INTRODUCTION
Human rotavirus (family Reoviridae) is the most common cause of severe, acute gastroenteritis (AGE) in children worldwide, causing the death of nearly half a million children aged <5 years annually, mostly in low-income countries [Reference Parashar1]. In industrialized countries, the incidence of the infection is similar but mortality is infrequent [Reference Parashar1, Reference Williams, Lobanov and Pebody2] due to better nutritional status and easier access to healthcare in the paediatric population. Even so, in industrialized countries, rotavirus causes a substantial burden on healthcare systems due to the high number of hospitalizations, nosocomial infections and outpatient visits, produced during annual epidemics. Currently, two live vaccines against rotavirus have been licensed, which prevent between 46% and 96% of hospitalizations due to rotavirus [Reference Ruiz-Palacios3–Reference Patel5]. Therefore, in the next few years, several countries will consider their introduction and will consequently require accurate and current epidemiological data. Such information will also be required to monitor the impact of vaccination.
Although numerous studies have been performed to estimate the burden of severe rotavirus diarrhoea in industrialized countries, few have been population based, with laboratory confirmation of cases, and performed throughout the calendar year over sufficiently prolonged periods to be able to describe tendencies in the rates and epidemiology of the disease. Similarly, surveillance of the genotypes of circulating rotavirus strains is important to predict the possible effect of vaccination and to detect changes in circulating rotaviruses once vaccination has been introduced. The current study, performed year-round for 12 years, was designed to directly monitor the incidence of hospitalization caused by rotavirus in a well-characterized population in a region where the vaccine has hardly been used.
METHODS
Study area
This study was performed in the regions of San Sebastián, Tolosa and Urola-Costa (Autonomous Community of the Basque Country, northern Spain). The infants and children from these high-income regions represented 20·6% and 0·9% of the paediatric population of the Basque Country (Spain) and the entire Spanish territory, respectively [population 405 745 inhabitants, of which 19 738 were aged <5 years, according to data from the municipal censuses from 2006 (Basque Institute of Statistics)]. Hospital Donostia (San Sebastián, 79 paediatric beds) is the only public hospital in this area and caters for about 97% of paediatric hospitalizations. This study was performed between July 1996 and June 2008 (12 years) and was divided into four study periods (trienniums): July 1996–June 1999, July 1999–June 2002, July 2002–June 2005 and July 2005–June 2008. Children aged between 1 month and 5 years that were hospitalized for >24 h for AGE were candidates for inclusion. Although two rotavirus vaccines are available in Spain [Rotarix (GlaxoSmithKline, Belgium) since July 2006 and RotaTeq (Sanofi Pasteur-Merck, France) since February 2007], these vaccines were not included in the vaccination schedule during the study period, and the mean coverage for both vaccines in the last 2 years of the study was estimated to be <20%.
Data sources
Hospital discharge data
Children hospitalized for gastroenteritis were identified by searching electronic hospital discharge data. Hospitalization discharge records with an International Classification of Diseases, ninth revision, Clinical Modification code for gastroenteritis were initially selected. We used the codes for infectious diarrhoea of determined aetiology (001–009, excluding 003.2, 006.3 and 006.6) and the codes for diarrhoea of undetermined aetiology, including those cases of presumed infectious origin (009.0–009.3) and non-infectious origin (558.9 and 787.91). All children with AGE as the main discharge code as well as those with AGE as a secondary code but whose medical record showed a clear association with AGE (dehydration requiring intravenous fluid replacement) were included. Re-admissions due to the same enteropathogenic agent within a 6-month period were excluded, despite the small risk that some episodes were not recorded.
Hospital virology laboratory data
Children with rotavirus gastroenteritis were identified by searching the computerized records of the microbiology laboratory of Hospital Donostia. In the Paediatrics department, stool cultures are taken of all hospitalized patients with AGE as part of routine procedures. The presence of rotavirus antigen was investigated in all of these patients with a commercial enzyme immunoassay (EIA) kit, according to the manufacturer's instructions (IDEIATM Rotavirus, Dako Diagnostics Ltd, UK) [Reference Cilla6]. The samples in which EIA offered the highest optical density (>2000) were frozen at −80°C and were subsequently analysed for G and P types through multiplex, reverse transcription–polymerase chain reaction methods [Reference Iturriza-Gomara, Kang and Gray7]. In order to exclude nosocomial infections, only stool samples obtained within <5 days of hospital admission were considered. Patients who were simultaneously positive for rotavirus and enteropathogenic bacteria were included in the study, although the presence of rotavirus antigen in some of these patients could have been incidental. Demographic data were gathered from each patient with rotavirus-related AGE occurring within the study period and the number of the medical record was noted if the patient had been hospitalized. Finally, both data sources (discharge and laboratory data) were linked by using the medical record number and a single database was constructed with the patients' demographic, clinical and virological data.
