Policy statements from the Society of Healthcare Epidemiology of America, Infectious Diseases Society of America, and Pediatric Infectious Diseases Society on antimicrobial stewardship programs (ASP) have recommended that the Centers for Medicare and Medicaid Services encourage healthcare institutions to develop and implement stewardship efforts. 1 An optimal ASP is composed of a multidisciplinary team with dedicated oversight, policies, procedures, and accountability for reporting outcomes to continuously evaluate and promote optimal antimicrobial use. However, many community hospitals lack administrative or financial support, or the expertise, such as dedicated infectious diseases specialists, to successfully implement these strategies. 1 – Reference Bartlett and Siola 4
While the impact of ASPs is often benchmarked on antimicrobial utilization data, disease state–driven approaches targeting timely antibiotic therapy for patients with bloodstream infections (BSIs) are essential. Delays in treatment have been associated with increased morbidity and mortality resulting in detrimental outcomes for patients.Reference Ibrahim, Sherman, Ward, Fraser and Kollef 5 , Reference Lodise, McKinnon, Swiderski and Rybak 6 With mortality increasing by the hour for patients with BSI, reducing treatment time is essential to optimize outcomes.Reference Kumar, Roberts and Wood 7 Novel approaches to ASP that have emphasized close collaboration between the microbiology laboratory and pharmacy have shown successful outcomes. These effects are more pronounced in patients with BSI where use of rapid diagnostics coupled with pharmacy intervention to improve antibiotic optimization resulted in significant reductions in hospital length of stay (LOS), healthcare costs, and mortality.Reference Perez, Olsen, Musick, Cernoch, Davis, Peterson and Musser 8 – Reference Vlek, Bonten and Boel 13
To date, the role of rapid diagnostics in ASPs has largely been studied in tertiary or quaternary care medical centers with dedicated infectious diseases pharmacists, complex patient populations, and robust clinical laboratory services. Thus, limited data are available in lower acuity community hospital settings. Subsequent to the success of the ASP at our quaternary-care flagship hospital with use of matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry and near real-time infectious diseases pharmacist notification, we endeavored to capitalize on the existing centralized clinical laboratory infrastructure and expertise described in previous publications and implement a proactive ASP in 2 representative community hospitals.Reference Perez, Olsen, Musick, Cernoch, Davis, Peterson and Musser 8 , Reference Perez, Olsen and Musick 9 In this study, the impact of rapid diagnostics with pharmacist intervention on patients with Gram-negative bacteremia is assessed in this community hospital setting.
METHODS
Houston Methodist (HM) consists of 7 hospitals: Houston Methodist Hospital, its flagship academic hospital in the Texas Medical Center (TMC) where clinical laboratory services are centralized, and 6 community hospitals throughout the Greater Houston metropolitan area. This study was comprised of 2 groups of patients ≥18 years of age with Gram-negative bacteremia who were admitted to HM Sugar Land Hospital (235 beds, 20 miles from TMC) and HM Willowbrook Hospital (241 beds, 30 miles from TMC). A pre-intervention group was admitted between January 1, 2011, and December 31, 2011, and an intervention cohort was admitted between January 1, 2014, and December 31, 2014. The pre-intervention time frame was chosen because ASP efforts were not established nor was MALDI-TOF validated for routine identification (ID). A patient was excluded if his or her culture showed a pathogen that was not a facultative anaerobic or aerobic Gram-negative bacillus. Additionally, any polymicrobial bacteremia or subsequent bacteremia episodes after a subject’s initial inclusion were not included in the analysis. Any patients who died prior to the blood culture becoming positive, and/or for whom criteria for discharge were determined by institutional policies rather than at the treating physician’s discretion (ie, artificial life-sustaining device implantation, neurological complications, and/or elective procedures) were not included. This study was approved by the Institutional Review Board of HM Research Institute [IRB(2)1014-0219].
