Introduction
Fire, as a ubiquitous driver of disturbance in terrestrial ecosystems, has been increasingly realized as a major evolutionary force across the globe (Bond and Keeley, Reference Bond and Keeley2005; Bowman et al., Reference Bowman, Balch, Artaxo, WJ, JM, MA, CM, RS, JC, SP, FH, JE, MA, CA, JB, MA, IC, CI, AC, TW, GR and SJ2009; Li et al., Reference Li, Bond-Lamberty and Levis2014). Fire-related cues function as germination stimuli of many postfire species (Collette and Ooi, Reference Collette and Ooi2017; Manela et al., Reference Manela, Dagon, Semesh and Ovadia2019). Many plants have evolved specific traits, such as fire-stimulated flowering, seed release and germination, to time and adapt their growth with postfire environments (Baldwin and Morse, Reference Baldwin and Morse1994; Bytebier et al., Reference Bytebier, Antonelli, Bellstedt and Linder2011; He et al., Reference He, Pausas, Belcher, Schwilk and Lamont2012; Tonnabel et al., Reference Tonnabel, Mignot, Douzery, AG, FM, Midgley, Illing, Justy, Orcel and Olivieri2014). Perhaps, most spectacular among these traits is seed germination that is stimulated by fire-derived smoke, a trait that enables plants to colonize the nutrient-rich habitats that follow fires (Preston and Baldwin, Reference Preston and Baldwin1999; Lamont and He, Reference Lamont and He2017). Ever since smoke was found to promote seed germination of some postfire plants (Zimmerman and Laven, Reference Zimmerman and Laven1987; Keeley and Fotheringham, Reference Keeley and Fotheringham1998), extensive efforts were motivated to identify the active compounds responsible for smoke-stimulated seed germination (Baldwin et al., Reference Baldwin, Staszak-Kozinski and Davidson1994; Keeley and Fotheringham, Reference Keeley and Fotheringham1997; Flematti et al., Reference Flematti, Merritt, Piggott, RD, SM, KW and EL2011).
The recent discovery of karrikins has reignited interests in understanding the mechanisms underlying smoke-promoted seed germination and its evolution (Flematti et al., Reference Flematti, Ghisalberti, Dixon and Trengove2004; van Staden et al., Reference van Staden, Jäger, Light, Burger, Brown and Thomas2004; Nelson et al., Reference Nelson, Flematti, Riseborough, Ghisalberti, Dixon and Smith2010; Morffy et al., Reference Morffy, Faure and Nelson2016; Lamont and He, Reference Lamont and He2017). The powerful germination activity of karrikins has sometimes led to the assumption that karrikins are the universal (or the primary) chemical signal responsible for smoke-promoted seed germination (Nelson et al., Reference Nelson, Riseborough, Flematti, Stevens, EL, KW and SM2009; Lamont and He, Reference Lamont and He2017; Keeley and Pausas, Reference Keeley and Pausas2018). Accumulating evidence has suggested that smoke-promoted seed germination is a more complex trait than a universal karrikin-driven process (as extensively reviewed by Keeley and Pausas, Reference Keeley and Pausas2018). It is increasingly realized that other active chemicals might be responsible for smoke-derived seed germination (Downes et al., Reference Downes, Lamont, Light and van Staden2010; Keeley et al., Reference Keeley, Pausas, Rundel, Bond and Bradstock2011), which is supported by recent reports of some other active compounds in smoke that can promote seed germination, including NOx and glyceronitrile (Keeley and Fotheringham, Reference Keeley and Fotheringham1997; Flematti et al., Reference Flematti, Merritt, Piggott, RD, SM, KW and EL2011). The ecological relevance of these compounds has been debated (Keeley et al., Reference Keeley, Pausas, Rundel, Bond and Bradstock2011; Lamont and He, Reference Lamont and He2017; Keeley and Pausas, Reference Keeley and Pausas2018). Further studies are needed to explore the potential smoke-derived germination cues of postfire-adapted species in the appropriate ecological contexts.
