CLINICIAN'S CAPSULE
What is known about the topic?
The influence of renal impairment upon emergency department-based outcomes in patients with atrial fibrillation or flutter (AFF) is unknown.
What did this study ask?
What was the impact of renal impairment upon adverse events (AE) and rate and rhythm control (RRC) attempts in emergency department (ED) AFF patients?
What did this study find?
When ED AFF patients with renal impairment are administered RRC, they have more than 10% excess AE risk.
Why does this study matter to clinicians?
Emergency physicians should be cautious about attempting RRC in ED AFF patients with renal impairment.
INTRODUCTION
Atrial fibrillation or flutter (AFF) are commonly encountered dysrhythmias.Reference McDonald, Pelletier and Ellinor1 Most emergency department (ED) research has focused on patients with acute-onset AFF and determined that rate or rhythm control (RRC) are both safe and effective.Reference Connolly and Gillis2–Reference Skanes, Healy and Cairns8 However, in patients with AFF either provoked by or concomitant with an acute underlying illness,Reference Atzema, Lam and Young9 RRC has a higher chance of undesirable ED-based outcomes.Reference Scheuermeyer, Pourvali and Rowe10
Community AFF patients with renal impairment have higher rates of stroke and death,Reference Nakagawa, Hirai and Takashima11–Reference Oleson, Kip and Kamper15 whereas ED patients with elevated creatinine have higher mortality.Reference Atzema, Lam, Young and Kester-Greene16 The recent CAEP guidelinesReference Stiell, Scheuermeyer and Vadeboncouer4 do not mention patients with renal disease but given this elevated risk, we sought to investigate whether RRC was also associated with poor ED-based outcomes.
We analysed an ED AFF cohort and hypothesized that patients with renal impairment undergoing RRC would have more adverse events (AE) and higher rates of treatment failure.
METHODS
Study design and setting
This cohort study was conducted at two urban Canadian university-affiliated EDs. St. Paul's Hospital is a referral centre with 70,000 annual ED visits and comprehensive cardiology and renal services. Mount St. Joseph's Hospital is a community centre with 25,000 yearly visits and a general internal medicine unit. This is a secondary analysis of a previously described cohortReference Scheuermeyer, Pourvali and Rowe10, Reference Scheuermeyer, Grafstein and Stenstrom17, Reference Scheuermeyer, Grafstein and Stenstrom18 approved by the Providence Health Care/University of British Columbia research ethics board.
Patient selection
During the study period, every electrocardiogram (ECG) conducted was stored in the MUSE (GE Healthcare Clinical Systems, Waukesha, WI) database, along with the patient's unique identifying number, and date and time of acquisition. A cardiologist confirms all ECGs within 24 hours. We interrogated the database to identify ED-based ECGs showing AFF between January 1, 2009 and December 31, 2009.Reference Scheuermeyer, Pourvali and Rowe10, Reference Scheuermeyer, Grafstein and Stenstrom17, Reference Scheuermeyer, Grafstein and Stenstrom18 We entered this list into a spreadsheet (Excel, Microsoft Corporation, Redmond WA), removed all identifiers except the unique number, date, and time, and reviewed the ED chart. Each patient then had a chart review of the ED encounter.
Exclusions
We excluded patients with recent cardiac procedures because cardiologists or surgeons directed treatment, and those referred to the ED for direct specialty admission. We also excluded patients who re-attended the ED within 1 year, those from outside our six-ED health region, and those who only attended to monitor anticoagulation.
Interventions
ED physicians managed AFF patients at their discretion, including decisions to 1) order any investigations; 1) administer the following care: no RRC; rate control using intravenous or oral rate control agents; or rhythm control using oral or intravenous antiarrhythmic agents, or electrical countershock under procedural sedation; and 3) discharge or refer the patient.
Testing of renal function
Both hospitals used a rapid serum creatinine test on a blood gas analyser (Radiometer ABL800 Flex analyzer, Radiometer Canada, London, ON). To account for differences in gender and body mass, the estimated glomerular filtration rate (eGFR) was automatically calculated using the abbreviated Modification of Diet in Renal Disease (MDRD) equation, eGFR = 175x (SCr/88.4)−1.154 x (age)−0.203 for males, multiplied by 0.742 if the patient was female.Reference Levey, Bosch and Lewis19 Results were routinely available within 10 minutes.
