Introduction
More than 820 million people in the world were still hungry in 2018, underscoring the immense challenge of achieving the Zero Hunger target by 2030 (FAO et al., 2019). Additionally, it is unanimously recognized by many researchers and organizations that only 12 crops contribute most to the current global food production, with only three of them (rice, wheat and maize) providing more than 50% of the world's calories (Singh et al., Reference Singh, Dubey, Chaurasia, Dubey, Pandey, Singh and Abhilash2019). A rapid reduction of the gene pool in both plant and animal genetic resources is observed as only a dozen species of animals provide 90% of the animal protein consumed globally and just four crop species provide half of the plant-based calories in the human diet (FAO, 2009). Hence, directing researchers attention to plant protein sources becomes a necessity as the animal protein sources face challenges of preference (for vegetarians) and affordability. As such, the contribution of legumes as plant protein source is indubitable and domestication of new species is becoming an imperative due the challenges faced by the domesticated ones (Harouna et al., Reference Harouna, Venkataramana, Ndakidemi and Matemu2018; Singh et al., Reference Singh, Dubey, Chaurasia, Dubey, Pandey, Singh and Abhilash2019; Takahashi et al., Reference Takahashi, Sakai, Yoshitsu, Muto, Anai, Pandiyan, Senthil, Tomooka and Naito2019). Legumes (family: Fabaceae) constitute the third largest family of flowering plants (Bhat and Karim, Reference Bhat and Karim2009). It is sometimes thought that legumes have very important nutritional value for both humans and animals and are referred to as the ‘poor man's meat’. The most commonly domesticated grown and commercialized legumes such as soybeans, cowpeas, common beans and others have demonstrated considerable contribution to the global food security (Harouna et al., Reference Harouna, Venkataramana, Ndakidemi and Matemu2018). Phaseolus and Vigna genera comprise the most widely consumed legumes, namely common beans (Phaseolus vulgaris L.) and cowpea (Vigna unguiculata (L.) Walp) (Garcia et al., Reference Garcia, Pena-Valdivia, Rogelio and Muruaga1997; Gepts, Reference Gepts and Tolba2001; Harouna et al., Reference Harouna, Venkataramana, Ndakidemi and Matemu2018). Within each genus, there are fewer domesticated edible species than the non-domesticated wild species. Some domesticated and semi- domesticated species are being neglected and underutilized species despite the persistent decrease of the genetic diversity in the food systems. The little attention paid to such species, coupled to the complete ignorance of their existence by agricultural researchers, plant breeders and policymakers has contributed enormously to their under-exploitation and utilization (Padulosi et al., Reference Padulosi, Thompson and Rudebjer2013). A deep exploration into these under-exploited species diversity could be a significantly considerable alternative option that would contribute to the mitigation of food and nutrition insecurity. The Vigna genus comprises more than 100 wild species, which do not have common names, apart from their scientific appellation (Tomooka et al., Reference Tomooka, Naito, Kaga, Sakai, Isemura, Ogiso-Tanaka, Iseki and Takahashi2014). They are given different denotations, such as the under-exploited wild Vigna species, undomesticated Vigna species, wild Vigna or alien species, depending on the scientist (Pratap et al., Reference Pratap, Malviya, Tomar, Gupta, Jitendra, Pratap and Kumar2014; Harouna et al., Reference Harouna, Venkataramana, Ndakidemi and Matemu2018). Preliminary investigations on V. racemosa (G. Don) Hutch. & Dalziel, V. ambacensis Welw. ex Baker, V. reticulata Hook.f. and V. vexillata (L.) A.Rich. accessions were carried out in this study to unveil their proximate composition potential based on their availability and accessibility in the nearest gene bank. Earlier reports have demonstrated their potential usages, farmers’ preferences, cooking and agro- morphological characteristics (Harouna