The term limbic encephalitis was first used by Reference Corsellis, Goldberg and NortonCorsellis et al (1968) to describe a neuropsychiatric syndrome characterised by subacute onset of memory disturbance, hallucinations and seizures, with evidence of inflammation in the medial temporal lobes. The use of the word limbic could be criticised in this context, as inflammation has been described elsewhere in the brain, but the term appears to have been used to denote encephalitis with prominent psychiatric symptoms.
Cases of this type had been described earlier, especially in relation to malignancy. Reference Brain and HensonBrain & Henson (1958) described how different combinations of non-metastatic symptoms could occur including neuropathy, myopathy and encephalitis. It is thought that the antibodies are generated as a response to tumour antigens and later develop molecular mimicry against autoantigens, thereby causing the neurological syndrome. Reference Brain, Adams, Brain and NorrisBrain & Adams (1965) demonstrated that a group of patients with this problem could present initially for psychiatric hospital admission with symptoms such as irritability, depression and anxiety. The usual primary tumour was found to be an oat cell carcinoma of the lung but other cases were described with primaries in ovary, testis, thymus, uterus, colon and larynx. The postmortem pathology suggested an inflammatory process and Reference DropchoDropcho (1989) suggested that this might reflect an autoimmune aetiology. A number of antibodies were described in the subsequent decade; examples are anti-Hu associated with lung cancer (Reference Graus, Cordon-Cardo and PosnerGraus 1985), anti-Ma2 associated with testicular cancer (Reference Voltz, Gultekin and RosenfeldVoltz 1999) and anti-CRMP5/CV2 in thymoma (Reference Antoine, Honnorat and AnterionAntoine 1995).
The next development was the discovery that the same antibodies could lead to a similar syndrome in the absence of malignancy. Reference Vincent, Buckley and SchottVincent et al (2004) described ten patients with antibodies against voltage-gated potassium channels (VGKCs) who had a similar syndrome, one of whom also had neuromyotonia, a cramping condition caused by peripheral nerve hyperexcitability. Most patients had low plasma sodium concentrations and abnormal high-signal change in the medial temporal lobes on magnetic resonance imaging (MRI). Symptoms in this group included depression, memory loss, delusions (in one patient) and preceding ‘flu-like’ symptoms. The majority of the patients improved with immunosuppressive therapy. (As discussed below, the exact targets for these antibodies were identified a few years later.)
In 2007, a new syndrome was described almost exclusively in young women with ovarian teratoma presenting with psychiatric disturbance, amnesia, seizures, dyskinesia, autonomic disturbance and respiratory failure. Patients were found to have antibodies directed against the NR1 and NR2 subunits of the N-methyl-D-aspartate (NMDA) receptor (also known as NMDAR) (Reference Dalmau, Tuzun and WuDalmau 2007). Subsequently, the epitope was identified to be the extracellular N-terminal domain of the NR1 subunit (Reference Dalmau, Gleichman and HughesDalmau 2008).
Autoantibodies associated with encephalitis
The initial antibodies associated with neurological syndrome and cancer had intracellular antigenic targets and were unlikely to be directly pathogenic, but were useful as diagnostic markers. The more recent discoveries, such as anti-VGKC and anti-NMDA receptor antibodies, act against cell surface antigens and, importantly, are responsive to immunomodulatory therapies. Although initially these antibodies were thought to be mainly against ion channels (hence the term autoimmune channelopathies; Reference Vincent, Lang and KleopaVincent 2003), it has subsequently been found that the antibodies can be targeted against complexing proteins linked to the ion channels. Psychiatric features are seen in a substantial proportion of these syndromes, with varying degrees of severity. A summary of the clinical features associated with the common antibodies is shown in Table 1.
TABLE 1 Clinical characteristics of syndromes due to antibodies against neuronal cell membrane antigens

Anti-VGKC-complex antibodies
These were initially described in patients with neuromyotonia (Reference Newsom-DavisNewsom-Davis 1997) and were later identified in patients with an immunotherapy-responsive form of encephalitis characterised by memory loss, seizures, confusion and hyponatraemia (Reference Buckley, Oger and CloverBuckley 2001). Later experiments confirmed that the antibodies were directed against the complexing proteins associated with VGKC rather than the VGKC itself (Reference Irani, Alexander and WatersIrani 2010a; Reference Lai, Huijbers and LancasterLai 2010).