Data capture
The sites share an electronic database that records patient demographics, triage complaint, and all ED investigations with results including eGFR. For each patient, emergency physicians completed an electronic discharge summary recording all diagnoses, medications administered, procedures, and consultations. The nursing record provided initial and all subsequent vital signs.
Chart review
We followed the criteria of GilbertReference Gilbert, Lowenstein and Koziol-McLain20 and Worster.Reference Worster, Bledsoe and Cleve21 Four reviewers blinded to study hypothesis and outcomes independently abstracted charts onto standardized electronic spreadsheets.Reference Scheuermeyer, Pourvali and Rowe10, Reference Scheuermeyer, Grafstein and Stenstrom17, Reference Scheuermeyer, Grafstein and Stenstrom18 We trained reviewers on the first 10 charts, the primary investigator was available at any time to discuss unclear data, and reviewers submitted blocks of charts on regular intervals. With the assistance of electronic records dating to 1999, we regularly clarified missing or discrepant data, and identified obvious issues such as a CHADS2 scoreReference Gage, Waterman and Shannon22 in an 80-year old patient. A second abstractor reviewed a random 10% of all charts, and we obtained kappa values for the key variable eGFR, dichotomized as greater or less than 60. A second staff emergency physician reviewed all potential AE. In case of disagreement, two specialists independently reviewed the case.
Outcomes and variable definitions
The primary outcome was the number of patients with at least one AE within 4 hours of RRCReference Scheuermeyer, Pourvali and Rowe10, Reference Scheuermeyer, Grafstein and Stenstrom17, Reference Scheuermeyer, Grafstein and Stenstrom18 or within 4 hours of arrival if no RRC was administered. These were defined according to prespecified criteria reflecting likely complications of RRC and classified into major and minor AE (Box 1).Reference Scheuermeyer, Pourvali and Rowe10, Reference Scheuermeyer, Grafstein and Stenstrom17, Reference Scheuermeyer, Grafstein and Stenstrom18 We combined patients undergoing RRC, similar to a previous study using the same cohort.Reference Scheuermeyer, Pourvali and Rowe10 Patients with more than one AE were counted as having a single AE.
Box 1. Emergency department adverse eventsReference Scheuermeyer, Pourvali and Rowe10, Reference Scheuermeyer, Grafstein and Stenstrom17, Reference Scheuermeyer, Grafstein and Stenstrom18
Major adverse events
Hypotension requiring inotropic agents
Respiratory distress requiring
Non-invasive positive-pressure ventilation
Endotracheal intubation
New bradycardia requiring pharmacologic intervention or pacing
Confirmed thromboembolic event
Chest compressions
Death
Minor adverse events
Respiratory distress requiring
Bag-valve mask
Oral airway
Hypotension requiring intravenous fluid bolus (crystalloid or colloid)
The secondary outcome was treatment failure with RRC. As per standards, successful rate control was defined as decreasing ventricular rate to 100 beats per minute or fewerReference Stiell, Scheuermeyer and Vadeboncouer4 within 4 hours of medication administration. (We did not distinguish resting or active heart rates.) Successful rhythm control was defined as establishment and maintenance of sinus rhythm in patients who had a rhythm control attempt.Reference Scheuermeyer, Pourvali and Rowe10, Reference Scheuermeyer, Grafstein and Stenstrom17
For all outcomes, we divided the cohort into patients with renal impairment (eGFR less than 60 mL/min/1.73 m2; “low eGFR”) and patients without renal impairment (eGFR 60 or greater), as per the Kidney Disease/Improving Global Outcomes (KDIGO) guidelines.Reference Levey, Becker and Inker23, 24 Because physicians might not have access to a prior eGFR value or a thorough medical history, we did not attempt to differentiate between chronic kidney diseaseReference Levey, Becker and Inker23, 24 and acute kidney injury.Reference Lassnigg, Schmidlin and Mouhieddine25 Greenslade used a similar approach in an ED cohort of chest pain patients.Reference Greenslade, Cullen and Kalinowski26 Because physicians might not order an eGFR in well-appearing patients, we treated patients without an eGFR as having a normal value.
Data analysis
We used Microsoft Excel 2011 (Microsoft Corp, Redmond, WA) for data entry, and reported discrete variables were reported as percentages. We presented continuous variables as means with standard deviations or medians with interquartile ranges where applicable. We performed statistical analyses using SAS 9.3. (SAS Corporation, Cary, NC).