The postfire germination behaviour of the annual Nicotiana attenuata is particularly well studied (Baldwin et al., Reference Baldwin, Staszak-Kozinski and Davidson1994; Baldwin and Morse, Reference Baldwin and Morse1994; Preston and Baldwin, Reference Preston and Baldwin1999; Krock et al., Reference Krock, Schmidt, Hertweck and Baldwin2002; Preston et al., Reference Preston, Becker and Baldwin2004; Schwachtje and Baldwin, Reference Schwachtje and Baldwin2004). The postfire seedling emergence of N. attenuata is restricted to burned areas and normally occurs within 3 years after a fire, after which long-lived seedbanks are established. This restricted distribution of N. attenuata seedlings after fires could be explained by a combination of fire-associated germination stimulants and the pyrolytic removal of allelopathic germination inhibitors from the litter layer (Preston and Baldwin, Reference Preston and Baldwin1999; Krock et al., Reference Krock, Schmidt, Hertweck and Baldwin2002; Preston et al., Reference Preston, Betts and Baldwin2002). However, an alternative explanation would be that fires also produce leach-resistant germination cues, which could be retained in soils and adsorbed to seed coats so as only to promote germination in areas actually burned by fires. Preston and Baldwin (Reference Preston and Baldwin1999) demonstrated a large (10×) fitness cost associated with mis-timing of germination to occur outside the boundaries of burned areas for N. attenuata; hence, the use of cues that accurately reflect fire boundaries would be highly advantageous for fire-chasing plants with long-lived seed banks.
Here, we revisit the bioassay-driven fractionation of N. attenuata seed germination cues found in commercially available liquid smoke water (House of Herbs Inc., Passaic, NY, USA), work that was started a quarter of a century ago (Baldwin et al., Reference Baldwin, Staszak-Kozinski and Davidson1994), with the superior analytical chemistry and metabolomic tools that are currently available for the N. attenuata system. We re-discover a smoke germination cue first reported in the Baldwin et al. (Reference Baldwin, Staszak-Kozinski and Davidson1994) study and show its likely ecological relevance as a germination signal for this species.
Materials and methods
Seed material
Nicotiana attenuata Torr. Ex Watts. seeds were collected from plants grown in the glasshouse with growth conditions described in Krügel et al. (Reference Krügel, Lim, Gase, Halitschke and Baldwin2002). A well-characterized inbred line ‘G2’ was used in this study (Schuman et al., Reference Schuman, Heinzel, Gaquerel, Svatos and Baldwin2009; Bhattacharya and Baldwin, Reference Bhattacharya and Baldwin2012). The ‘G2’ line was inbred for three generations in the glasshouse after collection from a plant growing in a burn at Apex Mine, near Santa Clara (Utah, USA) in July 2007. This line shows that very low background germination percentages and its germination are strongly promoted by smoke in our laboratory bioassays; hence, seeds of this genotype were used in the bioassay-driven fractionation of the germination cues. Seeds were air-dried at room temperature in a desiccator for 10 d after collection, cleaned by removing empty seeds manually, and stored at −80°C until use (Scaffidi et al., Reference Scaffidi, Waters, Sun, BW, KW, EL, GR and SM2014).
Germination assays
For all germination tests, four replicate assays of 25 seeds each were imbibed in plastic petri dishes (5-cm diameter) filled with 3.7 g of analytical grade sand (Merck, Darmstadt, Germany) and 4 ml of Milli-Q water (or indicated solutions). Imbibed seeds were incubated in a Percival growth chamber (14 h day, 200 μM m−2 s−1 PAR, 30°C: 10 h night, 22°C) at conditions which previous research had established as being optimal germination conditions for N. attenuata seeds (Baldwin et al., Reference Baldwin, Staszak-Kozinski and Davidson1994) and germinating seeds counted every day for 7–10 d (depending upon the tests). Since tetrazolium (2,3,5-triphenyl-2H-tetrazolium chloride) staining (Berridge et al., Reference Berridge, Herst and Tan2005) revealed 100% viability of our cleaned seeds, the germination percentage was calculated as G% = Number of germinated seeds/25 × 100%.
Bioassay-guided fractionation of smoke water on HPLC
Previous research had demonstrated that commercially available liquid smoke fully mimicked the germination responses of freshly prepared aqueous smoke extracts, and even though no significant differences were found among five different brands of liquid smoke condiment (Baldwin et al., Reference Baldwin, Staszak-Kozinski and Davidson1994), the same brand (House of Herbs) that was used in the previous work was used in this study. House of Herbs liquid smoke was first partitioned using dichloromethane (DCM) with a ratio of 1:1 (v/v). The organic DCM partition was dried and re-dissolved in Milli-Q water (less than 2% MeOH was used to help solution), loaded on a Chromabond HR-X solid-phase extraction (SPE) column (Macherey-Nagel, Düren, Germany) and eluted with MeOH. After this cleaning process, the smoke sample was subjected to a coarse fractionation programme on an Agilent 1100 HPLC system equipped with a Luna C18 column (250 × 10 mm, 5 μm particle diameter; Phenomenex, USA). The following binary mobile-phase gradient was applied: 0–30 min, gradient phase from 85% A (Milli-Q water), 15% B (MeOH) to 100% B; 30–35 min, isocratic 100% B. The flow rate was 3 ml min−1. Fractions were collected from 0.5 to 30 min, every 30 s. Fractions were dried under N2, re-dissolved in equal volumes of Milli-Q water to the concentration equivalent to the initial smoke water and diluted 1:300 in Milli-Q water for germination tests. The active fraction was referred to as the crude active fraction (CAF; supplementary Fig. S1).