et al., Reference Harouna, Venkataramana, Matemu and Ndakidemi2019a; Harouna et al., Reference Harouna, Venkataramana, Matemu and Alois Ndakidemi2020). It is very important to note here that investigations focusing on the chemical composition of wild Vigna species accessions are scanty or not well documented. The proximate composition, fatty acid composition, total phenolic content, antioxidant activity and amino acid profile of an unknown accession of V. racemosa were reported in a recent study (Ade-Omowaye et al., Reference Ade-Omowaye, Tucker and Smetanska2015). Another study also reported recently on the chemical changes during open and controlled fermentation of V. racemosa seed collected from their natural environment, regardless of their genetic specification (Difo et al., Reference Difo, Onyike, Ameh, Njoku and Ndidi2015). Other studies focusing on qualitative evaluation of bioactive compounds of V. kirkii (Baker) Gillett, Kew Bull, V. marina (Burm.) Merr., V. gracilis (Guill. & Perr.) Hook. f., V. heterophylla A.Rich., V. parkeri Baker, V. hosei (Craib) Backer, V. adenantha (G.Mey.) Maréchal, Mascherpa & Stainier, V. venusta (Piper) Marichal, Mascherpa & Stainier, V. minima (Roxb.) Ohwi & H. Ohashi, V. glabrascens Maréchal, Mascherpa & Stainier and V. triphylla (R.Wilczek) Verdc. have revealed the presence of biochemicals such as Robinin, Kaempferol-3- rutinoside, Isorhamnetin-3- rutinoside, Hyperoside, Delphinidin and Cyanidin (Lattanzio et al., Reference Lattanzio, Cardinali, Linsalata, Ng, Perrino, Singh, Mohan Raj, Dashiel and Jackai1997; Macorni et al., Reference Macorni, Ruggeri and Carnovale1997; Bravo et al., Reference Bravo, Siddhuraju and Saura-Calixto1999). Many other biochemical parameters of these wild Vigna species also need to be investigated to enhance their usages. Therefore, this study aimed at exploring the proximate composition of some wild Vigna species (V. racemosa, V. ambacensis, V. reticulata and V. vexillata) in order to detect accessions for potential domestication and/or crop improvement.
Materials and methods
Seeds preparation for proximate composition analysis
One hundred and six (106) accessions of the four species of wild Vigna legumes (V. racemosa, V. ambacensis, V. reticulata and V. vexillata) were obtained from genebanks as presented in Table S4. Approximately 20–100 seeds of each accession were supplied by the genebanks and planted in an experimental plot, following the augmented block design arrangement, and allowed to grow until full maturity as described in an earlier study (Harouna et al., Reference Harouna, Venkataramana, Matemu and Alois Ndakidemi2020).
The seeds were planted at the Tanzania Agricultural Research Institute (TARI), Selian in the Arusha region, located in the northern part of Tanzania. TARI-Selian lies at a latitude of 3°21′50.08” N and longitude of 36°38′06.29″ E at an elevation of 1390 m above sea level (a.s.l.). Eighty seven (87) accessions of matured seeds of wild Vigna species of legumes harvested were selected based on their productivity in the field to carry out the proximate composition. In addition to the wild accessions, three domesticated Vigna legumes that is, cowpea (V. unguiculata), rice bean (V. umbellata), and a semi-domesticated landrace (V. vexillata) were used as checks. The checks were obtained from the Genetic Resource Center (GRC-IITA), Nigeria (cowpea), the National Bureau of Plant Genetic Resources (NBPGR), India (rice bean), and the Australian Grain Gene bank (AGG), Australia (semi-domesticated landrace V. vexillata).
The matured fruits were harvested with their pods, sun-dried and the seeds were removed from the pods, threshed and winnowed, then free from broken seeds, dust and other foreign materials to obtain clean seeds. The seeds were then stored in a plastic bags at room temperature (27 °C – 30 °C) for subsequent analysis. After that, the seed samples were grinded using a kitchen blender (3 in 1 Electric Chopper Juice Blender HB-38, 350W, Jar Capacity: 1.5L, Guangdong, China) and sieved, and the 1 mm fraction were collected for analysis.