We compared risk of AE in low eGFR patients with the risk in normal-eGFR patients by several methods, as follows:
1) The adjusted causal risk difference is the excess risk of AE as a result of RRC administration in patients with low versus normal eGFR after adjustment for the presumed overall greater illness of the low eGFR population. Expressed another way, this is the AE rate if all AFF patients, dichotomized into low- and normal-eGFR groups, received RRC, minus the AE rate if no AFF patients received RRC.
2) The population attributable risk is the excess AE risk due to RRC use, compared with no RRC use, in patients with both low and normal eGFR after adjustment for comorbidities – or alternatively, the proportion of AEs that would not have occurred had RRC not been attempted. While similar to 1), this only measures patients who actually had RRC, rather than if, “all” patients had RRC.
3) The exposure effect is the excess AE risk when RRC is used in patients with low versus normal eGFR. (This only applies to patients who received RRC.) We used three different analyses because this is a heterogeneous group of acute and chronic AFF patients with and without acute underlying illnesses, and we also combined RRC treatments. Similar direction and magnitude of the association between eGFR and outcomes in each separate analysis would suggest a strong relationship between eGFR and outcomes of RRC.
Firstly, we calculated crude AE rates in patients who received RRC and those who did not and obtained crude risk difference. Secondly, we fit a logistic regression model for AE risk using the following: age, sex, prior AFF, hypertension, diabetes, initial systolic blood pressure, and use of RRC. Thirdly, we used the fitted model to estimate standardized (to the covariate distribution) risks under different exposure assumptions (actual exposure to RRC, if all patients were exposed to RRC, if no patients were exposed to RRC) and subgroups (all patients, and only those who received RRC) to calculate the previous three measures of effect (adjusted causal risk, population attributable risk, and exposure effect) and compare these effect measures between the low and normal eGFR groups. We used the percentile bootstrap method to obtain 95% confidence intervals (CI).
To analyse treatment failure, we calculated the adjusted odds ratio of treatment failure with RRC, comparing patients with low versus normal eGFR. We fit a logistic regression model for treatment failure as a function of normal versus decreased renal ability, using the same covariates.