Further fractionation of the CAF was conducted on an Agilent 1100 HPLC system equipped with a Nucleodur Sphinx RP18 column (150 × 4.6 mm, 5 μm particle diameter, Macherey-Nagel, Germany). The following binary mobile-phase gradient was applied: 0–30 min, gradient phase from 70% A (Milli-Q water), 30% B (MeOH) to 50% A, 50% B; 30–35 min, gradient phase from 50% A, 50% B to 100% B; 30–35 min, isocratic 100% B. The flow rate was 900 μl min−1. Fractions were collected with 1 min collection windows; each fraction was tested for germination activity as described above. Based on the elution behaviour of the germination-active fractions, we constructed a matrix of fractions with differential germination activities. A first batch (Batch 1) of five fractions was collected from 8 to 13 min (1 min for each fraction). By shifting collecting times 10 s backward but retaining the 1 min collection frequency, a second batch of five fractions was collected. This process was reiterated until a total of seven batches were collected, with a total of 35 fractions.
Mass spectrometry profiling of the matrix fractions
Mass spectrometry (MS) profiling of the 35 fractions was performed on a Dionex Ultimate 3000 UHPLC system with an Acclaim RSLC 120A C18 column (150 × 2.1 mm, particle size 2.2 μm, ThermoFisher) equipped with a Security Guard ULTRA guard column (Phenomenex). The following binary mobile-phase gradient was applied: 0–0.5 min, isocratic 90% A [Milli-Q water, 0.1% (v/v) acetonitrile and 0.05% formic acid], 10% B (acetonitrile and 0.05% formic acid); 0.5–13.5 min, gradient phase to 10% A, 90% B; 13.5–15 min, isocratic 10% A, 90% B. The flow rate was 400 μl min−1. Eluted compounds were detected by a high-resolution quadrupole-time-of-flight (qTOF) mass spectrometer (microTOF-Q II, Bruker, Bremen, Germany) equipped with an electrospray ionization source operated in positive ionization mode. Typical Q-TOF instrument settings were as follows: capillary voltage 4500 V, capillary exit 130 V, dry gas temperature 180°C and dry gas flow of 8 l min−1. Ions were detected from m/z 50 to 1400 at a 1 Hz acquisition rate. Mass calibration was performed using sodium formate clusters [10 mM solution of NaOH in 50/50% (v/v) isopropanol/water containing 0.2% (v/v) formic acid]. Raw data files were converted to the netCDF format using the export function of the Data Analysis v4.0 software (Bruker).
Structure elucidation by LC–MS/MS analysis
A targeted MS/MS analysis was conducted on the LC–MS system described for the MS profiling to gain structural information of the active compound. Fragmentation data of the parent ion m/z 183 were acquired with different collision-induced dissociation (CID) settings (20, 30 and 40 eV).
Validation of the active compound by LC–MS/MS analysis
Since the MS spectra of the active compound showed strong similarity to syringaldehyde (SAL) in the available MS spectra database, commercial SAL was analysed on the LC–MS/MS to validate the active compound. The same LC–MS/MS programme was performed as used for the structure elucidation experiment.
Germination activity of SAL
Germination activity was tested towards commercial SAL on N. attenuata seeds. SAL solution was prepared in Milli-Q water to 50 ng μl−1, and dilutions of 1×, 5×, 10×, 25×, and 50× were made using Milli-Q water. Four replicates of 25 seeds each were used in the SAL germination activity test as described in the germination assay section.
Detection of SAL in postfire soils
To test whether wildfires produce SAL in the natural habitat of N. attenuata, we analysed SAL abundance in postfire soils from two natural fires in Arizona and Utah, USA. One batch of postfire soil was collected in Arizona on 2 July 2016, 9 d after a small wildfire that had burned a cluster of creosote bushes (Larrea tridentata) was extinguished. Three soil samples (about 100 g each, including burned soil and semi-burned litter) were collected at each of three locations at the inside, outside and on the edge of the burn. A second batch was collected in Utah 8 d after an 800 acre fire on 22 May 2020 burned a natural habitat near Toquerville, Utah. Burned soil and semi-burned litter were collected from underneath the charred remains of five individual shrubs of the following species: Mormon tea (Ephedra nevadensis), sand sage (Artemesia filifolia), blackbrush (Coleogyne ramosissima), yucca (Yucca utahensis), juniper (Juniperus osteosperma), bitterbrush (Purshia tridentata), indigo bush (Psorothamnus fremontii) and a brome grass (Bromus spp.) dominated shrub-free area. Unburned soil from a nearby wash was sampled as a control.