The three main antigenic targets include leucine-rich glioma inactivated protein 1 (LGI1), contactin-associated protein-like 2 receptor (CASPR2) and Contactin-2, of which LGI1 is responsible for a predominantly limbic encephalitis phenotype. Many patients with autoimmune encephalitis were also found to have a characteristic presentation of faciobrachial seizures (brief, dystonic seizures affecting ipsilateral arm and face) (Reference Irani, Michell and LangIrani 2011), which were often unresponsive to anti-epileptic medications but were very sensitive to corticosteroids (Reference Irani, Buckley and VincentIrani 2008). These have already been described as tonic seizures (Reference Andrade, Tai and DalmauAndrade 2011). Nearly 80% of patients developed faciobrachial dystonic seizures before the onset of the amnestic syndrome (Reference Irani, Michell and LangIrani 2011), thereby possibly providing a therapeutic window for immunosuppressive therapy before developing serious cognitive sequelae.
The other phenotype of VGKC-complex antibody disease is that of Morvan’s syndrome characterised by neuromyotonia associated with hallucinations, insomnia, delirium and autonomic disturbances. Unlike autoimmune encephalitis, Morvan’s syndrome is more commonly associated with CASPR2 antibodies. The distribution of the proteins might partially explain this difference in antibody specificity – LGI1 is a glycoprotein mainly expressed in the central nervous system (and in particular the hippocampus) and CASPR2 is expressed more widely in the central and peripheral nervous system (Reference Irani, Alexander and WatersIrani 2010a). Contactin-2 antibodies are rare and are often seen in association with CASPR2 antibodies.
The assay for VGKC-complex antibodies is still useful for the diagnosis of these syndromes, which have an incidence of 1–2 per million per year in the UK. More specific assays for the individual targets are commonly used, although these may not identify non-LGI1/non-CASPR2 antibodies which may be picked up by radioimmunoassay. It still needs to be proven whether these non-LGI1/non-CASPR2 antibodies are pathogenically relevant. However, the newer method using immunocytofluorescence has the advantage of screening for a number of antibodies (LGI1, CASPR2, NMDA receptor, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, gamma-aminobutyric acid (GABA)-B1, GABA-B2) that can be associated with similar syndromes. In the absence of positive LGI1 or CASPR2 antibodies, the results of VGKC-complex antibodies in atypical presentations (i.e. without the classical limbic encephalitis or Morvan’s syndrome) should be discussed with an experienced neurologist before treatment decisions are undertaken.
Anti-NMDA receptor antibody encephalitis
Since its discovery, anti-NMDA receptor antibody encephalitis has been found to be one of the most common forms of autoimmune encephalitis, surpassing several viral aetiologies (Reference Granerod, Ambrose and DaviesGranerod 2010). Studies have shown that the syndrome can appear in children as young as 7 months (less than 10% of paediatric cases are paraneoplastic), that it is less likely to be paraneoplastic with increasing age and less likely in males (Reference Irani, Bera and WatersIrani 2010b; Reference Dalmau, Lancaster and Martinez-HernandezDalmau 2011), with women between 18 and 40 years old being at highest risk of harbouring an underlying malignancy (Reference Titulaer, McCracken and GabilondoTitulaer 2013). In contrast to patients with VGKC-complex antibody encephalitis – which mainly occurs in men older than 50 years of age (male to female ratio 2:1) – anti-NMDA receptor antibody encephalitis mainly affects women younger than 50 years of age (male to female ratio 1:4).
Anti-NMDA receptor antibody encephalitis has been described to occur in a multistage process (Reference Irani, Bera and WatersIrani 2010b), although this may not always be the case in all patients. The disorder can start with a ‘flu-like’ illness but is generally followed by psychiatric symptoms (particularly psychosis, mood disorder and personality change), amnesia, confusion or seizures. The next stage occurs 2–3 weeks later and is characterised by movement disorders including dystonia, chorea, rigidity and catatonia (including posturing and echopraxia). In children, orofacial dyskinesias are often the first symptom. The syndrome classically culminates in autonomic disturbance (e.g. cardiac dysrhythmia, hyperthermia, unstable blood pressure, hyperhidrosis and sialorrhoea), reduced conscious levels and can lead to death.
