A survey in 1996 reported that 44% of adult men and 32% of women in Scotland were overweight and a further 14% of men and 17% of women were obese (Scottish Intercollegiate Guidelines Network, 1996). Treatment with specific drugs is a potential reason for obesity and any practice that may exacerbate obesity warrants serious scrutiny (Reference Allison and CaseyAllison & Casey, 2001).
There has been an increasing number of reports of an association between treatment with atypical antipsychotics, weight gain and hyperglycaemia. Moreover, de novo cases of diabetes mellitus have been reported in patients receiving these medications (Reference Bushe and LeonardBushe & Leonard, 2004). Recommendations for regular weight and plasma glucose monitoring in patients receiving atypical and typical antipsychotics have been made (Reference Haupt and NewcomerHaupt & Newcomer, 2001; Reference LebovitzLebovitz, 2003; Reference Casey, Haupt and NewcomerCasey et al, 2004).
Lebovitz (Reference Lebovitz2003) suggested monitoring of body weight, plasma fasting glucose and lipids with the initiation of atypical antipsychotics and continuing monitoring throughout treatment. Others have supported baseline and continuation monitoring of body weight, body mass index (BMI), waist circumference, fasting blood glucose and fasting lipid profile (Reference Marder, Essock and MillerMarder et al, 2002; Reference Taylor, Paton and KerwinTaylor et al, 2003; American Diabetes Association et al, 2004; Reference Casey, Haupt and NewcomerCasey et al, 2004; Reference Menza, Vreeland and MinskyMenza et al, 2004). However, a recent study (Reference Taylor, Young and EsopTaylor et al, 2004) revealed that less than half of hospitalised patients prescribed antipsychotic drugs were tested for diabetes.
Our previous survey demonstrated that there was no reliable monitoring of weight or blood glucose among psychiatric in-patients in Aberdeen (Reference Boilson and HamiltonBoilson & Hamilton, 2003). In the present study we developed a protocol for weight and blood glucose monitoring for psychiatric in-patients receiving antipsychotic medication. We then repeated the monitoring survey in the same in-patient settings as in our previous study.
Methods
Developing the protocol
Our recommendations from the previous survey (Reference Boilson and HamiltonBoilson & Hamilton, 2003) were used for the development of the protocol, together with consultations with consultant endocrinologists and a working group involving hospital dieticians, pharmacists and psychiatrists. Consideration was given to the Grampian Guidelines for the Management of Diabetes Mellitus (further details available from the authors) and Obesity in Scotland (Scottish Intercollegiate Guidelines Network, 1996) when drawing up an action plan and defining risk groups.
The draft protocol was circulated among all general adult consultant psychiatrists and the ward managers of the four acute wards, and feedback was invited. The protocol was redrafted, approved and implemented on all four acute wards from 1 June 2004.
Copies of the protocol, BMI ready reckoners and essential information packages were distributed to all acute wards and to each general adult consultant psychiatrist prior to the start date. Each consultant, all junior doctors and ward managers were informed about the project by letters and individually. Reminder letters were sent to all junior doctors and senior trainees at the time of the medical staff rotation that occurred during the study period.
Copies of the protocol are available from the authors on request. Essentially an algorithm is provided for each patient for whom initiation or a change of antipsychotic is being considered. Baseline weight and fasting blood glucose are indicated with a timetable of monitoring thereafter for normal and ‘ high-risk’ patients.
Survey
The second survey was performed retrospectively in October 2004 using the same study methodology as previously (Reference Boilson and HamiltonBoilson & Hamilton, 2003). A list of all people with an ICD–10 (World Health Organization, 1992) diagnosis of schizophrenia, schizoaffective disorder, persistent delusional disorder, acute and transient psychotic disorder or induced delusional disorder who were admitted to the four acute general adult wards at the Royal Cornhill Hospital from 1 June 2004 to 31 August 2004 was obtained from medical records. Consent to examine medical records was obtained from the responsible medical officers. Fifty-nine patients were identified but 6 sets of medical notes were unavailable. Of the remaining 53 patients, 9 were readmitted during the specified period. Each admission was dealt with individually. For 8 of the readmitted patients both admissions were included in the study; for 1 only one admission was included owing to lack of appropriate notes at readmission. In total there was a data-set for 61 admissions.
Case notes and ward weight books were reviewed for any recordings of weight during the index admission. A record of blood glucose measurement was sought in both case notes and the regional laboratory computer database for the period of the index admission. Recording of weight and measurement of random and fasting blood glucose was compared for all admissions in the 2001 and 2004 study periods and for patients newly started on or changing antipsychotic drug by χ2-tests.
Results
All patients included in the survey had been prescribed antipsychotic medication. Twenty-four were initiated on, or had a change to antipsychotic medication during admission; this is the group for whom the new protocol was intended. However, an overall improvement in monitoring for all patients receiving antipsychotic medication had been anticipated. Hence data are presented for the entire sample and then specifically for patients with a new prescription or drug change (Table 1).