Moisture content determination
The method employed for the determination of moisture content in the sample based on the measurement of the loss in weight due to drying at a temperature of about 105 °C as describe in the Association of Official Analytical Chemists (AOAC) methods (method 950.46) (AOAC, 2000). A watch glass was washed and dried in an oven (DRY-Line 56, STEP Systems GmbH, Nuremberg, Germany) at about 105 °C for 3 h, it was cooled in a desicator and weighed empty.
About 2.0 g of sample was weighed into a clean watch glass. The watch glass and its content were dried in an Air-circulated oven (DRY-Line 56, STEP Systems GmbH, Nuremberg, Germany) at about 105 °C to constant weight. The watch glass and its content was cooled in a desiccator and reweighed.
The percentage moisture was obtained using the expression below;

Ash content determination
The term ash refers to the residue left after combustion of the oven dried sample and is a measure of the total mineral content. Determination of the ash content was done according to AOAC method 923.03 (AOAC, 2000).
Three different crucibles were preheated in a Muffle furnace (Nabertherm GmbH, Lilienthal, Germany) at about 550 °C. Each crucible was cooled in a desiccator and weighed. Approximately 2.0 g of each sample was weighed into different crucibles. The crucibles and their contents were transferred into a Muffle furnace (Nabertherm GmbH, Lilienthal, Germany) set at 550 °C and allowed to stay for 6 h. After cooling the heated crucibles, the weights of crucibles and their content were taken and recorded. The percentage ash was calculated using the following expression;

Crude lipid content determination
The crude lipid content of wild Vigna legumes samples was determined according to the AOAC method 960.39. A Soxtec™ extraction system (Model 2043 Extraction Unit; Tecator, Sweden), and 30 ml of Petroleum ether (Mallinckrodt, Paris, KE, USA) were used to extract the oil from the samples. The amount of extracted oil was determined gravimetrically.
The percentage of lipid was obtained following the equation below;

Crude protein content determination
The protein content of the wild Vigna legume samples was analysed according to the AOAC method 928.08 (AOAC, 2000). The samples were digested with concentrated sulphuric acid (Pharmco-AAPER, USA), Hydrogen peroxide (Fisher Scientific, Fair Lawn, NJ, USA), and two Kjeldahl catalyst tablets (FisherTab ST-35; Fisher Scientific, Sweden) using a Kjeltec block digester unit (Model 2020 Digester; Tecator, Sweden). The total nitrogen amount in the sample was determined by distillation and titration of the extracts using a Kjeltec instrument (Kjeltic™ 8200 Auto Distillation Unit) (Ng, Dunford, and Chenault, Reference Ng, Dunford and Chenault2008). A conversion factor of 6.25 was used to convert the amount of nitrogen to amount of protein present in the samples.
The amount of protein in the samples (dry basis) is calculated from the following formula:

where, T, Volume of the standard hydrochloric acid used in the sample titration. B, Volume of the standard hydrochloric acid used in the blank titration. M, Molarity of the acid in four decimal places. W, mass of the sample used in the determination in milligrams. 6.25, factor used to convert per cent N to per cent crude protein. Most proteins contain 16% N, so the conversion factor is 6.25 (100/16 = 6.25). MCF, Moisture Correction Factor = 100/(100 - % Moisture).