It is important to note that psychiatric symptoms, predominantly psychosis, are the most common presenting symptom of anti-NMDA receptor antibody encephalitis in adults, with the first large case series of 100 patients reporting 80% presenting to psychiatric services (Reference Dalmau, Gleichman and HughesDalmau 2008), and recently a larger series has reported similar high levels of 65% (Reference Titulaer, McCracken and GabilondoTitulaer 2013). Although psychiatric symptoms become less frequent with decreasing age at onset, they are still a common presenting feature in children.
Similar symptoms (including both motor and psychiatric phenomena) have been observed after treatment with phencyclidine, an NMDA receptor antagonist (Reference Baldridge and BessenBaldridge 1990).
Other antibodies
Patients with anti-AMPA receptor antibodies are comparatively rare (they are predominantly women and often present with a paraneoplastic syndrome) and mostly have a relapsing course, with prominent psychosis in addition to the other limbic encephalitis symptoms (Reference Graus, Boronat and XifroGraus 2010). However, psychosis is comparatively less common in GABA-B receptor (GABA-BR) antibody-mediated encephalitis, which is less frequent and seen in a paraneoplastic setting (often small cell lung cancer) with prominent seizures (Reference Lancaster, Lai and PengLancaster 2010). Both these syndromes respond well to immunosuppressive therapies.
Various neurological disorders such as stiff-person syndrome, complex partial seizures, limbic encephalitis and cerebellar ataxia have been described in patients with high titre anti-glutamic acid decarboxylase (anti-GAD) antibodies (>1000 U/ml) (Reference Saiz, Blanco and SabaterSaiz 2008). Most patients have a chronic non-remitting course but immunotherapies and plasma exchange may have some benefit. Many patients with anti-GAD antibody syndrome have been diagnosed with a psychogenic illness at the beginning and often have a long interval before the actual diagnosis is made (S.J., unpublished observation).
The trigger for antibody production could be due to ectopic antigen in the associated tumour (e.g. NMDAR, AMPAR, GABA-BR) or due to molecular mimicry from a prodromal (likely unidentified) infection in the majority of patients. However, the organism is highly variable (Reference Prüss, Dalmau and HarmsPrüss 2010) and hence a simple molecular mimicry is difficult to prove. An increasing number of patients are also being identified with confirmed viral infections preceding the illness (Reference Prüss, Dalmau and HarmsPrüss 2010; S.J., unpublished observation), possibly suggesting a role for systemic immune response opening up the blood–brain barrier and thereby triggering the symptoms arising from the limbic system. In children, a link between post-herpes simplex virus encephalitis, choreoathetosis and NMDA receptor antibodies has been recently described (Reference Armangue, Titulaer and MálagaArmangue 2013).
Autoantibodies are a possible cause of isolated psychiatric symptoms
It is possible that there might be forms of auto immune brain disorders that present predominantly, or exclusively, with a specific symptom. There is already some evidence that a neurological syndrome with isolated seizures can occur that would, until the antibody was found, be described as ‘cryptogenic’ epilepsy. As mentioned earlier, seizures are a prominent feature of most autoimmune encephalitides. There have now been several replicated studies finding a relatively wide range of autoantibodies associated with patients with first-onset seizure and patients with established chronic epilepsy, most notably anti-VGKC-complex, but also including anti-NMDA receptor, antibodies at rates of 5–10% (Reference CorrellCorrell 2013).
There is a parallel literature emerging for an association of these antibodies with cases of isolated psychosis in patients that would otherwise be diagnosed with schizophrenia. The association of antibodies and neuropsychiatric symptoms has recently been reviewed (Reference Kayser and DalmauKayser 2011, Reference Kayser, Titulaer and Gresa-Arribas2013). In the 2013 review, 571 patients with NMDA receptor antibodies were studied and 23 (4%) patients with isolated psychiatric episodes were identified, 5 at disease onset and 18 during relapse. Delusional thinking, mood disturbance and aggression were the predominant symptoms (Reference Kayser, Titulaer and Gresa-ArribasKayser 2013). Of 22 patients, 10 (45%) had abnormal MRI findings and 17 (77%) had raised white blood cell counts in the cerebrospinal fluid (CSF). Eighty-three per cent of patients improved after immunotherapy or tumour removal. This was not a controlled study and is very likely to have underrepresented those patients with isolated psychiatric episodes (who would not have been identified as ‘at risk’ and therefore tested).