Data on consumption of oral rehydration solutions in Gipuzkoa between 2001 and 2008 were obtained by checking the sales of pharmacies and parapharmacies in the region (data supplied by the Pharmaceutical Union of Gipuzkoa after reviewing 70% of the total sales in Gipuzkoa).
Data analysis
Population-based hospitalization rates and 95% exact binomial confidence intervals were calculated for each of the four study periods as the estimated number of hospitalizations per 10 000 children, based on the number of children with AGE and those positive for rotavirus antigen, stratified by age group. As the denominator, we used the population living within the study area in each study period, estimated from the corresponding censuses. Weights were calculated to account for the number of children without a stool sample for virological analysis (3·6–5·4% depending on the triennium) and for the children presumably hospitalized outside Hospital Donostia (3% overall throughout the study period), as we assumed that missing EIA results would have the same proportion of rotavirus-positive samples as those samples for which results were available. The χ2 test was used to compare percentages (qualitative variables). A P value of <0·05 was considered statistically significant.
RESULTS
Hospitalization attributable to gastroenteritis based on hospital discharge data
Between July 1996 and June 2008 there were 1290 hospitalizations receiving a discharge code for AGE. In 1114 (86·4%) (1067 children, with a mean age of 16·6±13·1 months), AGE was considered to be the cause of hospital admission, while the remainder were eliminated because the stool sample had been taken ⩾5 days after admission (n=68, possible nosocomial origin) or because AGE was not considered to be the primary cause of hospital admission (n=108). Of excluded patients, there were 60 who had received the specific discharge code of rotavirus (008·61). AGE episodes were more frequent in cold months (Fig. 1) and 59·2% (n=660) occurred in boys.
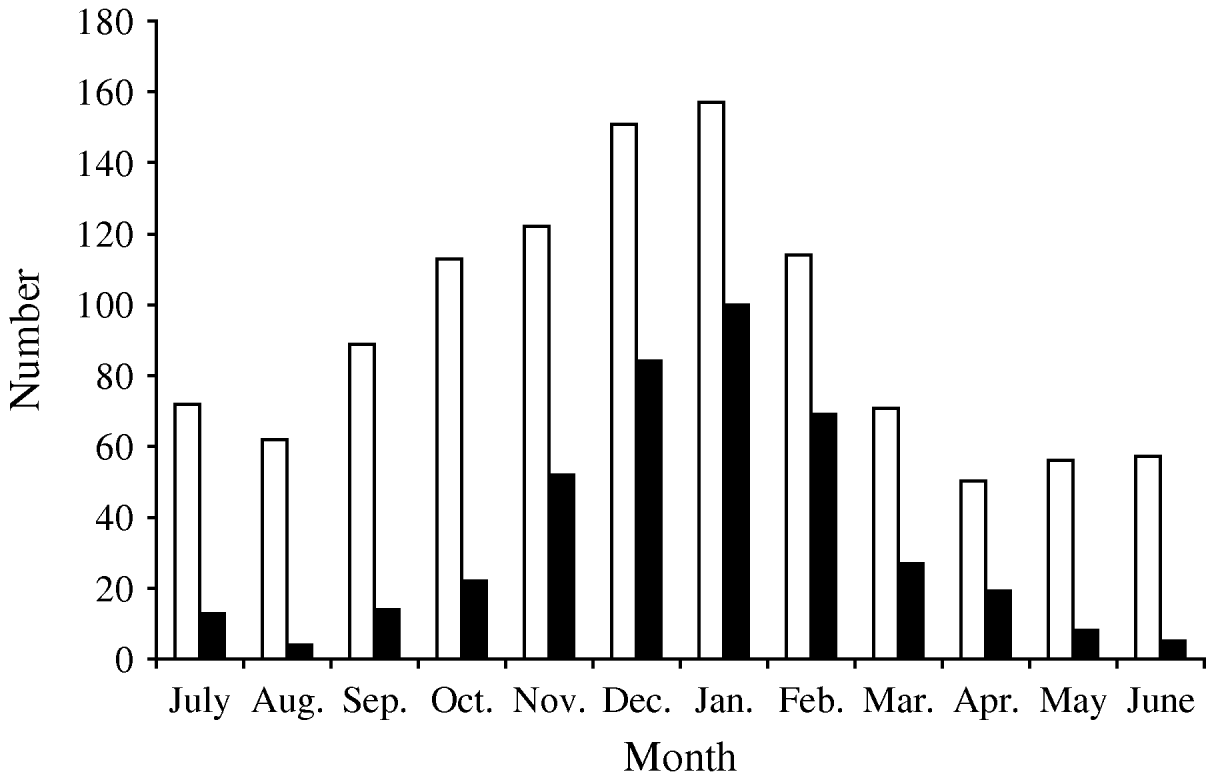
Fig. 1. Seasonal distribution of hospitalization episodes due to acute gastroenteritis (□) and rotavirus-positive (▪) episodes in children aged <5 years, Gipuzkoa (Basque Country, Spain) between July 1996 and June 2008.
