Background
Dementia is a syndrome characterised by progressive cognitive and functional decline constituting one of the leading causes of disability worldwide (Duong, Patel, & Chang, Reference Duong, Patel and Chang2017; World Health Organization [WHO], 2017). The prevalence of dementia is estimated to double every 20 years, making it a major public health concern, with estimated worldwide costs reaching $1 trillion per year in 2018 (Wimo et al., Reference Wimo, Guerchet, Ali, Wu, Prina, Winblad and Prince2017; Ferri et al., Reference Ferri, Prince, Brayne, Brodaty, Fratiglioni, Ganguli and Scazufca2005; Prince et al., Reference Prince, Wimo, Guerchet, Ali, Wu and Prina2015). Several psychiatric disorders have been found to increase risk of developing dementia, with depression, anxiety, and post-traumatic stress disorder (PTSD) being among those most extensively studied. There is also increasing research interest in the relationship between non-affective psychotic disorders and future dementia risk. Schizophrenia and related non-affective psychotic disorders such as schizoaffective disorder, and delusional disorder are commonly characterised by symptoms such as hallucinations and delusions, a lack of motivation, and impairments in cognitive functioning (Arciniegas, Reference Arciniegas2015). Cognitive impairment is a core symptom occurring both in dementia and psychotic disorders, although the underlying cause and course of cognitive impairment in psychotic disorders remains unclear (Alkan, Davies, & Evans, Reference Alkan, Davies and Evans2021; Kahn, Reference Kahn2019). Understanding whether psychotic disorders represent a risk factor for dementia is important for its prevention and timely treatment and could lead to improved clinical management and a better understanding of the nature of cognitive impairment in psychotic disorders (Livingston et al., Reference Livingston, Huntley, Sommerlad, Ames, Ballard, Banerjee and Mukadam2020).
Despite increasing research interest in the association between psychotic disorders and subsequent cognitive decline and dementia, research in this area remains relatively sparse. Although a previous meta-analysis found an increased risk of dementia in people with schizophrenia, only six studies were identified, with heterogeneous designs (Cai & Huang, Reference Cai and Huang2018). Since the publication of the previous review, multiple large longitudinal studies have been published examining the association between psychotic disorders and subsequent dementia.
In addition, little is known about whether dementia risk differs for people with late-onset (LOS) and very late-onset schizophrenia-like psychoses (VLOSLP), which have symptom onset after age 40 and 60 years respectively, relative to psychotic disorders with a more typical age-at-onset, occurring in late adolescence and early adulthood (Howard, Rabins, Seeman, Jeste, & the International Late-Onset Schizophrenia Group, Reference Howard, Rabins, Seeman and Jeste2000; Kessler et al., Reference Kessler, Amminger, Aguilar-Gaxiola, Alonso, Lee and Ustün2007). Differences in epidemiology and presentation have been found in those with LOS and VLOSLP, including a higher prevalence among women and fewer negative symptoms (Howard et al., Reference Howard, Rabins, Seeman and Jeste2000; Stafford, Howard, & Kirkbride, Reference Stafford, Howard and Kirkbride2018; Stafford, Howard, Dalman, & Kirkbride, Reference Stafford, Howard, Dalman and Kirkbride2019; Vahia et al., Reference Vahia, Palmer, Depp, Fellows, Golshan, Kraemer and Jeste2010). Given these differences, LOS and VLOSLP might be expected to show different patterns and strength of association with dementia compared to psychotic disorders with a more typical age-at-onset.
Furthermore, given that dementia is commonly preceded by neuropsychiatric symptoms, which can include hallucinations and delusions (Wise, Rosenberg, Lyketsos, & Leoutsakos, Reference Wise, Rosenberg, Lyketsos and Leoutsakos2019), examining variation in associations by psychotic disorder age-at-onset and duration between psychotic disorders and dementia could provide insight into whether associations are likely to be causal, or to reflect reverse causation due to neuropsychiatric symptoms as part of the dementia prodrome. For example, stronger associations with dementia for LOS and VLOSLP, and when the duration between psychotic disorders and dementia is shorter, would be more indicative of reverse causation, whereas longstanding associations would be more consistent with a causal relationship.
A comprehensive and up-to-date systematic review and meta-analysis is therefore now required in order to provide an accurate quantitative estimate of the association between non-affective psychotic disorders and future risk of dementia, with a particular focus on investigating potential underlying mechanisms and for updating current life course models of dementia prevention.