Data Collection and Definitions
Patients with Gram-negative BSIs were identified using Vigilanz, a real-time surveillance and notification software (Vigilanz, Minneapolis, MN). Data were collected on demographic characteristics, comorbidities, source and manifestations of BSI, microbiology, antibiotic therapy, and severity of illness by the Acute Physiology and Chronic Health Evaluation II (APACHE II) score.Reference Knaus, Draper, Wagner and Zimmerman 14 , 15 For the intervention time period, assessment of the clinical pharmacist’s response to Vigilanz notifications were evaluated. BSI onset was defined as the time the first blood sample yielding the study isolate (index blood culture) was collected. Infection-related characteristics examined included infection source; pathogen species and susceptibility data; and time, dose, and route with antibiotics relative to time of index culture collection. The source of bacteremia was determined according to the definitions published by the Centers for Disease Control and Prevention.Reference Garner, Jarvis, Emori, Horan and Hughes 16 In each case, an effort was made to establish a primary source of infection.
We previously validated a method to identify Gram-negative bacteria directly from positive blood culture medium using MALDI-TOF Biotyper (Bruker Daltonics, Fremont, CA) and to rapidly perform susceptibility testing using the BD-Phoenix system in 2012. We have since successfully implemented in our clinical microbiology laboratory that serves as the reference laboratory for the HM system.Reference Wimmer, Long and Cernoch 17 Prior to using MALDI-TOF, positive blood culture specimens were inoculated on appropriate solid agar media and subsequently identified by conventional clinical microbiology procedures.
Prior to the implementation of ASP notification, Gram-stain results were called to the nursing unit by the microbiology staff followed by passive reporting in the electronic medical record without further notification to the care team. Since the implementation of pharmacist intervention, Gram-stain results have been reported to the nursing unit in a process identical to that in the pre-intervention group. Once the results become available, a page prompted by Vigilanz, is sent to the pharmacist on-call 24/7 with the organism ID and subsequent susceptibility profile. If necessary, the pharmacist contacts the treating provider to communicate the clinical interpretation of the results with recommendations for the most effective and targeted antimicrobial therapy via prospective audit and feedback strategy. Any decision to change antibiotic therapy remains at the discretion of the treating physician. Infectious disease pharmacists are available for consultation as needed and provide training to the on-call clinical pharmacists on the workflow process and interpretation of blood cultures and antibiotic selection.
In this study, therapy was appropriate when the administered regimen was active in vitro and, when available, was in accordance with current clinical guidelines regarding dosing and route of administration.Reference Perez, Olsen and Musick 9 , Reference McGregor, Rich and Harris 18 Therapy was defined as inactive if the blood isolate was resistant to the agent(s) used or in the absence of any antibacterial medications. Empiric therapy was defined as antibiotics administered in the time period before the identification of the blood culture isolate and susceptibility results were available. De-escalation was defined as switching to a narrower-spectrum agent or decreasing the number of antibiotics from ≥2 agents to a single agent when clinically appropriate.
The duration of total hospital and ICU LOS were defined as by the difference in days between admission and discharge. Because death can artificially decrease LOS, LOS analyses were conducted with patients who survived to discharge. We used the American Hospital Association definition for a community and hospital system. 19
Cost analysis was based on total hospital costs across all centers, including room and board, pharmacy, radiology, and laboratory. Cost data were obtained from an individual in the HM accounting department who was independent of the study team. All reported costs represent actual costs for the administration of patient care as determined by the individual departmental finance sections.Reference Perez, Olsen, Musick, Cernoch, Davis, Peterson and Musser 8 , Reference Perez, Olsen and Musick 9
Summary statistics for continuous variables were reported as mean±standard deviation (SD), and results for categorical variables were presented as frequencies. The Mann-Whitney test was employed to identify significantly different central locations between groups for continuously scaled variables, whereas the χ2 test was used to determine significantly different configurations across groups of categorical data. All tests were 2-tailed, and P≤.05 represented statistical significance. P values for the χ2 test were based on Fisher’s exact test.