Anti-NMDA receptor antibodies and isolated psychosis
To date, three studies have found an association between isolated psychosis and anti-NMDA receptor antibodies and three studies have failed to find an association. In terms of positive studies, the first study was of 46 patients with first-episode psychosis, 3 of whom were positive for NMDA receptor antibodies (Reference Zandi, Irani and LangZandi 2011). It was noteworthy that none of these positive cases had any neurological symptoms or signs that distinguished them from negative cases.
The second study was of similar-sized group of 51 patients with schizophrenia and schizoaffective disorder which found 4 patients with positive results (Reference Tsutsui, Kanbayashi and TanakaTsutsui 2012). Unfortunately, this study did not give details on duration of illness or whether there were any potential biases in case selection. However, three of these four patients had other neurological features such as generalised tonic–clonic seizures or orofacial dyskinesias.
Most recently, a third, larger study of 74 patients with established (chronic) schizophrenia and 47 patients with first-episode psychosis (with a combined mean illness duration of 9 years) found a small number of patients (n = 4) positive for immunoglobulin G (IgG) NMDA receptor antibodies (Reference Steiner, Walter and GlanzSteiner 2013). Importantly, this study also had healthy and psychiatric control groups. Two of 70 patients with major depression, none of 38 patients with borderline personality disorder and one of 230 healthy controls had NMDA receptor antibodies which were against IgA or IgM. Eight patients in the schizophrenia/first-episode psychosis cohort also had IgA/IgM antibodies against NMDA receptors, the potential pathological significance of which is less clear.
These three studies should be balanced against two studies reporting no patients with anti-NMDA receptor antibodies in a sample of 50 patients with schizophrenia with a mean duration of illness of 9 years (Reference Haussleiter, Emons and SchaubHaussleiter 2012) and 80 patients with first-episode psychosis (Reference Masdeu, Gonzalez-Pinto and MatuteMasdeu 2012). There is another study reporting no positive antibodies in schizophrenia but this was only in a sample of seven patients, which clearly limits the conclusions that can be drawn from it (Reference Rhoads, Guirgis and McKnightRhoads 2011).
Anti-VGKC-complex antibodies and isolated psychosis
There is one patient reported so far with anti-VGKC-complex antibodies and isolated psychosis in a study by Reference Zandi, Irani and LangZandi et al (2011). This patient did not have any clinical features to distinguish them from patients without antibodies.
Treatment of autoimmune encephalitis
Patients with the full syndrome of anti-VGKC-complex encephalitis (i.e. those with LGI1 or CASPR2 antibodies) tend to respond favourably to immunosuppressive therapy (especially corticosteroids) and often have a monophasic course. The antibody titres and symptoms progressively improve and in most patients remain quiescent for a prolonged period of time (Reference Irani, Buckley and VincentIrani 2008, Reference Irani, Alexander and Waters2010a). In some patients, symptoms tend to relapse on gradual withdrawal of steroids and may necessitate steroid sparing therapy, for example azathioprine or methotrexate.
Patients with anti-NMDA receptor encephalitis generally have similarly good responses to treatment, especially if diagnosed and treated promptly. It is also important to screen for an underlying malignancy, initially using a wholebody computed tomography (CT) scan and if negative, a positron emission tomography (PET) scan. MRI scans are useful in young women, since occasionally teratomas may be missed by CT and PET scans and also to reduce radiation exposure (Reference Titulaer, Soffietti and DalmauTitulaer 2011). Early and aggressive immunotherapy in the form of plasma exchange or intravenous Ig (IVIg) should be instituted with the help of the local neurological unit. In the minority of patients with underlying neoplasia, removal of the tumour often aids prompt recovery (Reference Dalmau, Gleichman and HughesDalmau 2008). Without immunosuppression, patients often remain in hospital for several months, with invasive respiratory support and very slow recovery. Up to a quarter of patients might succumb to the disease or have a relapsing course and hence intensive immunotherapy is essential. Patients who are treatment resistant require therapy with cyclophosphamide or rituximab which often helps in achieving remission. A proposed diagnostic and treatment algorithm is given in Fig. 1.