Table 1. Weight and blood glucose measurements of patients taking antipsychotic medication in 2001 and 2004
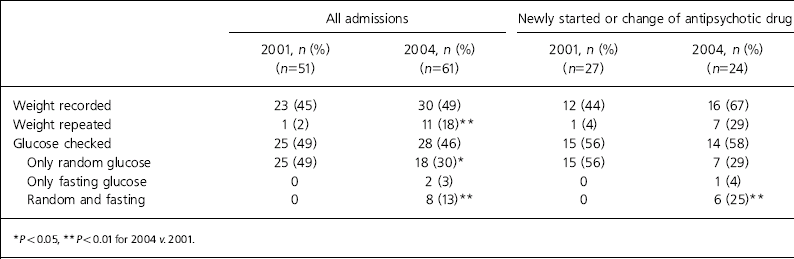
| All admissions | Newly started or change of antipsychotic drug | |||
|---|---|---|---|---|
| 2001, n (%) (n=51) | 2004, n (%) (n=61) | 2001, n (%) (n=27) | 2004, n (%) (n=24) | |
| Weight recorded | 23 (45) | 30 (49) | 12 (44) | 16 (67) |
| Weight repeated | 1 (2) | 11 (18)** | 1 (4) | 7 (29) |
| Glucose checked | 25 (49) | 28 (46) | 15 (56) | 14 (58) |
| Only random glucose | 25 (49) | 18 (30)* | 15 (56) | 7 (29) |
| Only fasting glucose | 0 | 2 (3) | 0 | 1 (4) |
| Random and fasting | 0 | 8 (13)** | 0 | 6 (25)** |
Weight
Of the 61 admissions in 2004, 30 (49%) had body weight recorded during admission compared with 45% in 2001 (χ2=0.18, P=NS). Eleven patients had their weight remeasured during admission in 2004 compared with only 1 patient in 2001. Of 24 patients who were newly started or initiated on different antipsychotics in 2004, 16 (67%) had their weight recorded compared with 44% in 2001.
Blood glucose
Of the 61 admissions in 2004, 28 (46%) had their blood glucose checked compared with 25 (49%) in 2001 (Table 1). Of the 28 patients whose blood glucose was checked, 18 had only a random glucose check, 2 had only fasting glucose measurements and 8 had random and fasting blood glucose tests.
Fifteen patients had random blood glucose levels over 5.5 mmol/l which, according to Grampian Guidelines for the Management of Diabetes Mellitus, indicates further investigation. Of the 15 patients with elevated random glucose levels, 6 had fasting blood glucose checked but 9 had no further investigation. Of the 24 patients who newly started or changed antipsychotic medication in 2004, 14 (58%) had some form of blood glucose test; in 3 cases the notes documented that patients had refused blood testing.
Two patients with diabetes mellitus were identified. One (BMI=30) had a fasting blood glucose of 14.2 mmol/l and had had an elevated random glucose level in 1999. A fasting blood glucose test was repeated and triglycerides, lipids and haemoglobin A1c were checked during the admission in 2004. The patient was switched from olanzapine to risperidone and referred to a dietician. Problems of obesity and diabetes were mentioned in the discharge letter to the general practitioner but without any indication of result or requirement for monitoring.
The second patient, who had been treated with olanzapine for 2 years, had a fasting blood glucose level of 7.1 mmol/l. During the admission in 2004 the medication was switched to oral risperidone and lipids were measured. Results were reported to the general practitioner and recommendations for monitoring were made.
Discussion
Haphazard monitoring of weight and blood glucose was identified in our first survey (Reference Boilson and HamiltonBoilson & Hamilton, 2003) but we postulated that this would have improved following warnings of a potential association between hyperglycaemia and olanzapine (Committee on Safety of Medicines, 2002) and the introduction of a local monitoring protocol.
However, in spite of these national and local endeavours we conclude that practice had changed little in the period immediately following the introduction of the protocol. There was a trend towards improvement in the proportion of patients weighed when initiating or changing antipsychotic treatment, but the difference was not statistically significant. Less than half of all patients were weighed at all. The total proportion of patients undergoing blood glucose monitoring was actually less after the protocol was introduced, but we did observe the performance of fasting tests for the first time.
Only when patients had baseline weight, BMI and fasting blood glucose recorded in the case notes was it deemed that the protocol had been followed. This was the case for only 7 patients (29%) newly starting or changing antipsychotic treatment.
On analysing the raw data from individual wards we noted considerable variance. Where adherence to the protocol was high there was a motivated clinician, usually a trainee psychiatrist, who had undertaken the responsibility for this monitoring. Consideration is being given as to whether an individual clinician could oversee this monitoring across the acute wards. Our protocol was well publicised with consultants and ward managers, but training nursing staff and junior doctors in weight measurement, BMI calculation and blood glucose testing may pay dividends.
We accept that some of our analyses confer risks of type 1 error through multiple comparisons and that the study may be underpowered because of a relatively small sample size.
A critical consideration was the decision to restrict our review of monitoring to the in-patient setting. Most prescribing of atypical antipsychotics takes place in outpatient and general practice settings. We would anticipate that the lower availability and use of weighing scales and feasibility of blood glucose monitoring, particularly fasting samples, would make adherence to the protocol even less likely among out-patients.
In summary, although adherence to a protocol for weight and blood glucose measurement is important, the ultimate solution may lie in the emergence of efficacious antipsychotic drugs which are free from these side-effects.
Declaration of interest
R.H. has received fees for speaking and attending meetings from Pfizer, Lundbeck, Lilly and AstraZeneca. M.B. has taken part in research projects funded by Pfizer.
Acknowledgements
We thank Dr John Eagles, Consultant Psychiatrist, Royal Cornhill Hospital, Aberdeen for his helpful comments and Pat Grant, Medical Records Department, Royal Cornhill Hospital, Aberdeen.




eLetters
No eLetters have been published for this article.