To address gaps in previous knowledge, this systematic review and meta-analysis aimed to investigate whether non-affective psychotic disorders are longitudinally associated with an increased risk of dementia and to provide an up-to-date estimate of the association. In addition, we aimed to compare differences in association among those with LOS and VLOSLP relative to psychotic disorders with a more typical age-at-onset. We additionally examined whether other non-affective psychotic disorder diagnoses, such as delusional disorder, in addition to schizophrenia, are associated with an increased risk of dementia. To further investigate potential sources of heterogeneity, we examined whether variables such as sex, study type, study quality, follow-up study interval, and anti-psychotic medication use influenced results.
Method
We followed current PRISMA guidelines (Page et al., Reference Page, McKenzie, Bossuyt, Boutron, Hoffmann, Mulrow and Moher2021) for the reporting of systematic reviews (online Supplementary Table S1) and registered our review with PROSPERO: CRD42021255719.
Search strategy
Four databases, Medline, Embase, PsycInfo, and CINAHL plus, were searched for published literature and two additional databases, Open Grey and Ethos, were searched for grey literature up to 29th of June 2021. We re-ran the search using Medline until March 2022 to check for recent papers. The search terms used were broadly related to non-affective psychotic disorders, dementia, and type of study (see online Supplementary Table S2 for details of search terms used). Full-texts were searched for all identified studies, and two authors independently screened all full-text articles for relevant studies. Disagreements were discussed with a third reviewer. Reference lists of all relevant studies reaching the full-text phase were hand searched to ensure no relevant studies were missed.
Selection criteria
We included both prospective and retrospective longitudinal studies examining the association between psychotic disorders and dementia. The population of interest were adults aged ⩾18 years with a clinical diagnosis of a non-affective psychotic disorder (ICD-11, 6A20-6A2Z or equivalent), and the comparison group were adults without a non-affective psychotic disorder (American Psychological Association [APA], 2013; WHO, 2018). Dementia diagnosis was also based on clinical criteria (ICD-11, 8A20-8A2Z, NINCDS-ADRA or equivalent) (APA, 2013; Blacker et al., Reference Blacker, Albert, Bassett, Go, Harrell and Folstein1994; WHO, 2018).
Data extraction
Data were extracted independently by two authors and included: name of authors, year of publication, setting, sample size, study type, assessment/diagnostic criteria of psychotic disorder and dementia, length of follow-up, relevant effect estimates, risk of bias assessment, and control of confounders. Hazard ratios (HRs), risk ratios (RR), odds ratios (ORs), incidence rate ratios (IRRs), standardised mortality ratios (SMRs), and standardised incidence ratios (SIRs), along with standard errors and confidence intervals (CIs), were extracted for the meta-analysis. We combined various measures of relative risk in line with current guidelines, and the rare disease assumption, which states that various measures of relative risk will approximate each other when the outcome investigated is sufficiently rare (Greenland, Reference Greenland1987).
Risk of bias assessment
Two reviewers independently assessed risk of bias in all included studies using a modified version of the Newcastle-Ottawa Scale (NOS) (see online Supplementary Table S3), covering three risks of bias domains: selection of study groups, comparability and study design, and outcome (Stang, Reference Stang2010). Disagreements were resolved with a third reviewer. Small-study effects were assessed using Egger's weighted regression and visual inspection of a funnel plot (Lin & Chu, Reference Lin and Chu2018).
Data analysis
STATA (Version 17.1) and the ‘metan’ and ‘metareg’ commands were used to conduct the meta-analyses and meta-regressions. The generic inverse variance method and a random-effects model was used to obtain pooled relative risk estimates across studies that reported IRRs, HRs, RRs, ORs, SMRs, and SIRs. Heterogeneity was measured using the χ2 Cochran's Q test and the I 2-statistic. We examined sources of heterogeneity using subgroup analyses, sensitivity analyses, and meta-regression.