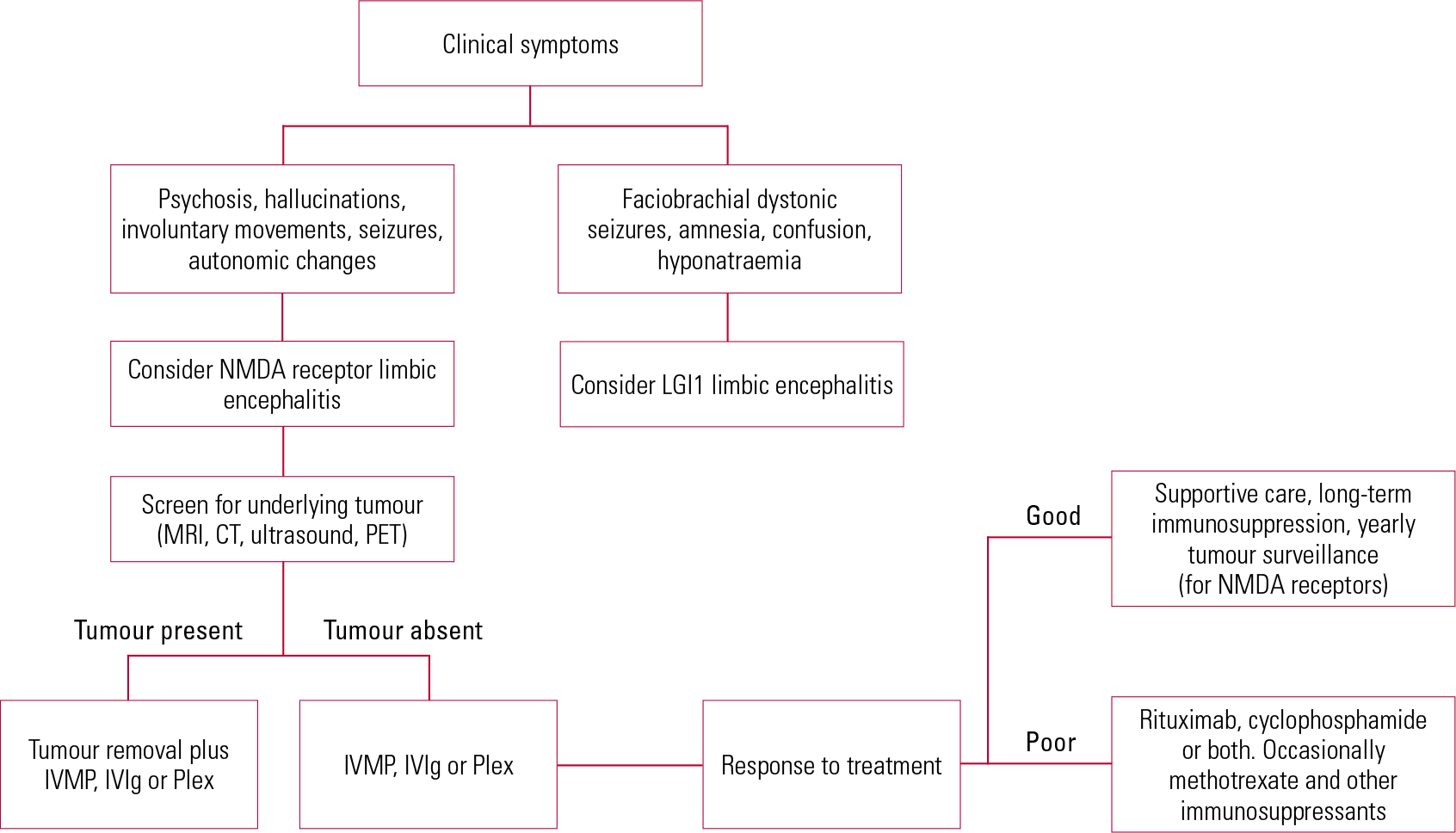
Fig 1 Suggested algorithm for treatment of autoimmune encephalitis. CT, computed tomography; IVIg, intravenous immunoglobulin; IVMP, intravenous methylprednisolone; LGI1, leucine-rich glioma inactivated protein 1; MRI, magnetic resonance imaging; NMDA, N-methyl-D-aspartate; PET, positron emission tomography; Plex, plasma exchange.