Hospitalization for rotavirus gastroenteritis
In 1070/1114 hospitalization episodes due to AGE (96·1%), a stool sample was available for analysis of gastroenteric pathogens, including rotavirus antigen determination. Rotavirus was detected in 419 (39·2%) episodes. In 302 of these episodes (72·1%), the discharge code was for rotavirus (008·61). Rotavirus was detected in all months of the year, oscillating between 62·5% (253/405) of hospital admissions for AGE occurring in the epidemic peak (December–February) and 14·1% (38/269) of those occurring during the warmest months (June–September) (Fig. 1). Rotavirus caused 44·9% (n=358) of hospitalizations attributable to AGE in children aged <2 years during the study period, this age group represented 85·4% of all hospitalizations due to rotavirus.
A total of 11·0% (46/419) of patients positive for rotavirus simultaneously showed an enteropathogenic bacteria in stool samples: Campylobacter spp. (n=31), Escherichia coli (n=6), Salmonella spp. (n=5) and Yersinia enterocolitica (n=4). There was only one re-admission episode due to AGE attributable to rotavirus in a boy (the two admissions were separated by an 18-month interval), although Campylobacter jejuni was also detected in the second admission. Moreover, three patients were admitted to hospital due to rotavirus gastroenteritis that had a prior rotavirus-associated AGE episode not requiring admission (episodes separated by 12, 18 and 48 months), one of whom had leukaemia. Patients hospitalized for rotavirus represented 9·2% (419/4570) of all the children aged <5 years in whom rotavirus was detected in the same time period and geographical area.
Incidence of hospitalization due to rotavirus
The overall annual incidence of AGE-related hospitalization and that due to rotavirus in the four study periods is shown in Table 1. The mean annual incidence of rotavirus-associated hospitalization decreased from 29·8 cases/10 000 children aged 1 month to <5 years in the period July 1996 to June 1999 to 13·6 cases from July 2002 to June 2005 (P<0·001), a period in which the rotavirus vaccines had not been commercialized, and this figure remained fairly constant in subsequent periods. Both the mean annual incidence of AGE-related hospitalization and that specifically due to rotavirus decreased with age (Table 2). The mean annual incidence of hospitalization due to rotavirus in children aged <2 years was 45·3 cases/10 000, decreasing to 0·6 cases/10 000 children in the fifth year of life.
Table 1. Mean annual incidence of hospitalization (rate/10 000 children and 95% confidence intervals) for acute gastroenteritis (AGE) and rotavirus AGE in children aged 1 month to <5 years in Gipuzkoa (Basque Country, Spain) between July 1996 and June 2008
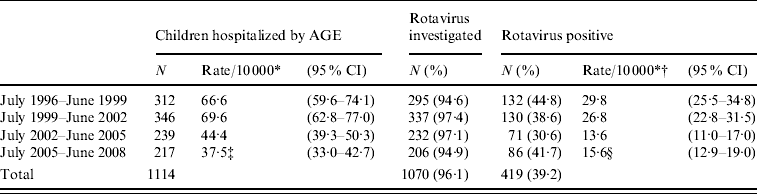
* Rate corrected (weight) for the percentage of hospitalization episodes in other hospitals (3%).
† Rate corrected for the percentage of episodes in which the presence of rotavirus was not investigated.
‡ χ2 with 3 degrees of freedom=75·44, P<0·001. Comparison of each triennium with the following triennium showed a significant difference between the second and third trienniums: χ2=29·0, P<0·001.
§ χ2 with 3 degrees of freedom=44·93, P<0·001. Comparison of each triennium with the following triennium showed a significant difference between the second and third trienniums: χ2=22·3, P<0·001.