Results
Study selection
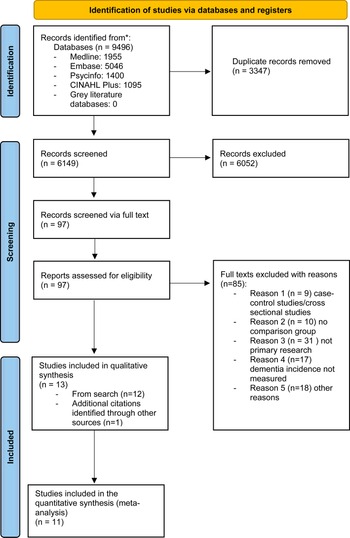
The study selection process followed the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) (Page et al., Reference Page, McKenzie, Bossuyt, Boutron, Hoffmann, Mulrow and Moher2021). A total of 9496 studies were identified through the search process, with a total of 6149 studies remaining after removal of duplicates (Fig. 1). Following the initial screening of titles and abstracts, a total of 73 studies remained, which were screened for eligibility. When we re-ran our search to update it, an additional 24 studies were identified that were eligible for full-text screening. Of all the full-text studies identified 85 were excluded due to not meeting eligibility criteria (see online Supplementary Table S4), leaving 12 studies meeting our inclusion criteria. One newly published study was identified after the initial screening (Richmond-Rakerd, D'Souza, Milne, Caspi, & Moffitt, Reference Richmond-Rakerd, D'Souza, Milne, Caspi and Moffitt2022), resulting in a total of 13 included studies of which 11 were eligible to be included in the meta-analysis. Three of the identified studies had the same comparison population, but two of these focused specifically on late-onset acute and transient psychosis (LOATP) and late-onset delusional disorder (LODD) (Kørner, Lopez, Lauritzen, Andersen, & Kessing, Reference Kørner, Lopez, Lauritzen, Andersen and Kessing2008, Reference Kørner, Lopez, Lauritzen, Andersen and Kessing2009a, Reference Kørner, Lopez, Lauritzen, Andersen and Kessing2009b). These two studies were not included in the meta-analysis but were retained in the narrative synthesis, given that no other studies examined these specific disorders.

Fig. 1. PRISMA flow diagram of the study selection process.
Study characteristics
Information on study characteristics is presented in Table 1. Of the 13 included studies, three were prospective and 10 were retrospective. Studies were published between 2003 and 2022, with four studies published between 2003 and 2014, four published between 2015 and 2019, and five published in 2020 or after. Sample sizes across studies ranged from 61 to 8 011 773 participants, with a total of 12 997 101 participants. Study follow-up periods ranged from 1.57 to 33 years (median: 11 years). Studies were conducted in Europe (Denmark, Finland, Sweden, and the United Kingdom), the United States, Australia, Taiwan, New Zealand, and Israel.
Table 1. Characteristics of included studies

Note. ICD-8/9/10, International Classification of Diseases, 8th/9th/10th revision; OR, odds ratio; HR, hazard ratio; SIR, standardised incidence ratio; RR, risk ratio; IRR, incidence rate ratio; CI, confidence interval; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, 4th Revision; NINCDS-ADRDA, National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders; Charlson comorbidity index: predicts mortality from a range of medical comorbidities (diabetes, liver disease, heart failure, etc.). N, number of participants; –, data not reported.
a If not stated otherwise, effect estimate of dementia incidence in non-affective psychotic disorder compared to general population.
b Not included in the meta-analyses.
Study participants were aged 18 years and above, with most being over the age of 60. The percentage of females across studies ranged from 0 in an only male cohort to 78% (Almeida et al., Reference Almeida, Ford, Hankey, Yeap, Golledge and Flicker2018). Studies focused on a range of non-affective psychotic disorder diagnoses including LOATP (n = 1; age-at-onset ⩾ 60) (Kørner, Lopez, Lauritzen, Andersen, & Kessing, Reference Kørner, Lopez, Lauritzen, Andersen and Kessing2009a), LODD (n = 1; age-at-onset ⩾ 60) (Kørner et al., Reference Kørner, Lopez, Lauritzen, Andersen and Kessing2008), LOS and/or VLOSLP (n = 5; age-at-onset ⩾ 40, or >60) (Almeida et al., Reference Almeida, Ford, Hankey, Yeap, Golledge and Flicker2018; Brodaty, Sachdev, Koschera, Monk, & Cullen, Reference Brodaty, Sachdev, Koschera, Monk and Cullen2003; Kodesh et al., Reference Kodesh, Goldberg, Rotstein, Weinstein, Reichenberg, Sandin and Levine2020; Kørner, Lopez, Lauritzen, Andersen, & Kessing, Reference Kørner, Lopez, Lauritzen, Andersen and Kessing2009b; Stafford et al., Reference Stafford, Dykxhoorn, Sommerlad, Dalman, Kirkbride and Howard2021), schizophrenia (n = 1) (Lin, Chung, Chen, & Chi, Reference Lin, Chung, Chen and Chi2018), psychotic disorders (n = 2) (Richmond-Rakerd et al., Reference Richmond-Rakerd, D'Souza, Milne, Caspi and Moffitt2022; Tapiainen, Hartikainen, Taipale, Tiihonen, & Tolppanen, Reference Tapiainen, Hartikainen, Taipale, Tiihonen and Tolppanen2017), and schizophrenia in older people (n = 3) (Kershenbaum et al., Reference Kershenbaum, Cardinal, Chen, Underwood, Seyedsalehi, Lewis and Rubinsztein2020; Ribe et al., Reference Ribe, Laursen, Charles, Katon, Fenger-Grøn, Davydow and Vestergaard2015; Stroup et al., Reference Stroup, Olfson, Huang, Wall, Goldberg, Devanand and Gerhard2021). Most studies investigated incidence of all-cause dementia, with one study focusing on incidence of Alzheimer's disease (Tapiainen et al., Reference Tapiainen, Hartikainen, Taipale, Tiihonen and Tolppanen2017).