RESULTS
In total, 571 patients with Gram-negative BSIs were evaluated for inclusion. After exclusion criteria were applied, 390 patients were included in the final analysis. Of these, 149 patients comprised the pre-intervention group and 241 in the intervention group (Figure 1). Baseline demographics were similar between the 2 study groups with the exception of the proportion of patients with ICU admissions (55.7% in the pre-intervention group vs 37.8% in the intervention group, P<.001) (Table 1). The most common infection site during both study periods was from genitourinary sources representing 48.3% in the pre-intervention group and 54.4% in the intervention group, followed by respiratory tract infections (11.4% vs 12.5%, respectively). Escherichia coli was the most frequently isolated pathogen, accounting for 53.7% in the pre-intervention group and 58.1% in the intervention group, followed by Klebsiella spp. (16.8% vs 20.8%, respectively). Both groups had similar frequencies of healthcare-associated and nosocomial infections.
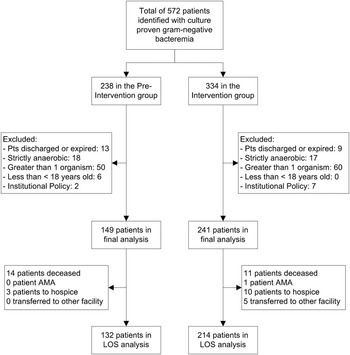
FIGURE 1 Eligibility and inclusion of study participants. Patients excluded due to being discharged or expired were those prior to time-to-positivity of index blood culture. AMA, against medical advice; LOS, length of stay.
TABLE 1 Demographics and Baseline CharacteristicsFootnote a
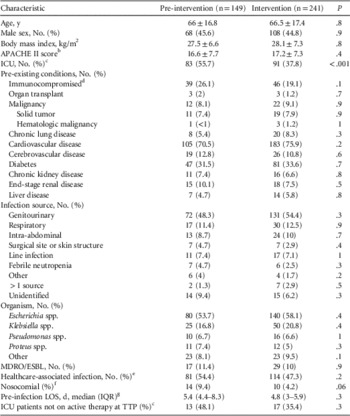
NOTE. APACHE II, Acute Physiology and Chronic Health Evaluation II; HIV/AIDS, human immunodeficiency virus/acquired immunodeficiency syndrome; IQR, interquartile range; LOS, length of stay; MDRO/ESBL, multidrug-resistant organism/extended-spectrum beta-lactamase–producing organism; TTP, time to positivity.
a Plus-minus values are means±SDs.
b The APACHE II score was calculated for ICU patients based on clinical data present during the 24 hours preceding the index blood culture. Missing variables were assumed to be normal.
c Includes patients with an intensive care unit admission at any time during the index hospitalization.
d Immunosuppressive therapy was defined as receipt of cytotoxic agents within 6 weeks, corticosteroids at a dosage of 15 mg or more of prednisolone (or equivalent) daily for longer than 1 week within 4 weeks, or other immunosuppressive agents within 2 weeks before bacteremia onset.
e Includes patients with recent contact with the healthcare system: recipients of recent intravenous therapy, dialysis, or home wound care; residence at long-term care facilities; and recent hospitalizations. 15
f Limited to patients hospitalized for ≥48 hours prior to collection of the index culture (onset of bloodstream infection).
g Pre-infection LOS limited to patients with nosocomial acquisition of bloodstream infection and who survived to hospital discharge (pre-intervention group [n=14] and intervention group [n=10]).
The mean time to culture positivity was not significantly different between the pre-intervention and intervention periods (22 vs 19 hours, respectively). The mean time to pathogen identification was significantly reduced after MALDI-TOF/ASP implementation, from 32 hours (±16 hours) to 6.5 hours (±5.4 hours) (P<.001); mean time to antibiotic susceptibility results was significantly reduced from 48 (±22) to 22 (±14) hours (P<.001); and time to therapy adjustment was significantly reduced from 71 (±59) hours to 30 (±30) hours (P<.001). Therapy adjustment included de-escalation and/or escalation of antibiotic therapy, dosing and/or administration route modifications, and/or discontinuation of unnecessary Gram-positive coverage.