Crude fibre content determination
The fibre content was evaluated using AOAC method (AOAC, 2000). Fat-free grinded wild Vigna samples of 1.0 g were weighed into a clean pre-weighed crucible. The crucible with sample was then transferred into the hot-extraction unit (Fibertec M6, 200–230 V, FOSS, Denmark) and the sample was left to digest for 30 min with 150 ml of solution containing 12.5% Sulphuric acid (Sigma Aldrich, Germany) and 0.25 ml of octanol (Sigma Aldrich, Germany). The condenser was switched off after 30 min and allowed to cool. The acid solution was filtered and washed with hot distilled water using suction. Then the samples were digested for 30 min with 150 ml alkali solution (12.5% NaOH) (Sigma Aldrich, Germany) and 0.25 ml of octanol to dissolve the alkali-soluble matter from the samples. The porcelain crucibles’ final residues were dried at 105 °C in an oven (DRY-Line 56, STEP Systems GmbH, Nuremberg, Germany) for 1 h, cooled in a desiccator and then weighed. The final residues were dried at 105 °C in an oven for 60 min. The residues were ignited in a pre-heated Muffle furnace (Carbolite, UK) at 550 °C for 3 h and weighed. The per cent of crude fibre content was calculated using the following equation:

where, W1, Sample weight. W2, Crucible weight with ash. W3, Empty crucible weight.
Carbohydrate content determination
The percentage carbohydrate was obtained by difference (AOAC, 2000)
Percentage carbohydrate = 100 – (% Moisture + %Protein + %Fat + %Ash + %Crude fiber)
Data analysis
The data on proximate composition parameters were collected in triplicate. Planned, single degree of freedom F test (orthogonal test) was used for mean separation. The one-way analysis of variance, the hierarchical clustering analysis and principal component analysis (PCA) were performed using XLSTAT.
Results
Proximate composition domesticated Vigna species used as checks
The proximate composition for the various Vigna species studied is summarized in Tables 1–4. The proximate composition of the three domesticated legumes included in this study for comparison is presented in the tables presenting the results for each species in order to ease the appreciation. The three domesticated legumes used here as checks are: a semi-domesticated landrace of V. vexillata (Check 1), cowpea (Check 2), and rice bean (Check 3). A keen examination of the proximate composition of these three checks shows that there is no significant difference in lipid, fibre and carbohydrate content of Check 1 and Check 2, which are significantly different (P < 0.05) from that of Check 3. Their lipid content is significantly higher than that of Check 3 while their carbohydrate and fibre contents are lower than that of Check 3. The ash and moisture contents of the three checks are significantly apparently similar. This can be elucidated by evaluating the individual minerals. The protein content of the three checks is significantly different with Check 1 having the highest protein content (Table 1–4).
Table 1. Proximate composition of Vigna ambacensis accessions (g/100 g)

Results are represented as the mean value of triplicates ± standard error. Mean values without any letter in common within each column are significantly different (p = 0.05). Check 1: Landrace of Vigna vexillata ; Check 2[Domesticated variety]: Cowpea (Vigna unguiculata); Check 3[Domesticated variety]: Rice Bean (Vigna umbellata).
Table 2. Proximate composition of Vigna racemosa accessions (g/100)

Results are represented as the mean value of triplicates ± standard error. Mean values without any letter in common within each column are significantly different (p = 0.05). Check 1: Landrace of Vigna vexillata ; Check 2[Domesticated variety]: Cowpea (Vigna unguiculata); Check 3[Domesticated variety]: Rice Bean (Vigna umbellata).
Table 3. Proximate composition of some studied Vigna reticulata accessions (g/100 g)

Results are represented as the mean value of triplicates ± standard error. Mean values without any letter in common within each column are significantly different (p = 0.05). Check 1: Landrace of Vigna vexillata ; Check 2[Domesticated variety]: Cowpea (Vigna unguiculata); Check 3[Domesticated variety]: Rice Bean (Vigna umbellata).