Characteristics of the sample, exposure, and outcome across studies
Nine studies used population registers with records of clinical diagnoses of psychotic disorders and dementia based on ICD-8, -9, or -10 (Almeida et al., Reference Almeida, Ford, Hankey, Yeap, Golledge and Flicker2018; Kodesh et al., Reference Kodesh, Goldberg, Rotstein, Weinstein, Reichenberg, Sandin and Levine2020; Kørner et al., Reference Kørner, Lopez, Lauritzen, Andersen and Kessing2008, Reference Kørner, Lopez, Lauritzen, Andersen and Kessing2009a, Reference Kørner, Lopez, Lauritzen, Andersen and Kessing2009b; Ribe et al., Reference Ribe, Laursen, Charles, Katon, Fenger-Grøn, Davydow and Vestergaard2015; Richmond-Rakerd et al., Reference Richmond-Rakerd, D'Souza, Milne, Caspi and Moffitt2022; Stafford et al., Reference Stafford, Dykxhoorn, Sommerlad, Dalman, Kirkbride and Howard2021; Tapiainen et al., Reference Tapiainen, Hartikainen, Taipale, Tiihonen and Tolppanen2017). One study used medical insurance data (Stroup et al., Reference Stroup, Olfson, Huang, Wall, Goldberg, Devanand and Gerhard2021) and three studies used data from mental health services, where both psychotic disorders and dementia were clinically diagnosed using ICD-9 or ICD-10, or DSM-III and DSM-IV criteria (Brodaty et al., Reference Brodaty, Sachdev, Koschera, Monk and Cullen2003; Kershenbaum et al., Reference Kershenbaum, Cardinal, Chen, Underwood, Seyedsalehi, Lewis and Rubinsztein2020; Lin et al., Reference Lin, Chung, Chen and Chi2018).
Risk of bias and adjustment for confounders
Ten studies scored in the higher range of methodological quality, two studies (Kershenbaum et al., Reference Kershenbaum, Cardinal, Chen, Underwood, Seyedsalehi, Lewis and Rubinsztein2020; Stroup et al., Reference Stroup, Olfson, Huang, Wall, Goldberg, Devanand and Gerhard2021) were rated as fair, and one as poor (Brodaty et al., Reference Brodaty, Sachdev, Koschera, Monk and Cullen2003) (Table 2). Almost all studies accounted for basic sociodemographic confounders such as age and sex (see Table 1). Several studies adjusted for comorbidities (Almeida et al., Reference Almeida, Ford, Hankey, Yeap, Golledge and Flicker2018; Lin et al., Reference Lin, Chung, Chen and Chi2018; Ribe et al., Reference Ribe, Laursen, Charles, Katon, Fenger-Grøn, Davydow and Vestergaard2015; Richmond-Rakerd et al., Reference Richmond-Rakerd, D'Souza, Milne, Caspi and Moffitt2022; Tapiainen et al., Reference Tapiainen, Hartikainen, Taipale, Tiihonen and Tolppanen2017), alcohol or substance use disorders (Almeida et al., Reference Almeida, Ford, Hankey, Yeap, Golledge and Flicker2018; Ribe et al., Reference Ribe, Laursen, Charles, Katon, Fenger-Grøn, Davydow and Vestergaard2015; Tapiainen et al., Reference Tapiainen, Hartikainen, Taipale, Tiihonen and Tolppanen2017), medications (Kodesh et al., Reference Kodesh, Goldberg, Rotstein, Weinstein, Reichenberg, Sandin and Levine2020; Lin et al., Reference Lin, Chung, Chen and Chi2018), smoking status (Kodesh et al., Reference Kodesh, Goldberg, Rotstein, Weinstein, Reichenberg, Sandin and Levine2020), and income and education level (Stafford et al., Reference Stafford, Dykxhoorn, Sommerlad, Dalman, Kirkbride and Howard2021).