What should psychiatrists do?
Although definitive studies are ongoing in this area, there is emerging evidence that there might be a subgroup of patients, presenting predominantly with psychotic symptoms, who should be treated with immunosuppressive therapy. In our opinion, the following ‘red flags’ should indicate potential screening for autoimmune encephalitis in patients with psychotic symptoms:
-
• sudden-onset paranoid psychosis or rapid deterioration
-
• prodromal headache or raised temperature prior to onset of psychosis
-
• cognitive impairment (short-term memory, disorientation) including evidence of delirium
-
• catatonia, particularly orofacial dyskinesia
-
• seizures, particularly faciobrachial seizures
-
• autonomic disturbance (hypo-/hyperthermia, unstable blood pressure, raised respiratory rate, tachycardia); suspected neuroleptic malignant syndrome
-
• hyponatraemia (an indicator of anti-VGKC-complex (LGI1) antibody encephalitis).
Which tests should be performed?
Anti-VGKC-complex and anti-NMDA receptor antibodies are available for testing clinically (Box 1 lists the main laboratories in the UK offering such tests). Other antibodies (AMPAR, GABA-B, glycine or dopamine D2) are much rarer, and not described in association with primary psychiatric presentations.
BOX 1 Main laboratories in the UK offering antibody tests for autoimmune encephalitisa
-
• Nuffield Department of Clinical Neurology, John Radcliffe Hospital, Oxford: VGKC (radioimmunoassay), NMDA receptor and other antibodies (cell-based assay) (www.oxfordlaboratorymedicine.co.uk/laboratoryservices/immunology/neuroimmunology)
-
• Neuroimmunology Department, Medical School, University of Birmingham: autoimmune encephalitis screen using immunofluorescence chip (NMDAR, LGI1, CASPR2, AMPAR1, AMPAR2 and GABA-BR) (www.birmingham.ac.uk/facilities/clinical-immunologyservices/neuroimmunology)
a. Check with your local laboratory before samples are sent, since some might be starting to perform these tests themselves.
Which other investigations are useful?
Clinical presentation, alongside serum antibody assay, is the mainstay of diagnosis. However, supporting evidence can be obtained from other investigations.
-
• Electroencephalogram (EEG) may show epileptiform activity or slow waves as an indicator of an encephalopathic picture. A recent series describes a unique electrographic pattern named ‘extreme delta brush, which is associated with a prolonged illness course (Reference Schmitt, Pargeon and FrechetteSchmitt 2012).
-
• Medial temporal hyperintensity on MRI.
-
• CSF examination for specific antibodies (many authors believe that CSF is more sensitive than serum, although this might not be practically possible in a psychiatric setting in all patients, unless accompanied by neurological symptoms).
-
• A raised CSF protein may be useful but is nonspecific.
-
• Pleocytosis (increased white blood cells) and oligoclonal bands in CSF, indicating an inflammatory process in the central nervous system.
Serum markers of inflammation such as erythrocyte sedimentation rate or C-reactive protein are usually normal.
If the initial screen for NMDA receptor antibodies is positive, we recommend that the patient be referred to a clinician with a special interest in antibody-mediated neuroimmunological conditions, who may be able to advise on further investigations (including CSF examination) and treatment.
When to treat/not to treat
If a patient presents with psychosis and any other features of encephalopathy such as reduced consciousness level, seizures, autonomic instability, catatonia or investigations to support encephalitis such as abnormality in EEG, MRI or CSF, then they should be treated as other patients with encephalopathy, with assertive immunotherapy.
However, with a positive serum antibody test but in the absence of other features of encephalitis, it is not known whether patients with antibodies and psychosis should be treated with immunotherapy or antipsychotics. The few case reports and anecdotal evidence indicate that patients respond to treatment with either (e.g. Reference Braakman, Moers-Hornikx and ArtsBraakman 2010; Reference Creten, Van der Zwaan and BlankespoorCreten 2011). Furthermore, there are emerging cases where the antibodies have been detected, but have not been thought to be clinically relevant. For example, there are case reports of patients with post-mortem-confirmed Creutzfeldt–Jakob disease (Reference Mackay, Ahmad and StoneMackay 2012) or herpes simplex encephalitis (Reference Prüss, Finke and HoltjePrüss 2012), also testing positive for anti-NMDA receptor antibodies. Although these antibodies are not reported in healthy populations, it is possible that they may also be epiphenomenal to the underlying illness process in patients with psychosis.