Table 2. Mean annual incidence of hospitalization stratified by age (rate/10 000 children and 95% confidence intervals) for acute gastroenteritis (AGE) and rotavirus AGE in children aged <5 years in Gipuzkoa (Basque Country, Spain) between July 1996 and June 2008

* Rate corrected for the percentage of hospitalization episodes in other hospitals (3%).
† Rate corrected for the percentage of episodes in which the presence of rotavirus was not investigated.
Overall, AGE and rotavirus gastroenteritis caused 8·4% and 3·1% of 13 309 paediatric admissions; in children aged <2 years, the percentages were 9·1% and 3·9%, respectively. The mean length of stay for children hospitalized with rotavirus infections was 4·7±4·9 days (mode 3 days). Fourteen children (3·4%) required admission to the paediatric intensive care unit; there were no deaths.
Rotavirus genotypes
G and P types were obtained in 124/137 samples in which these types were investigated (Table 3). In genotyped episodes, the most frequently detected combination was G1[P8] (54·8%), which circulated in all the annual epidemics and predominated in the first part of the study (74·5%) in the seasons 1996–1997 to 2002–2003. From the season 2003–2004 onwards, a greater number of genotypes were detected due to the presence of strains with the combinations G9[P8], G2[P4] and G3[P8] (60·9% of genotyped episodes).
Table 3. Combinations of G and P types of rotavirus detected in children hospitalized for acute gastroenteritis

* G3+G4[P8].
† The P type was not obtained in one sample (periods 2000–2001 and 2002–2003).
‡ G2[P8].
§ G12 P not typed.
∥ Three were [P8] and two were [P4].
Consumption of oral rehydration solutions
Between 2001 and 2008 there was a marked increase in the sale of oral rehydration solutions, acquisition of these items increasing from 8001 in 2001 to 31 979 in 2004 and 33 472 in 2008. Of these products, 83·4% were acquired from pharmacies and the remainder were acquired from parapharmacies.
DISCUSSION
One of the best ways to survey severe rotavirus diarrhoea and to assess the impact of rotavirus immunization is to analyse the information provided by sentinel hospitals that routinely test for rotavirus [8–Reference Chang11]. However, few hospitals routinely perform this test as it is often not useful for treatment and requires substantial economic resources to be expended [Reference Patel10]. In the current study, 96% of the children hospitalized for AGE year-round for 12 years were studied virologically and the fraction of these tested for rotavirus hardly changed throughout the four trienniums (94·6–97·4%), allowing the burden of rotavirus disease to be directly assessed. This percentage is clearly higher than that of the few laboratory-confirmed studies that have recently been published [Reference Patel10, Reference Payne12].
A total of 27·9% (n=117) of children admitted to hospital for rotavirus infection received a discharge code of AGE other than rotavirus, usually unspecified (i.e. 558·9), hampering their identification. In contrast, 16·6% (60/362) of the episodes in which the discharge code of rotavirus was used corresponded to children in whom AGE was not considered the cause of hospital admission. If AGE had been considered as the cause of these episodes of hospitalization, the percentage of hospitalizations attributable to rotavirus would have erroneously increased. These data and the differences found in the coding practices of distinct hospitals highlight the difficulty of estimating the burden of rotavirus disease accurately when discharge hospitalization codes are the only information source used [Reference Hsu13].
The fraction of AGE episodes attributable to rotavirus (39·2% overall, 44·9% in children aged <2 years, 62·5% during the cold trimester) confirms the major role of this pathogen in the aetiology of severe childhood AGE. These figures are in line with those reported in recent studies performed in different countries [8, Reference Payne12, Reference Parashar14, Reference Forster15], the highest figures being those obtained in the current decade [8, Reference Parashar14]. Substantial variations were observed throughout the study, the median detection rate oscillating between 44·8% and 30·6% in the first and third trienniums of the study, respectively, which may have been due to variations both in rotavirus activity and in the incidence of diarrhoea of other causes. These variations did not indicate a particular tendency since the detection rate was similar in the first and fourth trienniums of the study (Table 1), but do reveal the importance of performing studies over several seasons.