Table 2. Results of NOS assessment for study quality

a Adjusted for at least two covariates.
b Up to two points for follow-up.
Primary meta-analysis of psychotic disorders and risk of dementia
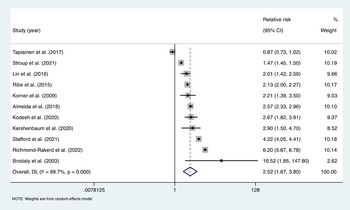
Pooling estimates from 11 studies showed that psychotic disorders were associated with an increased risk of all-cause dementia; pooled RR = 2.52, 95% CI (1.67–3.80), I 2 = 99.7%, p < 0.001; a total of 12 997 101 participants and around 119 274 individuals with psychotic disorders; median follow-up of 11 years (Fig. 2). For the Tapiainen et al. (Reference Tapiainen, Hartikainen, Taipale, Tiihonen and Tolppanen2017) study, we used the estimated risk based on the 10-year time interval, given that it was the longest period reported. For the Kørner et al. (Reference Kørner, Lopez, Lauritzen, Andersen and Kessing2009b) study we used the estimate for LOS, and for the Stroup et al. (Reference Stroup, Olfson, Huang, Wall, Goldberg, Devanand and Gerhard2021) study we calculated an IRR based on the incidence rate data, which was provided separately for those with and without schizophrenia. Of the 11 studies included, 10 reported that psychotic disorders increased risk of dementia, while one study by Tapiainen et al. (Reference Tapiainen, Hartikainen, Taipale, Tiihonen and Tolppanen2017) reported no significant association.

Fig. 2. Meta-analysis of effect estimates of non-affective psychotic disorders compared to no non-affective psychotic disorders on risk of dementia.
The Brodaty et al. (Reference Brodaty, Sachdev, Koschera, Monk and Cullen2003) study was excluded from the funnel plot for small-study effects, due to being an outlier that influenced the scale of the plot (see online Supplementary Fig. S1). Visual inspection of the funnel plot indicated some asymmetry, however the Egger's test did not provide evidence of small-study effects (t = 1.22, p = 0.252) (Ioannidis & Trikalinos, Reference Ioannidis and Trikalinos2007).
Subgroup analyses and meta-regression
Our subgroup analyses indicated that the risk of dementia was higher in individuals with typical and late-onset psychotic disorders (onset <40 years and >40 years) compared to individuals with VLOSLP (onset >60 years); pooled RR = 3.10, 95% CI (2.33–4.14), I 2 = 77.5%, p = .0.004, v. pooled RR = 2.77, 95% CI (1.74–4.40), I 2 = 98.9%, p < 0.001, n = 3). Studies with a follow-up of <10 years showed a larger effect compared to studies with a follow-up of ⩾10 years; pooled RR = 2.64, 95% CI (1.98–3.51), I 2 = 10.9%, p = 0.338, v. pooled RR = 2.33, 95% CI (1.43–3.82), I 2 = 99.8%, p < 0.001. Risk was higher in studies conducted in non-European countries compared to those recruiting in European countries; pooled RR = 2.96, 95% CI (1.56–5.62), I 2 = 99.4%, p < 0.001, v. pooled RR = 2.04, 95% CI (1.12–3.71), I 2 = 99.5%, p < 0.001. Studies in which ⩾60% of the sample were female showed a higher risk compared to studies with less than <60% of the sample being female; pooled RR = 3.24, 95% CI (2.09–5.02), I 2 = 79.0%, p = 0.003, v. pooled RR = 2.10, 95% CI (1.42–3.12), I 2 = 96.7%, p < 0.001. In addition, the association between psychotic disorders and dementia was stronger in studies published in 2020 or after, relative to those published before 2020; pooled RR = 3.13, 95% CI (1.55–6.33), I 2 = 99.9%, p < 0.001, v. pooled RR = 1.93, 95% CI (1.34–2.77), I 2 = 95.8%, p < 0.001.