Table 4. Proximate composition of some studied Vigna vexillata accessions (g/100 g)
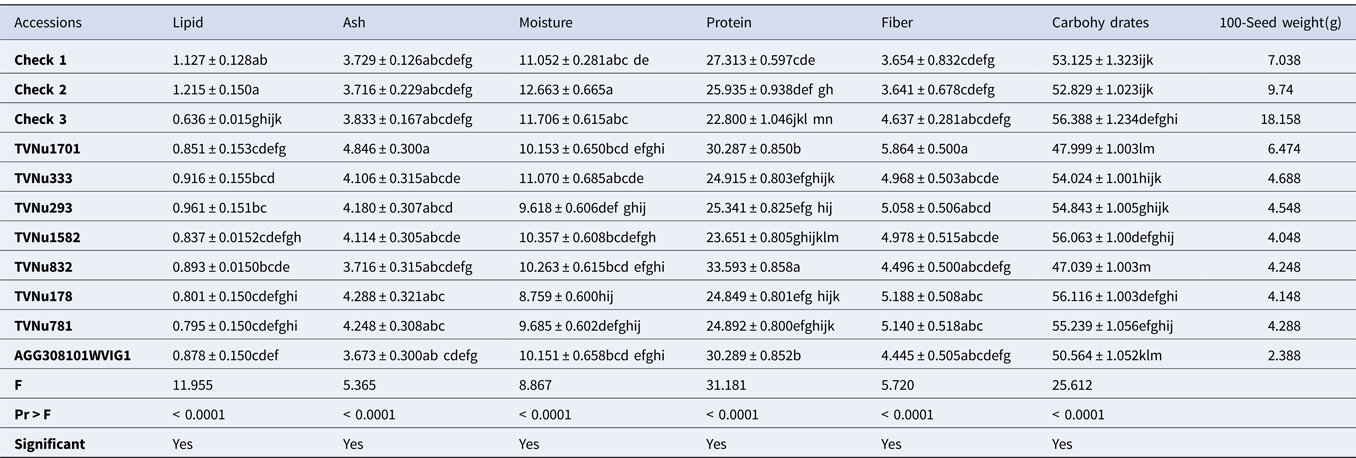
Results are represented as the mean value of triplicates ± standard error. Mean values without any letter in common within each column are significantly different (p = 0.05). Check 1: Landrace of Vigna vexillata; Check 2[Domesticated variety]: Cowpea (Vigna unguiculata); Check 3[Domesticated variety]: Rice Bean (Vigna umbellata).
Proximate composition of Vigna ambacensis accessions
Table 1 summarizes the proximate composition of V. ambacensis accessions (11 accessions). It shows that the lipid content of all the wild accessions is significantly similar to those of Check 1 and 2 while it is significantly (P < 0.05) higher than that of Check 3. All the accessions showed significant lower ash content than the three checks except for accessions TVNu219 and TVNu877 which is comparable to that of the checks. The moisture and protein content of the wild accessions are significantly lower than that of the checks while the carbohydrate and fibre content of the wild accessions were higher than that of the checks.
Proximate composition of Vigna racemosa accessions
Table 2 summarizes the proximate composition of V. racemosa accessions. It was found that the lipid content of AGG53597WVIG1 and AGG51603WVIG1 accessions was significantly comparable to that of Check 1 and Check 2 whereas ‘Unknown_Vigna_racemosa’, AGG52867WVIG1 and ‘Unknown Vigna” showed comparable lipid content to that of Check 3. By analogy to the V. reticulata species, all the accessions showed comparable ash content to that of the three checks indicating that none of the accessions had higher ash content than the checks. Accession AGG53597WVIG1 showed higher moisture content as compared with Check 1, Check 3 and all the other wild accessions. Moreover, it was comparable to that of Check 2. The accession AGG51603WVIG1 (36.689%) showed substantially significant higher protein content than that of all the checks whereas accession AGG53597WVIG1 (28.852%) had a protein content comparable to that of Check 1. The rest of the accessions had significant low protein content which is comparable to that of Check 3. Furthermore, the greater number of wild accessions presents significantly higher fibre and carbohydrates contents as compared to the checks.