Given the nature of the treatments required and current uncertainties about who to treat, discussions and decisions about treatment should be made jointly between psychiatric and neurological teams specialising in these disorders.
Plasma exchange or IVIg is often undertaken in a specialised neurology unit. Plasma exchange use is limited by its lack of easy availability and may have side-effects related to central venous line placement (thrombosis, air embolism, bleeding and vagus nerve injury) and also thrombotic complications. Occasionally, the replacement fluids may cause nausea, vomiting, diarrhoea, fever or chills. The side-effects of IVIg are often as a result of increased plasma viscosity (especially in products containing sucrose as a stabiliser), leading to renal dysfunction and thrombotic complications. Other less severe complications include aseptic meningitis, bronchospasm, transient elevation of liver enzymes, bone marrow suppression and allergic skin reactions. It has been noted that patients with severe isolated IgA deficiency are more prone to anaphylactic reactions after IVIg.
Undertaking plasma exchange or an infusion with IVIg requires a level of compliance. This can be a challenge when patients are acutely disturbed; atypical antipsychotics (e.g. olanzapine) and benzodiazepines have been used to sedate the patient sufficiently to allow immune therapy to occur, although care should be taken to monitor autonomic function, with the risk of exacerbating the autonomic instability that is seen in NMDA receptor encephalitis. Symptomatic treatment of psychiatric symptoms is based on anecdotal case reports and does not have a clear evidence base (Reference Chapman and VauseChapman 2011).
Multidisciplinary non-pharmacological interventions with physical, occupational and speech and language therapy inputs are thought to accelerate recovery. The setting for treating patients should be carefully considered.
Case vignettes
The following fictitious case vignettes illustrate typical clinical presentations.
Vignette 1: NMDA receptor antibodies associated with ovarian teratoma
A 24-year-old woman was admitted to the mental health unit with first-onset psychosis. She complained of auditory hallucinations. Soon after admission she was found to be showing involuntary movements with catatonic posturing. No response was noted from first-line antipsychotic agents. Within a week of presentation she became increasingly confused with an episode of generalised tonic–clonic seizures. Her initial investigations revealed normal MRI scan of the brain and the EEG showed diffuse slowing of the brain waves suggestive of a diffuse but non-specific encephalopathy. She was transferred to the neurology ward where a lumbar puncture showed that CSF contained 5 white blood cells, protein of 1.12 g/dl (normal range 15–45 mg/dl), CSF glucose of 3.8 (simultaneous blood glucose of 4.9) without any oligoclonal bands. Renal function, electrolytes, liver function and peripheral blood count were normal. NMDA receptor antibodies were detected in the serum. During the hospital stay, she was found to be tachycardic and hypotensive and later required invasive ventilation for respiratory depression.
In view of the diagnosis of autoimmune encephalitis secondary to NMDA receptor antibodies, plasma exchange was initiated, followed by a course of highdose oral steroids. A CT scan of the chest, abdomen and pelvis revealed a suspicious (but non-diagnostic) swelling in the left ovary, which was confirmed to be hypermetabolic on a PET scan. Laparoscopic oophorectomy was performed, and histology revealed an ovarian teratoma. Removal of the teratoma and continuation of immunosuppression using steroids was followed by significant clinical improvement. At last follow-up, 12 months after the initial admission, she is able to perform her normal activities of daily living without any support and has restarted her previous job as a teacher on a part-time basis.
This patient demonstrates the typical presentation of NMDA receptor encephalitis with the initial psychiatric onset, followed by more widespread neurological symptoms of seizures and confusion, culminating in autonomic dysfunction and respiratory failure. Although ovarian teratomas were thought to be highly prevalent in patients with NMDA receptor encephalitis, an increasing number of patients are being diagnosed without any underlying neoplasia. However, if a tumour is identified, its removal aids in the management of the underlying neuropsychiatric syndrome.