Studies investigating broader non-affective psychotic disorders showed a higher risk compared to studies investigating schizophrenia; pooled RR = 2.78, 95% CI (1.57–4.91), I 2 = 99.4%, p < 0.001, v. pooled RR = 2.13, 95% CI (1.66–2.73), I 2 = 95.9%, p < 0.001. Risk was higher in prospective studies compared to retrospective studies; pooled RR = 2.65, 95% CI (2.10–3.35), I 2 = 28.3%, p = 0.248, v. pooled RR = 2.34, 95% CI (1.45–3.78), I 2 = 99.8%, p < 0.001. Our final set of analyses showed that studies in individuals with a minimum age <60 at baseline showed a higher risk of dementia compared to studies with a minimum age ⩾60; pooled RR = 3.16, 95% CI (1.59–6.28), I 2 = 99.8%, p < 0.001, v. pooled RR = 2.61, 95% CI (1.39–4.92), I 2 = 99.8%, p < 0.001. Correlations between potential moderators are reported in online Supplementary Table S5.
In line with current recommendations, we conducted meta-regressions for characteristics that were examined in at least 10 studies (Higgins et al., Reference Higgins, Thomas, Chandler, Cumpston, Li, Page and Welch2019). We did not find evidence that heterogeneity between estimates was explained by type of psychotic disorder (meta-regression p = 0.66), follow-up time-point (p = 0.59), geographical region (p = 0.41), or year of study publication (p = 0.22) (see online Supplementary Table S6).
Sensitivity analysis
Sensitivity analyses showed that the strength and direction of the results, and between study heterogeneity (I 2 range: 96.7–99.6) remained the same after excluding any single study. Excluding low-quality studies (n = 3) (Brodaty et al., Reference Brodaty, Sachdev, Koschera, Monk and Cullen2003; Kershenbaum et al., Reference Kershenbaum, Cardinal, Chen, Underwood, Seyedsalehi, Lewis and Rubinsztein2020; Stroup et al., Reference Stroup, Olfson, Huang, Wall, Goldberg, Devanand and Gerhard2021) and those not adjusting for at least two confounders (n = 3) (Brodaty et al., Reference Brodaty, Sachdev, Koschera, Monk and Cullen2003; Kershenbaum et al., Reference Kershenbaum, Cardinal, Chen, Underwood, Seyedsalehi, Lewis and Rubinsztein2020; Stroup et al., Reference Stroup, Olfson, Huang, Wall, Goldberg, Devanand and Gerhard2021) did not influence the pooled estimate; pooled RR = 2.50, 95% CI (1.71–3.68), I 2 = 99.0%, p < 0.001; pooled RR = 2.50, 95% CI (1.71–3.68), I 2 = 99.0%, p < 0.001, respectively (see Table 3). We also conducted a sensitivity analysis in which the Tapiainen et al. paper was excluded due to reporting the probability of a diagnosis of schizophrenia given a dementia diagnosis, in contrast to the other studies included in the meta-analysis. In this analysis, the association between psychotic disorders and dementia was slightly stronger than the main estimate; pooled RR = 2.84, 95% CI (1.84–4.38), I 2 = 99.7%, p < 0.001.
Table 3. Meta-analysis, subgroup analysis, and sensitivity analyses of the association of non-affective psychotic disorders and dementia

a Five studies excluded due to insufficient data (Kershenbaum et al., Reference Kershenbaum, Cardinal, Chen, Underwood, Seyedsalehi, Lewis and Rubinsztein2020; Kodesh et al., Reference Kodesh, Goldberg, Rotstein, Weinstein, Reichenberg, Sandin and Levine2020; Richmond-Rakerd et al., Reference Richmond-Rakerd, D'Souza, Milne, Caspi and Moffitt2022; Stroup et al., Reference Stroup, Olfson, Huang, Wall, Goldberg, Devanand and Gerhard2021; Tapiainen et al., Reference Tapiainen, Hartikainen, Taipale, Tiihonen and Tolppanen2017).
b One study excluded due to lack of data on minimum age (Tapiainen et al., Reference Tapiainen, Hartikainen, Taipale, Tiihonen and Tolppanen2017).
c Three studies excluded due to insufficient data on proportion of females (Ribe et al., Reference Ribe, Laursen, Charles, Katon, Fenger-Grøn, Davydow and Vestergaard2015; Richmond-Rakerd et al., Reference Richmond-Rakerd, D'Souza, Milne, Caspi and Moffitt2022; Tapiainen et al., Reference Tapiainen, Hartikainen, Taipale, Tiihonen and Tolppanen2017).