Soil samples (2 g) were extracted with 10 ml of MeOH. Extracts were loaded on SPE columns (HR-X, Macherey-Nagel), washed with 4 ml of 5% aqueous MeOH and eluted with 4 ml of 50% MeOH. SPE-purified soil extracts were dried and re-dissolved in 1 ml of MeOH for MS analyses. SAL abundance of the first batch was estimated using 10 ng μl−1 SAL standards with the qTOF-MS profiling method described above. For the second batch, soil extracts were prepared as described for the first batch. Quantification was performed on a UPLC-triple-quadrupole MS/MS instrument (EVOQ Elite, Bruker) operated in a positive electrospray ionization (ESI) mode. Multiple reaction monitoring settings were optimized for the detection of SAL: quantifier m/z 183.1 → 123.1 (CID 9 V) and quantifier m/z 183.1 → 77.2 (CID 20 V). Quantification was based on a calibration curve using SAL standards of 0, 1, 2, 5, 10 and 20 ng μl−1 and processed through the same SPE-cleanup and UPLC–MS/MS programme.
Retention of SAL by seeds
N. attenuata seeds (145.14 ± 0.82 mg) were packed into a plastic tube (1.5 mm in diameter, 7 cm in length, referred to as ‘seed column’). Stock solutions of 20 ng μl−1 SAL were prepared in Milli-Q water. Three decade dilutions of test solutions, 1×, 10× and 100×, were prepared in Milli-Q water. For each assay, 400 μl of working solution was sequentially passed through three seed columns. Seeds were retrieved from the columns, rinsed thrice in 5 ml Milli-Q water and tested for germination. Four replicates of 25 seeds were taken from each seed column and used in replicate germination assays. A seed column passed through by 400 μl of Milli-Q water was used as a control group for germination tests. For comparison, 10 ng μl−1 KAR1 and its 1×, 10× and 100× dilutions were tested for retention by seeds in the same way.
Retention of SAL by soil
An unburned soil sample was collected from N. attenuata's native habitat in Arizona and mixed thoroughly before use. Ten μg of SAL in 1 ml MeOH were added to 1 g soil, and supernatant was discarded after over-night incubation. Then, the soils were subjected to leaching using 5 ml Milli-Q water. Spiked samples without leaching were used as controls. The leached and control soil samples were extracted using the SPE-cleanup process described above. The same amount of KAR1 was applied to the same procedure for comparison. The abundance of SAL and KAR1 was estimated using 10 ng μl−1 SAL or 1 ng μl−1 KAR1 standards, with the qTOF-MS profiling method described above.
Data analysis
Statistical analyses were performed using the SPSS17.0 software (SPSS Inc., Chicago, IL, USA). Data for signal intensities of mass features of the 35 smoke fractions were normalized before heat-map analyses and Pearson correlation analyses by the following equation:
where SIn is the normalized signal intensity of a specific mass feature in a given fraction, SIij the signal intensity of the mass feature in Fraction i Batch j, ∑ the sum of signal intensities for the mass feature in the five fractions of Batch j, and Max (∑) is the maximum of ∑ for the mass feature of the seven batches. By this normalization, data of signal intensities for all mass features ranged from 0 to 100.
Results
Identification of SAL as a germination cue in smoke
After a coarse separation of smoke water by HPLC, the resulting CAF was further separated by HPLC to produce seven batches of five fractions each (supplementary Fig. S1), with each batch being shifted by 10 s from the previous batch, to yield a matrix of 35 fractions (Fig. 1a). The obtained fractions were dried, re-dissolved in Milli-Q water and tested for germination activity using N. attenuata seeds. Germination bioassays indicated varied germination activities of the 35 fractions (Fig. 1b). MS profiling of the fractions revealed 199 mass features (m/z signals at specific retention time). Based on the abundance of their mass features, the 35 fractions clustered into three main clusters, of which cluster C1 included the nine fractions with germination activity (Fig. 1c). Pearson correlation analyses revealed nine mass features that correlated strongly with germination activity (Table 1). Since the most active fractions (Fraction 3 of Batch 1, B1F3) still showed some activity when diluted five times (Fig. 1d), we inferred that the associated m/z signal intensity for a relevant germination cue should be at least fivefold higher in the most active fraction than in non-active fractions. Using this criterion, we identified a 183 m/z mass feature for further exploration (Fig. 1e).