In addition, we also conducted two further analyses: 1) a secondary analysis of patients with an initial heart rate > 100 beats per minute (because they would be more likely to receive RRC), and 2) a sensitivity analysis for the cohort of patients with complete data, with all of the above outcomes.
RESULTS
During the 1-year study period, 1,508 consecutive patients had ECG-proven AFF, with 1,112 patient encounters meeting inclusion criteria (Figure 1). Overall, 966 patients (86.8%) had an eGFR, and none of the 146 patients without this test were admitted to hospital. The kappa value for eGFR was 1.0 (95% CI 0.97 to 1.0) and 2 of 73 potential AE required adjudication.
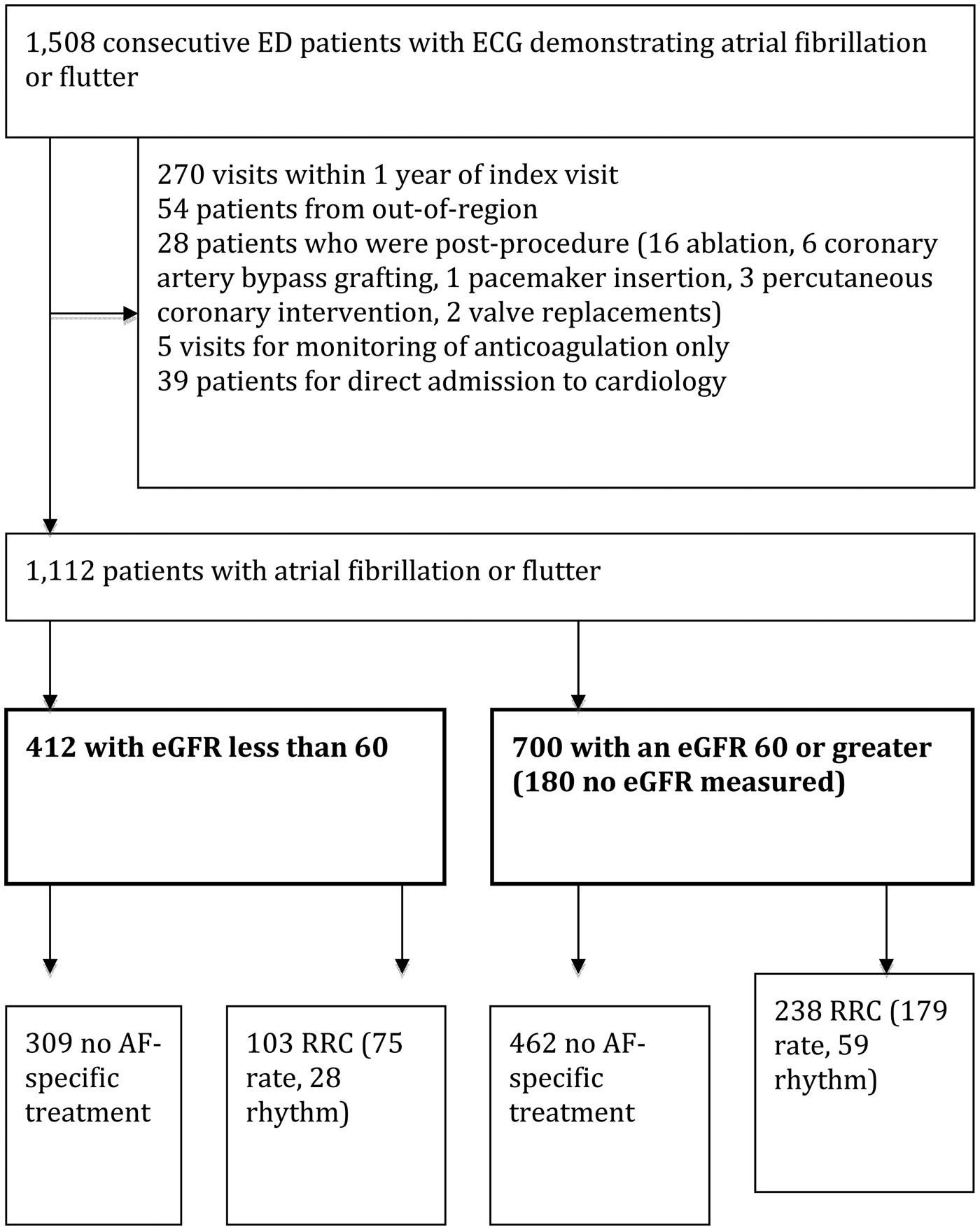
Figure 1. Study flow diagram.
Four hundred and twelve (37.1%) patients (349 fibrillation and 63 flutter) had low eGFR. Patients with low eGFR were older with more comorbidities. Overall, 254 patients received rate control (151 beta-blocker, 93 calcium channel blocker, 10 digoxin), and 87 patients received rhythm control (50 electrical, 37 chemical) (Table 1). Rates of RRC were 238/700 (34.0%) for patients with normal eGFR and 103/412 (25.0%) for patients with low eGFR.
Table 1. Baseline characteristics stratified according to estimated glomerular filtration rate, n = 1,112

Antiarrhythmics = propafenone, amiodarone, sotalol, flecanide, dronedarone; ASA = acetylsalicylic acid; CHADS2 = heart failure, hypertension, age > 75, diabetes, stroke/TIA; EMS = emergency medical services; TIA = transient ischemic attack.
Unadjusted AE rates were as follows (Table 2): For patients with low eGFR, AEs occurred in 26/103 (25.2%) who had RRC, and in 13/309 (4.2%) who did not. For normal patients, the proportion was 27/238 (11.3%) and 7/462, (1.5%), respectively. (Unadjusted AE results for both low normal eGFR for both RRC and “no treatment” are significant at p = 0.05.) The most common AE across all groups was “hypotension requiring fluid boluses” (Appendix 1), and patients receiving rhythm control had higher unadjusted AE rates than those receiving rate control. Unadjusted RRC failure rates were as follows (Table 3): 61/103 (59.2%) in patients with low eGFR and 78/238 (32.8%) for normal eGFR; treatment failures were higher for patients undergoing rhythm than rate control.
Table 2. Emergency department adverse events (unadjusted) stratified according to estimated glomerular filtration rate, n = 1,112

Difference is expressed as patients with impaired renal function minus patients with normal function. (Continuity correction used.)
AE = adverse event; eGFR is the estimated glomerular filtration rate in mL/min/1.73 m2; RRC = rate or rhythm control.