Vignette 2: NMDA receptor antibodies in the absence of malignancy
A 22-year-old graduate student presented to accident and emergency as she said that she did not feel safe at home, and requested admission. She gave a 1-week history of sudden onset of paranoid psychosis. She had loosely formed paranoid delusional beliefs – she believed that her family was plotting to kill her, perhaps through poisoning her food. She had disrupted sleep, with only a couple of hours a night for the past week. She heard a male voice talking to her, telling her that she was in danger, and also commenting on her actions. She had not been able to work for the past week, and was staying inside her flat keeping away from family and friends as she was worried that they meant her harm. She had no psychiatric history, had not been taking illicit substances and did not drink alcohol. On mental state examination she demonstrated features of catatonia. She was restless and paced during assessment, and occasional facial grimacing was noticed. She was hesitant in goal-directed movements. Her verbal fluency was impaired and she was perseverative in her speech. Her mood was subjectively and objectively euthymic. She did not describe any thought insertion or withdrawal, or somatic concerns. On cognitive examination she was disoriented to time and place. She had limited insight into her illness, but was requesting help. Her pulse, blood pressure and temperature were normal, and no focal abnormality was found on neurological examination. Blood investigations were unremarkable as was an MRI head scan. Urine drug screen was negative. EEG showed slow wave activity over frontal regions.
She was admitted to the psychiatric ward with a provisional diagnosis of schizophreniform psychosis and treated with olanzapine and regular benzodiazepines in view of the prominent catatonic symptoms. After 2 days’ treatment she was observed to collapse and temporarily lose consciousness. There was no seizure activity, but her blood pressure was low. Other observations (pulse, temperature, respiratory rate) were normal. Olanzapine was stopped with a query of neuroleptic malignant syndrome. However, creatine kinase was not significantly raised. Two weeks after admission she was found to have NMDA receptor antibodies in serum. She was transferred to the neurology ward and treated with high-dose oral steroids and plasma exchange. She was then transferred back to the psychiatric ward where over the next 2 weeks she improved in her psychotic and catatonic symptoms such that she was able to be discharged on maintenance steroids and no antipsychotics within a month of treatment. She was symptom-free within another month and able to return to her studies.
A challenge for neurologists and psychiatrists
Throughout most of the 20th century, neurology and psychiatry have developed parallel narratives to describe neuropsychiatric syndromes, and the idea that ‘one man’s schizophrenia is another man’s encephalitis’ goes back to at least the 1920s (Reference RogersRogers 1992). With the advent of clear biomarkers, this group of conditions offers an opportunity for the two disciplines to share skills and language, bringing together the traditional strengths of neurology (in molecular biology and the phenomenology of physical symptoms) with those of psychiatry (assessment and management of complex behaviours). We advocate the shared management of people with these conditions between interested neurologists and psychiatrists.
MCQs
Select the single best option for each question stem
-
1 Which of the following is not a ‘red flag’ sign for autoimmune encephalitis in patients with psychosis?
-
a Catatonia
-
b Hyponatraemia
-
c Hypercalcaemia
-
d Epilepsy
-
e Sudden onset psychosis.
-
-
2 Which of the following statements is true in relation to anti-NMDA receptor encephalitis?
-
a It most commonly occurs in men
-
b It most commonly occurs in people older than 50 years
-
c About 30% of patients with new-onset psychosis have anti-NMDA receptor encephalitis
-
d The most common presenting symptoms in adults are psychiatric
-
e Movement disorder is rare.
-
-
3 Which of the following is not a recognised treatment for autoimmune encephalitis?
-
a Corticosteroids
-
b Riluzole
-
c Rituximab
-
d Removal of primary tumour
-
e Plasma exchange.
-
-
4 Apart from serum and CSF antibody tests, which of the following is useful for the diagnosis of autoimmune encephalitis?
-
a CSF examination for oligoclonal bands
-
b Blood cultures
-
c Serum prolactin
-
d CT scan of the head
-
e Serum calcium.
-
-
5 Which of the following is not characteristic of anti-VGKC autoimmune encephalitis?
-
a Hyponatraemia
-
b Amnesia
-
c Faciobrachial seizures
-
d Delirium
-
e Hyperkalaemia.
-
MCQ answers
| 1 | c | 2 | d | 3 | b | 4 | a | 5 | e |





eLetters
No eLetters have been published for this article.