Fig. 1. Rapid and efficient bioassay-driven identification of germination cues from aqueous extracts of smoke by LC–MS. (a) A schematic showing differences in collection times of a matrix of 35 fractions from seven HPLC separations (Batches 1–7) of a CAF (supplementary Fig. S1). (b) Germination responses of N. attenuata seeds after 10 d of exposure to the 35 fractions of smoke extract from seven separation batches of B1–B7. Values are means ± SE (N = 4). The dashed line indicates the germination percentage of seeds exposed only to water for 10 d, and fractions with higher germination percentages than those of water were considered as active fractions. (c) A heat map depicts signal intensities of mass features in the 35 fractions. MS profiling revealed 199 mass features in the 35 smoke fractions and the active fractions clustered together (C1) based on m/z signal intensities. The signal intensities are colour coded with blue depicting low and orange depicting high intensities. (d) N. attenuata seed germination percentages in response to dilutions of the most active fraction, Fraction 3 of Batch 1 (B1F3). The original concentration of B1F3 was equivalent to a 1:300 dilution of the original smoke extract. Asterisks indicate significant differences (P < 0.05) between the treatment and the control of water imbibed seeds. (e) Signal intensities of nine mass features most strongly correlated with germination activity. Box plots show the medium, the upper and lower quartiles and the minimum and maximum signal intensities of the mass features in the non-active fractions. The blue dots show signal intensities of the mass features in the most active fraction B1F3. A red arrow depicts the 183 m/z mass feature, which had a more than fivefold difference in the signal intensity between B1F3 and the non-active fractions.
Table 1. Nine mass features displaying significant Pearson correlations with germination activity

Targeted MS/MS analysis of the active fraction B1F3 on the UHPLC-ESI-qTOF-MS elucidated the chemical structure of the 183 m/z mass feature. The [M+H]+1 molecular ion of m/z 183 suggested that a molecular formula as C9H10O4 and a spectral search in the GNPS database (Wang et al., Reference Wang, Carver, Phelan, LM, Garg, Peng, DD, Watrous, CA, Luzzatto-Knaan, Porto, Bouslimani, AV, MJ, W-T, Crüsemann, PD, Esquenazi, Sandoval-Calderón, RD, LA, RA, KR, C-C, DJ, RG, Kleigrewe, Northen, RJ, Parrot, EE, Aigle, CF, Jelsbak, Sohlenkamp, Pevzner, Edlund, Mclean, Piel, BT, Gerwick, C-C, Y-L, H-U, Maansson, RA, AC, AR, AM, BE, Klitgaard, CB, PCA, Torres-Mendoza, DJ, DB, LM, DP, Pociute, EC, Briand, EJN, EA, Glukhov, Ryffel, Houson, Mohimani, JJ, Zeng, JA, KL, Charusanti, KL, KF, Vuong, Elfeki, MF, Engene, Koyama, OB, Baric, RR, SJ, Tomasi, Jenkins, Macherla, Hoffman, Agarwal, PG, Dai, Neupane, Gurr, AM, CLamsa, Zhang, Dorrestein, BM, Almaliti, P-M, Phapale, L-F, Alexandrov, Litaudon, J-L, JE, TO, Peryea, D-T, Vanleer, Shinn, Jadhav, Müller, KM, Shi, Liu, Zhang, Knight, PR, BØ, Pogliano, RG, Gutiérrez, NP, WH, BS, PC and Bandeira2016) showed strong similarity to the NIST14 and MassBank database entries for SAL. Furthermore, the 183 m/z feature also showed an identical retention time and an MS2 fragmentation pattern to an authentic SAL standard (Fig. 2a,b). Germination assays revealed that N. attenuata seeds germinated to 76.0 ± 3.7% in 2 ng μl−1 SAL (25× dilution of a 50 ng μl−1 SAL solution), which was significantly (P < 0.05) higher than in water (Fig. 2c). The bioassay was repeated with a second seed lot of N. attenuata with similar results (supplementary Fig. S2).

Fig. 2. Identification of SAL as a germination cue. (a) MS2 spectra of the m/z 183 mass feature of the crude active fraction and an authentic SAL standard were recorded on a UHPLC-ESI-qTOF instrument (collision energy of 20 eV). (b) Extracted ion chromatograms of the LC–MS runs showed that the m/z 183 mass feature in the CAF had a same retention time as an authentic SAL standard. (c) Germination percentages of N. attenuata seeds were recorded after 7 d of being exposed to water (W), 1:300 smoke water (S) and 1- to 50-fold dilution of a 50 ng μl−1 SAL solution.
Detection of SAL in postfire soils
We detected SAL, in quantities as high as 40 ng g−1 in soil collected from a fresh wildfire of creosote bush (L. tridentata) in a natural N. attenuata habitat in Arizona (Fig. 3a,b). Interestingly, no SAL was detected outside the burn (Fig. 3b). From a wild fire that burned another natural N. attenuata habitat in Utah containing different shrub species, SAL abundance was found to vary in burned soils under different species, ranging from 0.43 ± 0.21 ng g−1 (for sand sage) to 23.44 ± 7.50 ng g−1 (for bitter brush) (Fig. 3c,d).

