Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines(Reference Page, McKenzie and Bossuyt32) after a prospectively registered protocol. We assessed the certainty of the evidence for each outcome of interest using the GRADE system(Reference Sterne, Savović and Page33). We identified and included all studies relevant to our research question, regardless of language. We used random effects for all outcomes, regardless of the heterogeneity expressed by I2, assuming that there is not one single true effect size across studies due to their clinical and methodological divergences. Our main limitation was the inability to access one Chinese publication (Reference Yan90), which probably could fulfill our eligibility criteria. Yet, we need to underline the limitations for the interpretation of our results. All the RCT included in this review had some concerns or high risk of bias, due to problems mainly related to the randomisation process (no information on the concealment of allocation sequence), due to deviations from interventions (unreported data or lack of blinding of participants and/or caregivers and/or people delivering the interventions and/or people assessing the outcomes) and due to missing outcome data (no information about follow-up loss or losses of follow-up higher than 13 %). Most included studies were small sample sized with highly variable intervention duration (1·29–48 weeks). The statistical heterogeneity remains high after subgroup analyses, suggesting the influence of other features between the studies, unexplored due to the impossibility to create subgroups (e.g. COPD severity, since it was unreported in the majority of the studies). Furthermore, the information unavailability about patients’ GOLD stage in most of the studies precluded the subgroup analysis for the disease severity.
The certainty of the evidence ranged from very low to low. The most common reasons for downgrading it were the identification of high or some concerns risk of bias in the included studies, imprecision of effect estimates and the inconsistency from individual studies, unexplained by sensitivity analysis. For these reasons, the available evidence herein summarised is insufficient to provide clear recommendations for clinical practice due to the limited certainty of evidence and should therefore be interpreted with caution. The potential benefit of nutritional supplementation/fortification directs to protein-based nutritional supplementation particularly for COPD patients with reduced BMI and clinically stable, which should be addressed in pragmatic trials.
In future RCT, investigations must be designed with adequate power to detect significant but realistic differences in outcomes relevant to individuals living with COPD; in addition to exploring the best protein substrate for supplements and its effective dose, if any, considering the patient’s nutritional needs, establishing strategies to optimise adherence; and effectively assess total food intake. Authors should also consider the intention-to-treat analysis and then perform subgroup and sensitivity analyses based on COPD metabolic phenotypes, pulmonary rehabilitation and adherence to the nutritional intervention, in addition to assessing the degree of maintenance of the identified benefits after treatment ends. When comparing the intervention to ‘routine care’, the latter should be clearly described to allow for transparent comparisons with other trials and appropriate inferences for real-life practice.
Conclusion
Based on limited evidence, this meta-analysis suggests that high-energy and/or high-protein intake improves anthropometric parameters and handgrip strength in COPD patients. Conversely, the nutritional interventions showed no benefit in fat-free mass.
Acknowledgements
We are grateful to Veronnike Kowalski for her valuable contribution in data extraction.
This work was carried out with the support of the Coordination for the Improvement of Higher Education Personnel – Brazil (CAPES). Flávia Moraes Silva received a productivity scholarship (PQ2) from the Brazilian National Council for Scientific and Technological Development (CNPq).
S. B. and F. M. S. designed the study; C. F. B. and S. B. performed the data extraction; F.M.S. was the third review author in case of disagreement; S. B. and F. M. S. were responsible for quality assessment; F. M. S., I. C. E. and S. B. analysed the data; F. M. S., I. C. E., P. Z. T. and S. B. drafted the manuscript, and all authors read and approved the final manuscript.
The authors have no conflict of interest to declare.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114522000976