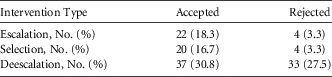
Clinical pharmacists made 120 recommendations to providers during the intervention period. Of those, 65% (n=79) were accepted (Table 2). Overall, the proportion of patients whose antibiotic regimen was adjusted was significantly increased from 63.8% in the pre-intervention group to 84.2% in the intervention group (P<.001). Additionally, the time to therapy adjustment was significantly decreased from an average of 71 hours in the pre-intervention group to 30 hours in the intervention group (P<.001).
TABLE 2 Clinical Pharmacist Interventions
The hospital LOS in survivors from infection onset did not differ significantly between the study groups with an average of 6.4±3.8 days (range, 0.8–24 days) in both groups and 3.7±3.4 days (range, 0.02–21.8 days) for ICU LOS (Table 3). Overall mortality did not differ significantly between the groups (9.4% vs 4.9%, P=.07).
TABLE 3 Patient Outcomes
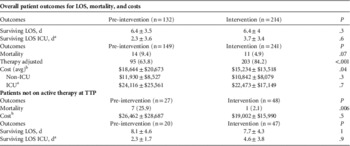
NOTE. LOS, length of stay; ICU, intensive care unit; TTP, time to positivity; Avg, average; Pts, patients.
a Patients in ICU group included any admissions to the ICU at any point within hospitalization.
b Costs in the pre-intervention group were adjusted to comparable dollars.Reference Perez, Olsen, Musick, Cernoch, Davis, Peterson and Musser 8 , Reference Perez, Olsen and Musick 9
Similar proportions of patients lacked active antibiotic therapy at culture positivity (27 of 149 [18%] pre-intervention and 48 of 241 [20%] intervention; Figure 2). The time to active treatment was significantly shorter in the intervention cohort. At 24 hours, time to active treatment was 8% shorter in the pre-intervention group vs 3% shorter in the intervention group with inactive therapy (P=.01). At 48 hours, time to active treatment was 4% shorter in the pre-intervention group vs <1% shorter in the intervention group with inactive therapy (P=.008). At 72 hours, time to active treatment was 3% shorter in the pre-intervention group and was no shorter in the intervention group with inactive therapy (P=.01). Mortality rates were significantly reduced from 25% (n=7 of 27) in the pre-intervention group to 2.1% in the intervention group (n=1 of 48) (P=.006).
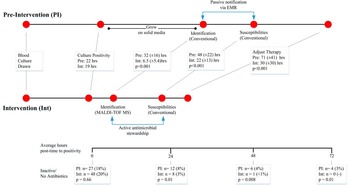
FIGURE 2 Timeline comparison of pre-intervention and intervention illustrating difference in laboratory, reporting, and interventions. Adjusted therapy includes de-escalation/escalation of antibiotic therapy, dosing/route modifications, and/or discontinuation of unnecessary Gram-positive coverage. Boxes represent average time in hours until corresponding information reported or adjustment of therapy. The bottom horizontal line represents the global study/patient timeline (hours) and includes point measurements (below) for patients on inactive therapy at 0, 24, and 48 hours in both groups. EMR, electronic medical record; MALDI-TOF, matrix-assisted laser desorption and ionization time-of-flight mass spectrometry.
Mean hospital costs per patient were $3,411 less in the intervention group than in the pre-intervention group ($18,645 vs $15,234; P=.04). Costs decreased in patients without ICU admissions ($11,930 vs $10,842; P=.3) and ICU patients ($24,116 vs $22,473; P=.7) although this decrease did not reach statistical significance. Costs were higher among patients without active empiric therapy ($26,462 vs $19,002; P=.5) (Table 3).