Fig. 3. Wildfires produce SAL in the natural habitats of N. attenuata. (a) Postfire soil samples were collected for SAL analyses from wildfires in Arizona (Burn 1) and Utah (Burn 2), USA. (b) SAL was detected in soil samples from within the burned area but not immediately outside the burned area. Values in the bars are means ± SE (N = 3); different lower case letters indicate significant differences (P < 0.05) among SAL concentrations revealed by least significant difference post-hoc comparisons. (c) Burn soils were collected in Burn 2 under the charred remains of individual plants (N = 5) from seven shrub species and a bulk collection from brome grass-dominated bare ground (images, ITB). (d) SAL abundance was quantified in soil samples collected from Burn 2 by UPLC–MS/MS. A soil sample of an unburned roadside wash was analysed as a control (Wash).
Retention of SAL by seeds
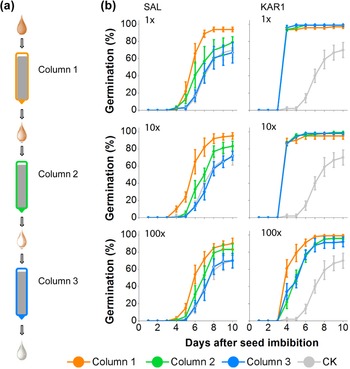
In our previous research, we established that the germination activity could be removed by passing smoke water through a column composed of seeds (Baldwin et al., Reference Baldwin, Staszak-Kozinski and Davidson1994). Here, we tested the germination activity of SAL and KAR1 solutions after passing through three sequential seed columns (Fig. 4a). More than 90% of the N. attenuata seeds packed into the first column germinated when we passed 1×, 10× and 100× dilutions of SAL (Fig. 4b). However, germination decreased considerably in the second column, and no significant germination stimulation was observed in the third column compared to the control seed column, through which only water was passed (Fig. 4b), indicating the retention of SAL by the seeds. An equivalent percentage of seeds germinated in the three sequential columns through which KAR1 solutions were passed, with a slightly lower germination activity in the most diluted concentrations (0.1 pg μl−1 KAR1) tested (Fig. 4b).

Fig. 4. Germination responses of N. attenuata seeds to the pass-through of SAL or KAR1 solutions applied to the column packed with dry seeds. (a) A schematic showing the workflow for passing solutions through three sequential seed columns. After solution passage, seeds were retrieved from each seed column, washed in Milli-Q water and tested for germination. (b) Germination responses of seeds recovered from the three sequential columns after passage were tested. Three decade dilutions (1×, 10× and 100×) were tested for each of SAL and KAR1, and the original concentrations (1×) were 10 ng μl−1 KAR1 and 20 ng μl−1 SAL solutions. A seed column with the Milli-Q water passage was tested as a control (CK). Note that the germination decreased for each passage of the SAL solution, but this was only the case for the most diluted (100×) solution of KAR1.
Retention of SAL by soil
We examined the retention of SAL and KAR1 in soil after water leaching (Fig. 5a). While no significant difference in the SAL contents of spiked soils was detected between water-leached and control samples (Fig. 5b), only 2.1 ± 0.07 μg KAR1 was detected in soil after water leaching, representing 22.2% of the KAR1-spiked soil without leaching treatment (Fig. 5b). This analysis demonstrates that SAL is sparingly water-soluble and retained in burned soil, closely reflecting the spatial boundaries of a burn. We further wanted to know how long SAL could be retained in soil samples. For this analysis, we retrieved soil samples collected from a wildfire in 1996 (see Fig. 1E from Preston and Baldwin, Reference Preston and Baldwin1999) and detected SAL concentrations as high as 135 ng g−1 soil from these soil samples that had been stored at room temperature for 21 years.

Fig. 5. Relative retention of KAR1 and SAL in native soil. (a) Native unburned soil samples were spiked with 10 μg KAR1 or SAL and subjected to a leaching treatment with water. Water-spiked soil samples (Water) were used to determine background levels of KAR1 and SAL. (b) The levels of KAR1 and SAL in the soil with (+) and without (−) leaching treatments were analysed by UHPLC-qTOF-MS. No KAR1 or SAL was detected in the non-spiked soil (Water). Data represent means ± SE (N = 3). The asterisk indicates a significant difference (Independent Samples t-test, P < 0.05) between the leaching treatment and the non-leaching control.