Proximate composition of Vigna reticulata accessions
The proximate composition of V. reticulata accessions has been summarized in Table 3 (eight accessions) to ease the readability of this article. The complete results presenting the proximate composition of the 36 studied accessions are displayed on Table S1. The results show that the lipid content of most of the wild accessions were not significantly different from those of Check 1 and Check 2. Four accessions (TVNu1394_VRe, TVNu324_VRe, TVNu57_VRe and TVNu141_VRe) exhibited comparable lipid content to that of the check 3. All the accessions showed comparable ash content to that of the three checks indicating that none of the accessions had higher ash content than that of the checks. All the accessions showed lower moisture content than the three checks. The accession TVNu1112_VRe (31.074%) had substantially higher protein content which is significantly higher than that of all the checks. On the other hand, five accessions (TVNu350_VRe, TVNu1852_VRe, TVNu324_VRe, TVNu57_VRe, andTVNu141_VRe) had protein content comparable to that of Check 1 and Check 2. The rest of the accessions had low protein content which is lower than that of Check 3. It was noticed that the greater number of wild accessions present a significantly higher fibre and carbohydrates contents respectively as compared to the checks.
Proximate composition of Vigna vexillata accessions
The proximate composition of V. vexillata accessions has been summarized in Table 4 (eight accessions) to ease the readability of this article. The complete results presenting the proximate composition of the 35 studied accessions are displayed on Table S2. It was found that the lipid content of the majority of wild accessions is significantly lower than that of Check 1 and check 2, except in the case of accessions AGG308096WVIG2, TVNu333, TVNu293 and TVNu 832 where the lipid content is statistically higher than that of the Check 3. Similar to the Vigna reticulata species, all the accessions showed comparable ash content to that of the three checks. A significant number of accessions showed comparable moisture content to that of the checks indicating phenotypic similarity in moisture content. The accessions TVNu832, TVNu1701, TVNu1546, AGG308101WVIG2 and AGG308099WVIG2 exhibited significant higher protein content than that of all the checks.
On the other hand, ten accessions (AGG308097WVIG2, TVNu1378, TVNu1529, TVNu1344, TVNu333, TVNu293, TVNu178, TVNu781, TVNu120 and TVNu1629) had protein content comparable to that of Check 1 and Check 2. The rest of the accessions had low protein content which is statistically lower than that of Check 3. It is similarly noticed that the greater number of wild accessions present a significantly higher fiber and carbohydrates contents as compared to the checks.
Concomitant analysis of the four Vigna species
To examine the relationship that could exist between the proximate composition and the accessions, as well as the relationship between the accessions themselves, a PCA (XLSTAT) was performed using the means values for nutrient component in each accession. Confidence ellipses and correlation circle, combined with an observation chart (Biplot), were obtained as shown in Fig. 1. These were obtained from the PCA analysis of the mean values for each species taken separately. The analysis showed that the first (F1 = 67.22%) and second (F2 = 17.99%) PCA dimensions represent 85.21% of the initial information, which is the best combination that explains the variation among the accessions and traits (proximate composition). It was found that there is a positive correlation between the traits ash, moisture and protein, except for the lipid, fibre and carbohydrate traits, which is due to the angles between their vectors (Fig. 1 and Table S3). It was also noted that all the checks, together with a set of wild accessions, are found on the left side of the F1 axis, forming a group of accessions with lower values for the examined nutrients traits, except for the lipid, fibre and carbohydrates. Those accessions could share common features with the checks. A second group, made up of only wild accessions, was found on the right side of the F1 axis, representing the accessions with higher values for the evaluated traits (Fig. 1).

Figure 1. The PCA analysis showing correlations between nutrients contents and wild Vigna accessions.