DISCUSSION
We evaluated the impact of rapid diagnostics with an active pharmacy intervention on patients with Gram-negative bacteremia in a community hospital setting and showed favorable outcomes. Significant improvements were achieved in time to infecting pathogen ID, antimicrobial susceptibilities, and overall management of antibiotic therapy. Importantly, a significant mortality benefit was observed among patients on inactive empiric treatment (25% in the pre-intervention group vs 2.1% in the intervention group; P=.006). These findings are consistent with previous studies describing the increased morbidity and mortality among patients with delays in appropriate antibiotic therapy; they further highlight the importance of real-time antimicrobial stewardship evaluation and use of rapid diagnostics.Reference Ibrahim, Sherman, Ward, Fraser and Kollef 5 – Reference Kumar, Roberts and Wood 7 In our previous studies by Perez et al,Reference Perez, Olsen, Musick, Cernoch, Davis, Peterson and Musser 8 MALDI-TOF was utilized for rapid pathogen ID in addition to real-time pharmacy intervention at a tertiary care referral center. The results of this study demonstrated significant decreases in (1) length of hospitalization from 11.9 days (pre-intervention group) to 9.3 days (intervention group), (2) 30-day all-cause mortality for the intervention group, and (3) mean cost per patient, witha decrease of $19,547.
In this study, we aimed to determine whether integrating rapid diagnostics with antimicrobial stewardship would improve outcomes in hospitalized patients in a community hospital setting that lacks pharmacists trained in infectious diseases. Our findings illustrate that with training, education, and availability of an infectious diseases pharmacist consultation, generalist clinical pharmacists can successfully assist in the notification and interpretation of culture results in a setting that applies to the majority of hospital facilities nationwide. 19 Despite barriers in community hospitals, ASPs have developed in many forms and have prospered with use of traditional interventions such as intravenous-to-oral conversions, educational programs, daily review of antimicrobial orders by a pharmacist, drug audits with feedback, and prior authorizations and/or restrictions.Reference Trivedi and Kuper 2 – Reference Bartlett and Siola 4 These simple and effective interventions have been shown to improve patient outcomes, to reduce costs, and to decrease the development of antimicrobial resistance.Reference Trivedi and Kuper 2 – Reference Bartlett and Siola 4 To our knowledge, only 1 study to date has evaluated the use of rapid diagnostics in a community hospital setting using the Nanosphere Verigene test to evaluate Gram-positive bacteremia in conjunction with ASP. Box et alReference Box, Sullivan and Ortwine 20 illustrated a similar decrease in time to therapy adjustment along with decreased LOS and hospital costs.
The relationship between prompt, appropriate initiation of antibiotic therapy and improved patient care confirms the importance of implementing rapid diagnostics in the community setting. Collaboration between the microbiology laboratory and pharmacy facilitated an expedited distribution of clinically actionable information to significantly improve outcomes. However, we did not observe a LOS reduction as we did in our previous studies conducted in a quaternary-care setting.Reference Perez, Olsen, Musick, Cernoch, Davis, Peterson and Musser 8 We did find many of the same improvements in process outcomes and therapeutic benefits in a very different patient population. When comparing baseline patient characteristics across studies, the most striking difference is that the lower patient acuity among community hospitals accounts for the much shorter overall LOS seen during both study periods (LOS, 6.5 days). Patients in the community hospital setting were less likely to be transplant recipients, immunocompromised, or have malignancies than patients in the quaternary-care setting. Additionally, the underlying source of BSI was markedly different; there were more genitourinary sources in the community hospitals and far fewer indwelling vascular devices. These findings are consistent with patient characteristics found in publications describing infectious etiologies among patients in community hospitals.Reference Rodríguez-Baño, López-Prieto and Portillo 21
Even with lower patient acuity, we observed an overall lower inpatient mortality rate for the intervention group that was not statistically significant (9.4% vs 4.9%; P=.07). Despite a similar and sizeable proportion of patients who were not receiving active antibiotic therapy at the time to positivity (TTP) during both study periods (18% and 20%; P=.07), we found a clear benefit to patients: the time to active therapy was significantly reduced during the intervention study period (Figure 2). The decreased time to active therapy was associated with a mortality benefit among those patients with less delay (25% in the pre-intervention group vs 2.1% in the intervention group; P=.006). Furthermore, there was a non-significant decrease of $7,470 per hospitalization among these patients (P=.5) leading to a projected estimated savings of $360,000 for the intervention cohort. Notably, no difference was observed in the number of patients admitted to the ICU in this subset of patients.