Table 3. Emergency department treatment failure (unadjusted) stratified according to estimated glomerular filtration rate, n = 1,112

Difference is expressed as patients with impaired renal function minus patients with normal function. (Continuity correction used.)
eGFR is the estimated glomerular filtration rate in mL/min/1.73m2.
Appendix 2 displays demographics, comorbidities, treatments, and outcomes for stepwise eGFR categories: as renal function decreased, patients were older, had more comorbidities, and had higher unadjusted rates of AE and treatment failure. The risk of AE (independent of whether patients received RRC) in patients with eGFR ≥ 60 was 34/700 (4.9%) compared with 8/173 (4.6%) in patients with eGFR 45–59, 13/135 (9.6%) in patients with eGFR 30–44, and 18/104 (17.3%) in patients with eGFR < 30. Among patients undergoing RRC, the AE risk was 27/238 (11.3%) in patients with eGFR ≥ 60, 6/50 (12%) for eGFR 45–59, 9/31 (29.0%) for eGFR 30–44, and 11/22 (50.0%) for eGFR < 30. The risk of treatment failure for patients undergoing RRC was 73/238 (30.6%) in patients with eGFR ≥ 60, 18/50 (36.0%) for eGFR 45–59, 22/31 (71.0%) for eGFR 30–44, and 16/22 for eGFR < 30.
For the adjusted outcomes, we excluded 81 (7.3%) patients from the primary analysis due to missing covariates. For patients with low eGFR, the excess causal risk difference for AE in patients with low eGFR who received RRC is 11.8% (95% CI 0.7 to 23.9%), the excess population attributable risk for RRC was 9.0% (95% CI 1.0 to 18.8%), and the excess exposure effect of RRC was 13.7% (95% CI 1.7 to 25.1%). When comparing patients with low versus normal eGFR, the adjusted odds ratio for RRC treatment failure was 3.11. (95% CI 1.79 to 5.57) (Table 4; stepwise calculations in Appendix 3.)
Table 4. Adjusted outcomes with 95% confidence intervals in main and secondary analyses, n = 1,112 overall

AE = adverse event; eGFR is the estimated glomerular filtration rate in mL/min/1.73 m2; hr = heart rate in beats per minute; RRC = rate or rhythm control.
The secondary analysis of 581 patients (18 [3.1%] excluded due to missing covariates) had similar results for both excess AE risk and treatment failure. The sensitivity analysis of 885 patients also had similar results for both outcomes (see Table 4 and Appendix 3).
DISCUSSION
In this cohort of 1,112 consecutive ED patients with AFF, over one-third had low eGFR and such patients were older with more cardiovascular comorbidities. The crude AE rate for AFF patients with low eGFR undergoing RRC is approximately 25% – rising to 50% at the lowest eGFR – a far higher rate than seen in other AFF groups.Reference Scheuermeyer, Grafstein and Stenstrom17, Reference Scheuermeyer, Grafstein and Stenstrom18 The crude RRC treatment failure for patients with low eGFR was nearly 60%.
We measured the influence of low eGFR upon RRC administration in AFF patients in three analyses, and, after controlling for baseline imbalances, AE rates were higher than had RRC not been administered.Reference McDonald, Pelletier and Ellinor1 The excess RRC risk was 12%, indicating that, had RRC theoretically been administered to every patient, there would have been 12% more AE in the low-eGFR group than the normal-eGFR group, than if RRC had not been administered to anyone. This implies that RRC has a greater AE effect upon patients with low eGFR.Reference Connolly and Gillis2 Because not all patients had RRC, the population attributable risk difference implies that the actual excess RRC risk for our study patients was 9%.Reference Heilbron, Klein and Talajic3 The exposure effect estimates that there is a nearly 14% excess risk when RRC is used in patients with low versus normal eGFR. Combined, these results, all statistically significant with the same clinical trend, indicate that administration of RRC in AFF patients with low eGFR is associated with a 9%–14% increase in AE rates when compared with RRC administration in patients with low eGFR. Furthermore, there is a threefold adjusted odds ratio of RRC treatment failure in low-eGFR patients. Our secondary analysis of patients with initial tachycardia or receiving rhythm or rate control had similar results, and our sensitivity analysis also supports these conclusions. These results should encourage clinicians to be very cautious in administering RRC treatments in AFF patients with low eGFR.