Discussion
The exciting discovery of karrikins as powerful germination stimulants for seeds (Flematti et al., Reference Flematti, Ghisalberti, Dixon and Trengove2004; van Staden et al., Reference van Staden, Jäger, Light, Burger, Brown and Thomas2004) reignited research into understanding how plants time their germination with postfire environments. Preston and Baldwin (Reference Preston and Baldwin1999) noted the large disconnect between the germination potential of soils collected from differently aged burns and different distances from fresh burns, soils which likely contained karrikins, with the occurrence of N. attenuata seedlings in nature. Postfire germination responses of natural seed banks occur up to 3 years after a fire and their occurrence is commonly limited to the burned area. The germination potential of soils from natural habitats was found to persist much longer (>30 years) and to spread further from a fire (40 m to 1 km) than native seed bank responses are commonly observed (Baldwin and Morse, Reference Baldwin and Morse1994; Preston and Baldwin, Reference Preston and Baldwin1999). This substantial ‘overshoot’ of germination responses outside the burned areas is likely a result of karrikins being transported by wind and water movement to adjacent areas surrounding fires and could explain the germination behaviour of N. attenuata seeds in washes and the consequent occurrence of wash populations (Baldwin and Morse, Reference Baldwin and Morse1994; Bahulikar et al., Reference Bahulikar, Stanculescu, Preston and Baldwin2004); however, this hypothesis needs to be tested with sensitive and KAR1-optimized detection procedures for soils from these ‘out-of-burn’ areas. Washes are commonly devoid of litter and competing vegetation, and germinating in these ‘out-of-burn’ areas is not likely to be maladaptive, as it clearly is in vegetated areas adjacent to burns (Lynds and Baldwin, Reference Lynds and Baldwin1998; Preston and Baldwin, Reference Preston and Baldwin1999). These observations lead to the question whether the seed bank germination behaviour could be shaped by processes other than the combination of highly dispersed positive germination signals and more focused negative germination signals from unburned litter, as proposed by Preston and Baldwin (Reference Preston and Baldwin1999). Could seeds be responding to other positive fire-associated germination signals that more faithfully reflected the actual area burned in a wildfire?
Here we re-discover SAL as such a positive germination cue. In our initial bioassay-driven fractionations of smoke water, we repeatedly purified germination-active fractions using a number of different chromatographic techniques in which SAL was identified (Baldwin et al., Reference Baldwin, Staszak-Kozinski and Davidson1994). However, we discounted SAL as a relevant germination cue, because SAL failed to germinate seeds in the bioassays across three decades of dilutions, as was observed for the smoke water extract (Baldwin et al., Reference Baldwin, Staszak-Kozinski and Davidson1994). A number of explanations may account for why in the current investigation, SAL was found to account for the germination behaviour of smoke water. A different genotype of N. attenuata seeds (‘G2’) was used to drive the current bioassay-driven fractionation. Secondly, all active fractions were previously tested in 9.8 mM KNO3 solutions to mimic the high N levels associated with postfire environments (Lynds and Baldwin, Reference Lynds and Baldwin1998), whereas in the current work, Milli-Q water was used in all bioassays. While Baldwin et al. (Reference Baldwin, Staszak-Kozinski and Davidson1994) reported that seed columns could scavenge the germination-active cue from smoke water extracts, the bioassay conditions which included KNO3 at high concentrations (9.8 mM) may not have allowed seeds to fully adsorb the SAL provided in the test solution and hence diminished the decadal germination response. While we know very little about how SAL is perceived by plants and how this perception is transduced into germination responses, KNO3 may have interfered with this process.
The narrow range of working concentrations of SAL provides some interesting clues for possible action modes of smoke in the nature. N. attenuata, as well as some other smoke-responsive species, responds to narrow ranges of smoke water (Light et al., Reference Light, Gardner, Jäger and van Staden2002; Flematti et al., Reference Flematti, Merritt, Piggott, RD, SM, KW and EL2011; Papenfus et al., Reference Papenfus, Naidoo, Pošta, Finnie and Staden2015). The lack of germination responses at high concentrations is unlikely due to toxicity as promoted germination is commonly observed when high-concentration smoke-treated seeds are rinsed and incubated in water (Light et al., Reference Light, Gardner, Jäger and van Staden2002; Schwachtje and Baldwin, Reference Schwachtje and Baldwin2004). Considering that SAL is sparingly water-soluble and can be retained by soil and seeds, N. attenuata seeds may rapidly sense the smoke cue shortly after wildfires and commence germination immediately when the abiotic and timing conditions are favourable. The ‘storage effect’ of the germination cue(s) (Light et al., Reference Light, Gardner, Jäger and van Staden2002) may be of critical ecological importance for postfire seed germination in the wild. N. attenuata seeds in the seedbank are exposed to smoke cues immediately after a fire (which normally occurs in July and August, the peak of the dry season in the plant's natural habitat in the USA), and then are leached by rainfall/snow melt during the wet cold winters before they germinate in March/April in the first growing season following the fire. Such delays in postfire regeneration are commonly observed in fire-chasers (Ooi et al., Reference Ooi, Auld and Whelan2004; Ooi, Reference Ooi2019). The ‘storage effect’ and leaching process of positive and negative germination cues from the soil during the intervals that commonly separate fires from the observed seed germination windows contribute to the challenges of designing ecologically relevant bioassay-driven fractionations. These considerations underscore the complexity of designing seed germination bioassays that are useful for bioassay-driven fractionations with seeds of native plants, which are likely using many different cues to regulate their germination.