In this study, we identified a significant difference in hospital costs that favored the intervention group by an overall average of $3,411 per patient cost savings. Although the exact reason for the cost savings remains unclear, a likely source is the greater number of patients with ICU admissions in the pre-intervention cohort. Because patients with ICU admissions inherently incur higher healthcare costs, we evaluated the groups separately (non-ICU patients and ICU patients). We found that a cost savings was maintained outside of ICU admissions for the intervention cohort, though the sample size was smaller and the difference did not reachstatistical significance.
Finally, in this study, we demonstrated the feasibility and utility of having a centralized clinical microbiology laboratory located in a metropolitan area within 30 miles of several facilities. This laboratory placement could potentially serve as a model for the existing 3,144 community hospitals across the United States where it may not be possible or cost-effective to have laboratories equipped with rapid diagnostics at in-house locations. 19
We are aware of several limitations to this study. First, this study took place in 2 community hospitals; its generalizability may be limited to similar system community settings. The patient data were analyzed retrospectively, which allows the possibility of information bias. Physician prescribing practice variations between sites, over time, and training/experience were not examined. In the analysis, we did not compare the impact of rapid diagnostics without ASP or vice versa, making it difficult to assess the individual impact of these elements. However, our objective was to evaluate the effect of the combined intervention. Finally, the clinical pharmacists responding to BSI notifications were not formally trained in infectious diseases. The use of general clinical pharmacists does make the study more generalizable; not all community hospitals have an infectious diseases trained pharmacist on their staff. Although this may be a disadvantage for the study, it highlights the necessity of trained infectious diseases pharmacists in the community setting. Perez et alReference Perez, Olsen, Musick, Cernoch, Davis, Peterson and Musser 8 found a recommendation acceptance rate of 91% when infectious diseases pharmacists intervened, compared with 65% acceptance in our study. Reasons for the differences are unclear and will be a focus of future efforts. ASP teams staffed by dedicated infectious diseases pharmacists are associated with better adherence to evidence-based antibiotic prescribing measures, including selection, de-escalation, modification at 24 hours, etc. This area requires further study to assess the impact these individuals would have to a program with rapid diagnostics.
In the growing use of rapid diagnostics, many studies have shown the benefit of their use in the treatment of BSI in tertiary and quaternary settings even though the evidence is lacking in the community setting. Our results demonstrate the benefit of rapid diagnostics coupled with ASP on time to therapy adjustment; the impact is greatest on patients who did not have active therapy at the time of culture positivity and on their clinical outcomes. The use of rapid diagnostics coupled with antimicrobial stewardship should be incorporated in the community setting to improve time to therapy adjustment in patients with Gram-negative bacteremia. Our data unambiguously demonstrate improved patient outcomes, most notably for those who lack active therapy.
ACKNOWLEDGMENTS
We thank the Clinical Microbiology Laboratory staff and the Clinical Pharmacy Services for assistance with the study. We thank James R. Davis, PhD, Department of Pathology and Genomic Medicine, HMH, for advice and assistance. We gratefully acknowledge K. E. Stockbauer, PhD, Office of Academic Development, Department of Pathology and Genomic Medicine, HMH, for assistance with manuscript preparation.
Financial support. No financial support was provided relevant to this article.
Potential conflicts of interest. All authors report no conflicts of interest relevant to this article.