It is important to note that these data reflect a population, and that select low eGFR-patients may in fact be at lower risk than normal eGFR-patients. To illustrate, it may be safer to administer rate control in a 55-year-old male with eGFR 59 than a hypotensive, hypoxic 90-year-old male with eGFR 60. Thus, although low eGFR is generally associated with worse outcomes, physicians must still regard this variable in the overall clinical context; other characteristics such as age and comorbidities also remain important.Reference Stiell, Scheuermeyer and Vadeboncouer4
We purposely combined patients with primary AFF presentations and with AFF precipitated by or concomitant with another acute condition. The latter group is likely sicker, and ED physicians should strive to identify underlying acute illnesses prior to initiation of AFF-specific treatment.Reference Scheuermeyer, Pourvali and Rowe10 There is likely overlap between patients with low eGFR and those with an underlying illness. However, distinguishing patients with AFF only (whether with low or normal eGFR), those with AFF and an underlying illness and acute low eGFR,Reference Lassnigg, Schmidlin and Mouhieddine25 and those with AFF and an underlying illness worsening chronic low eGFR can be very challenging in practice. Clinical histories, including prior eGFR, may not be available, ominous conditions such as sepsis may not declare themselves for many hours,Reference Villar, Clement and Stotts27 and often important ED AFF management decisions such as RRC are made very early during the ED encounter.Reference Scheuermeyer, Pourvali and Rowe10 Thus, the eGFR, a test that typically returns quickly, can be an important early clue that the patient is sicker (or healthier) than an initial physician assessment might indicate.
Previous data have shown that an ED serum creatinine >200 umol /L increases 90-day mortality risk for ED AFF patients.Reference Atzema, Lam, Young and Kester-Greene16 However, there is no prior data demonstrating an association between low eGFR and higher ED-based AE rates and treatment failures, which emergency physicians may have some control over. While recent ED guidelines identify a vulnerable group of ED AFF patients (with acute underlying medical conditions), using characteristics such as age and cardiovascular comorbidities,Reference Stiell, Scheuermeyer and Vadeboncouer4 there is no mention of low eGFR. Our study extends these findings by demonstrating that across a population of AFF patients with low eGFR, RRC is more likely to be associated with increased AE and treatment failure.
Limitations
The findings in this review of two urban Canadian EDs may be challenging to extrapolate. In particular, the proportion of patients with low eGFR may be higher than in other hospitals. We assumed patients without a recorded eGFR had normal renal function because only a small number had an AE and none were admitted, and our sensitivity analysis supports this. The MDRD equation was derived and validated in stable patients with chronic kidney disease,Reference Levey, Bosch and Lewis19 and its applicability in ED patients is unknown. Our definition of low eGFR (< 60 ml/min/1.73m2) reflects a stage of illness progression in outpatients with chronic kidney disease, and thus may misclassify some ED patients as high- or low-risk. We combined all patients with low eGFR, rather than distinguishing between acute and chronic renal insufficiency, to examine the risks associated with any renal insufficiency. This approach has been adopted in other ED cardiovascular cohort studies.Reference Greenslade, Cullen and Kalinowski26 Owing to small sample size but similar to a prior analysis,Reference Scheuermeyer, Pourvali and Rowe10 we combined rate control, chemical conversion, and electrical conversion. It is possible that unmeasured confounders, for example, physicianReference Scheuermeyer, Pourvali and Rowe10 or patient preference for RRC, or timing of RRC,Reference Scheuermeyer, Pourvali and Rowe10 influenced results, but our secondary analysis focusing on patients with higher heart rates and those who underwent RRC, should help ameliorate this concern.
CONCLUSION
In this cohort of ED AFF patients administered RRC, those with low eGFR had significantly increased adjusted excess risk of AE when compared with patients with normal renal function. Odds of treatment failure were also significantly increased.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/cem.2018.475.
COMPETING INTERESTS
None declared.
ACKNOWLEDGEMENTS
FS conceived the idea, designed the study, and composed the first draft. EG provided source data. HW and MW performed statistical analysis, while TB, JC, BG, and especially GI provided critical editorial input.
The authors gratefully acknowledge the contributions of Reza Pourvali, MD; Clare Heslop, MD, PhD; Jan McPhee, MD, MSc; Brett Heilbron, MD; and John Ward, MD.