The fact that dormancy depth of many native seeds is known to change seasonally and circa-annually (Baskin and Baskin, Reference Baskin and Baskin2014) challenges the use of bioassay-guided fractionations for the identification of smoke-related germination cue(s) that require repeated cycles of isolation, testing and re-fractionations, processes that can take several months, during which the dormancy depth of the bioassay seeds can wax and wane. Over the many years that we have conducted protracted bioassay-driven fractionations of smoke, we have commonly found that a given batch of seeds will undergo considerable fold-changes in dormancy depth, thereby confounding the analyses. Here, we provide a solution to this dilemma: an MS-assisted bioassay-guided method that efficiently identifies active germination cue(s) for a native postfire plant that only requires a single cycle of bioassay-driven fractionation and avoids the repeated rounds of purification and germination bioassays of traditional methods. By using several batches of active fractions containing different abundances of germination-active compounds in combination with high-resolution chromatography and MS technology, the method can pinpoint germination cue(s) by correlating the intensities of different MS signals to the germination activities of the fractions. This method, which uses the covariance of germination responses and mass features, requires only one round of seed germination bioassays, thereby obviating some of the frustrations associated with the dynamics of dormancy depth of native seeds.
It is very unlikely that most smoke-derived seed germination behaviours can be attributed to a few ‘primary’ or ‘general’ germination cues. It is sometimes assumed that karrikins are the ubiquitous or primary signals responsible for smoke-promoted seed germination, an assumption that provides rationale for studies attempting to understand the evolution of smoke-promoted seed germination and fire-adaptation of plants based on phylogenic analyses of karrikin signalling components (Morffy et al., Reference Morffy, Faure and Nelson2016; Sun et al., Reference Sun, Yao, Scaffidi, KT, SF, CS, SM, GR and MT2020). However, not all smoke-responsive seeds show karrikin-promoted germination (Downes et al., Reference Downes, Light, Posta, Kohout and van Staden2014; Papenfus et al., Reference Papenfus, Naidoo, Pošta, Finnie and Staden2015). The same caveat applies to SAL, which is a general microbial oxidation product of the syringyl unit of lignin. SAL accumulates in the soils of broad-leaved forests and grasslands, ecosystems in which plant litter accumulates (Heidke et al., Reference Heidke, Hartland, Scholz, Pearson, Hellstrom, SFM and Hoffmann2021), and in these ecosystems, SAL is unlikely to function as a fire-relevant germination cue. Considering that several distinct chemical compounds have been reported to promote seed germination of postfire species in different geographic zones, plants may have evolved different signal perception and transduction pathways for smoke-promoted seed germination.
Given the considerable differences in chemical structures between SAL and KARs, SAL's germination activity might be mediated by a signal cascade distinct from the KAR signalling pathway. Once we are able to silence the key components of the KAR signalling pathway, for example, MORE AXILLARY GROWTH2 (MAX2) and KARRIKIN-INSENSITIVE2 (KAI2), in the ‘G2’ genotype of N. attenuata used to drive the fractionation of SAL, we will be able to rigorously test whether KAR signalling is involved in the SAL-mediated germination response. The potential for the discovery of a new SAL-activated germination signalling pathway is one of the many areas of potential research opened up by this report.
Supplementary material
To view supplementary material for this article, please visit: https://doi.org/10.1017/S0960258521000271.
Acknowledgements
We thank Dr M. Schuman and Dr K. Gase for their insightful discussions during the project and Mr D. Hunsaker for his assistance in the smoke fractionation and germination tests.
Financial support
This work was funded by the Max Planck Society and the European Research Council advanced grant ClockworkGreen (No. 293926) and the Collaborative Research Centre ‘Chemical Mediators in Complex Biosystems – ChemBioSys’ (SFB 1127) from the DFG (awarded to I.T.B.).
Conflicts of interest
None declared.
Author contributions
D.C. and I.T.B. conceived and designed experiments. D.C., I.T.B., M.S., R.H., G.B., C.R., and D.L. performed experiments. D.C. analysed data. D.C. and I.T.B. wrote the manuscript.