Discussion
By assessing the proximate composition of 87 accessions from four wild unexplored Vigna species, this study revealed that some individual wild accessions have higher nutrient content as compared with domesticated ones which could be advantageous for bio-fortification or domestication. Likewise, the study examined the differences that may exist between the checks. Therefore, it was found that Check 1 and Check 2 might be related in terms of the phenotypic traits lipid, fibre and carbohydrates content though they are of different species (V. vexillata and V. unguiculata). In addition, it should be noted that Check 1 is landrace of V. vexillata which has not yet been fully domesticated as it is noticed that taxonomic arrangements within the Vigna genus are not completed (Gore et al., Reference Gore, Tripathi, Pratap, Bhat, Umdale, Gupta and and Pandey2019). Phylogenic proximity between V. vexillata and V. unguiculata has also been reported (Boukar et al., Reference Boukar, Bhattacharjee, Fatokun, Kumar, Gueye, Singh, Upadhyaya and Bisht2013). However, the differences observed between the three checks or between Checks 1 and 2 with Check 3 for the nutrients evaluated can simply be attributed to their species differences.
According to Table 1, the lipid content of all the wild accessions of V. ambacensis is significantly similar to those of check 1 and 2 while it is significantly (P < 0.05) higher than that of check 3. This is in line with reports that support the idea of constituents’ reduction in legumes due to domestication (Fernández-Marín et al., Reference Fernández-Marín, Milla, Martín-Robles, Arc, Kranner, Becerri and García-Plazaola2014). Other differences among the checks and the wild accessions may be due to species differences and phylogenic relationships.
In the case of V. racemosa accessions (Table 2), the same trend of result for the proximate composition was observed as in the cases of V. reticulata and V. vexillata. Following Table 3 and Table S1, the lipid content of most of the wild accessions of V. reticulata is not significantly different from that of Check 1 and 2 except for few accessions (TVNu1394_VRe, TVNu324_VRe, TVNu57_VRe and TVNu141_VRe) which are comparable to Check 3. All the accessions show comparable ash content to that of the three checks indicating that none of the accessions had higher ash content than that of the checks. This can be due to species or phylogenic proximity of V. reticulata with Check 1 and 2 (Table 3). All the accessions showed lower moisture content than that of the three checks. The low moisture content observed in wild accessions can be related to the seed characteristics and probably the genetic makeup of the V. reticulata accessions as it was earlier reported seed characteristics of wild legumes affect their composition and cooking characteristics (Ereifej, Reference Ereifej2004; Altuntas and Demirtola, Reference Altuntas and Demirtola2007; Harouna et al., Reference Harouna, Venkataramana, Matemu and Ndakidemi2019a). The low moisture content could also be a factor of good storing quality of the seeds. The accession with highest protein content (TVNu1112_VRe, 31.074%) might be a suitable genetic material for domestication or breeding and therefore should be further investigated through molecular marker as it high protein content might be due to its genomic difference. This might have been acquired based on the environmental background. For the accessions with protein content comparable to that of Check 1 and Check 2 (TVNu1852_VRe, TVNu141_VRe, TVNu57_VRe, TVNu324_VRe, and TVNu350_VRe), phylogenic studies as well as breeding and improvement is recommended. The rest of the accessions with very low protein content which is lower than that of Check 3 should be exploited for other nutritional elements. The greater number of wild accessions presented a significantly higher fibre and carbohydrates contents as compared to the checks. This is in line with earlier reports on wild legumes (Macorni et al., Reference Macorni, Ruggeri and Carnovale1997; Difo et al., Reference Difo, Onyike, Ameh, Njoku and Ndidi2015). It might be due to the biosynthesis of many polysaccharides by the wild legumes in order to protect the embryo and survive in harsh environments (Smykal et al., Reference Smykal, Vernoud, Blair, Soukup and Thompson2014). Therefore, it should be recommended to carry out sound examination of the carbohydrates and fibre fractions to ascertain the digestibility and clear their nutritive contribution in these seeds.