The incidence of hospitalization for AGE due to rotavirus is an essential component of the burden of the disease for healthcare systems, especially in high-income countries and, unlike the detection rate, does not depend on seasonal tendencies in the incidence of other enteropathogens, thus allowing its evolution to be monitored over time. The overall incidence of hospital admission was 45·3 and 21·5 cases/10 000 inhabitants in children aged from 1 month to <2 years and <5 years, respectively. On reaching the age of 5 years, one of every 95 children has been hospitalized for AGE due to rotavirus. The incidence found in the current study was somewhat higher than that found in a study with virological confirmation recently carried out in the USA [Reference Payne12] and was similar to the mean value obtained for 16 high-income European countries (19 hospitalizations/10 000 children aged <5 years) [Reference Williams, Lobanov and Pebody2].
The incidence of hospitalization for rotavirus AGE significantly decreased throughout the study, especially in the second half. The possible influence of vaccination on this decrease was considered minimal because the decrease in incidence was observed from 2002, while the rotavirus vaccine was available from 2006 and its coverage was low from 2006 to 2008. Previous data from Gipuzkoa also showed a high incidence rate (31·1 cases/10 000 children aged 1 month to 5 years from July 1993 to June 1996) [Reference Cilla6]. This decreasing tendency contrasts with the situation in other countries such as the USA where rotavirus-associated hospitalization remained relatively stable between 1995 and 2004 [Reference Pont16]. However, few regions have uniformly monitored the incidence of hospitalization for rotavirus AGE for prolonged periods. The decrease observed, in a society whose nutrition and hygiene levels did not significantly change during the study period, could be related to the rapid rise in the use of oral rehydration therapy in children with AGE. Indeed, the sale of oral rehydration solutions in pharmacies and parapharmacies quadrupled in Gipuzkoa between 2001 and 2004. As in Gipuzkoa, the use of oral rehydration has increased in France, especially after 1996 [Reference Martinot17]. Oral rehydration therapy is a key feature in the treatment of AGE in infants but continues to be underused globally, and specifically by physicians in high-income countries [Reference Bellemare18]. In Gipuzkoa, the incidence of rotavirus hospitalization has paralleled the decline in overall hospitalizations from diarrhoea, whether rotavirus positive or not. These results suggest that even in developed countries this therapeutic measure can currently reduce the impact of severe diarrhoea, whether due to rotavirus or other causes.
A notable finding was that we only observed four cases of hospitalization due to rotavirus in patients who had previously been infected by this virus, indicating considerable protection against subsequent infections [Reference Velázquez19]. Although no clearly defined pattern of viral circulation was detected, the G1[P8] combination, which is included in the monovalent Rotarix vaccine (GlaxoSmithKline Biologicals), was predominant (54·8%), in accord with other studies [8, Reference Cilla20, Reference Santos and Hoshino21], and circulated in all the annual epidemics. In specific seasons, we found a higher number of strains G3[P8] or the emerging G9[P8], which predominated in Spain for the first time in the 2005–2006 season, causing numerous hospitalizations [Reference Sánchez-Fauquier22]. Eighty-five percent of the typed strains (105/124) had both, a G and a P antigen included in the pentavalent vaccine (RotaTeq). For 86·3% and 100% of these strains, a G or P antigen was included in the monovalent or the pentavalent vaccine, respectively. A potentially limiting factor of vaccination is the age at which these cases occur, given that the recommended age of vaccination (2 and 4 months for the monovalent vaccine and 2, 4 and 6 months for the pentavalent vaccine) does not allow protection of previously infected children. Twenty per cent (85/419) of the episodes occurring in our study occurred in infants aged <6 months and similar figures have been reported in other studies performed in European countries [Reference Forster15, Reference Van Damme23].
The main limitations when comparing the data from the current study with those from other studies lies in the different hospitalization strategies, coding practices, criteria for patient searches and the frequency of performing virological tests used in distinct studies. Importantly, the current study was performed year-round for 12 years in a public hospital catering for >95% of the paediatric population of a wide geographical area, in which most AGE-related hospitalizations were virologically investigated (96%). In conclusion, in this European region, the incidence of hospital admission due to rotavirus-associated severe diarrhoea has decreased markedly, coinciding with an increase in the use of rehydration therapy. Despite this decrease, the impact of rotavirus AGE on the healthcare system continues to be substantial in the Basque Country. Given the high efficacy of rotavirus vaccines in infants in developed countries, their inclusion in immunization programmes should be considered.
ACKNOWLEDGMENTS
This study was partially supported by a grant from the Department of Health of the Basque Government (project 2007111026). The authors thank Adoración Jiménez (Pharmacy Department, Hospital Donostia) for help in obtaining data on the sale of oral rehydration solutions.
DECLARATION OF INTEREST
None.