In Table 4 and Table S2, the proximate composition of V. vexillata accessions is displayed. The low lipid content found in this result could be attributed to species and genomic differences as explained in the case of V. reticulata. Similar to the V. reticulata species, all the accessions showed comparable ash content to that of the three checks which can be explained by the same reasons as elaborated earlier. A significant number of accessions showed comparable moisture content to that of the checks indicating phenotypic similarity in moisture content.
The accessions TVNu1701, and AGG30801WVIG1 with highest protein content are speculated to be suitable candidate genetic materials for domestication or breeding. Therefore further investigation should be done through molecular marker as its high protein content might be due to its genomic difference. Furthermore, this might have been acquired based on the environmental background. For the accessions with protein content comparable to that of Check 1 and Check 2, phylogenic studies as well as breeding and improvement are recommended. As was also noticed some wild accessions present a significantly higher fibre and carbohydrates contents as compared with the checks. This finding concurred with earlier reports on wild legumes (Macorni et al., Reference Macorni, Ruggeri and Carnovale1997; Difo et al., Reference Difo, Onyike, Ameh, Njoku and Ndidi2015). It might be due to the biosynthesis of many polysaccharides by the wild legumes in other to protect the embryo and survive in harsh environments (Smykal et al., Reference Smykal, Vernoud, Blair, Soukup and Thompson2014) as explained earlier. Therefore, it is recommended to carryout sound examination of the carbohydrates and fibre fraction to ascertain the digestibility and clear nutritive contribution of the carbohydrates and fibre contained in these seeds.
It is necessary to note that domestication process could also affect other nutritional and health characteristics of the domesticated product as alerted by some researchers (Smýkal et al., Reference Smýkal, Nelson, Berger and Von Wettberg2018). The PCA provided indications relating to the domestication of these wild legumes by grouping them based on their quantitative proximate composition traits (Fig. 1).
It was shown that most of the nutrients analysed are positively correlated, and there is a degree of commonality between the checks and a group of some wild species. The check 3 showed close relationship with the V. vexillata and V. racemosa species, while the checks 1 and 2 showed close relationship with V. reticulata and V. ambacensis species as illustrated on the ellipses bootstrap of Fig. 1. This might be due to the relatively slight genetic similarities on their proximate composition which is presented on the biplot of Fig. 1.
Conclusion
The wild Vigna species studied presents a considerably high diversity in terms of proximate composition. Despite their under-exploitation for human benefits, the studied wild Vigna legumes demonstrated nutrient characteristics comparable with the domesticated ones. The study also demonstrated that the wild Vigna species possesses a large variation range of nutrient characteristics such as protein, ash and carbohydrate contents which could be exploited in the improvement of domesticated species or guide the domestication of new species. It was also found that some individual wild accessions have higher nutrients (protein, lipid, ash and carbohydrates) as compared with domesticated ones which could be advantageous for bio-fortification or domestication. The accessions that presented promising traits based on the protein content were: TVNu832, TVNu1701, AGG51603WVIG 1, AGG53597WVIG 1 and TVNu1112. Therefore, these accessions are the best suited for domestication. However, the accessions that showed poor nutrients contents should not be neglected and should be subjected to more study to unveil their potentials. Further investigations including studies involving not only their seeds content, but the other parts of the plant such as their leaves, roots and stem could be of great importance on unveiling their full potential in various aspects of life and science such as crop improvement and domestication, human and animal nutrition, biodiversity conservation and food security.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1479262124000029.
Acknowledgements
The authors are grateful to the Centre for Research, Agricultural Advancement, Teaching Excellence and Sustainability in Food and Nutrition Security (CREATES-FNS. The authors also acknowledge the additional funding support from the International Foundation for Science (IFS) through Grant No. I-3-B-6203-1. The authors are also grateful to the Genetic Resources Center, International Institute of Tropical Agriculture (IITA), Ibadan-Nigeria, as well as the Australian Grains Gene bank (AGG) for providing supporting information and seed materials for research.