Interval between incidence of psychotic disorders and dementia
For factors such as the interval between onset of non-affective psychotic disorders, and dementia diagnosis, and age-at-onset of dementia, there were insufficient data available to conduct further analyses, therefore we describe these narratively. Four studies investigated the interval between onset of a psychotic disorder and a dementia diagnosis and found that the association was stronger when the interval between the two diagnoses was shorter. Almeida et al. (Reference Almeida, Ford, Hankey, Yeap, Golledge and Flicker2018) stratified their analysis by those living with a psychotic disorder for <5 years, and ⩾10 years and found that the risk was higher for those living with a psychotic disorder for fewer years. Similarly, Stafford et al. (Reference Stafford, Dykxhoorn, Sommerlad, Dalman, Kirkbride and Howard2021) found that the strongest association was observed in the first few years following a psychotic disorder diagnosis. For both LODD and LOATP, associations were stronger at 0–6 months after receiving a psychotic disorder diagnosis compared to 12+ months (Kørner et al., Reference Kørner, Lopez, Lauritzen, Andersen and Kessing2008, Reference Kørner, Lopez, Lauritzen, Andersen and Kessing2009a).
Age-at-onset of dementia
Three studies investigated the effect of average age of dementia diagnosis. All three studies found that individuals with non-affective psychotic disorders were younger at dementia diagnosis, compared to those without (Ribe et al., Reference Ribe, Laursen, Charles, Katon, Fenger-Grøn, Davydow and Vestergaard2015; Stafford et al., Reference Stafford, Dykxhoorn, Sommerlad, Dalman, Kirkbride and Howard2021; Stroup et al., Reference Stroup, Olfson, Huang, Wall, Goldberg, Devanand and Gerhard2021). In the study by Stafford et al. (Reference Stafford, Dykxhoorn, Sommerlad, Dalman, Kirkbride and Howard2021) the median age of dementia diagnosis was lower in the VLOSLP group compared to those without VLOSLP. In the Ribe et al. (Reference Ribe, Laursen, Charles, Katon, Fenger-Grøn, Davydow and Vestergaard2015) study, dementia was diagnosed before the age of 65 for 22.4% of people with schizophrenia compared to 6.3% of people without schizophrenia, whereas in the Stroup et al. (Reference Stroup, Olfson, Huang, Wall, Goldberg, Devanand and Gerhard2021) study the prevalence of dementia in people with schizophrenia at 66 years was similar to the prevalence of dementia at 88 years for people in the general population.
Psychotic disorder diagnosis
Two studies focused on psychotic disorder subtypes other than schizophrenia or combined non-affective psychotic disorders (Kørner et al., Reference Kørner, Lopez, Lauritzen, Andersen and Kessing2008, Reference Kørner, Lopez, Lauritzen, Andersen and Kessing2009a). Both studies on LOATP and LODD reported an increased risk of dementia among people with LOATP or LODD compared to controls (SMR = 8.12, 95% CI (6.7–9.74), and IRR = 5.49, 95% CI (4.81–6.26), respectively).
Comorbidities
Two studies examined associations between psychotic disorders and dementia in relation to comorbidities (Lin et al., Reference Lin, Chung, Chen and Chi2018; Ribe et al., Reference Ribe, Laursen, Charles, Katon, Fenger-Grøn, Davydow and Vestergaard2015). One study found an increased risk of dementia for individuals diagnosed with schizophrenia with comorbid cardiovascular disease: HR = 4.27, 95% CI (3.64–4.99) v. no cardiovascular disease HR = 2.36 95% CI (1.45–3.87) (interaction p < 0.001). Associations between schizophrenia and dementia were also stronger in the presence of diabetes, hypertension, Parkinson's disease, traumatic head injury, and alcohol-related disorders (all interactions p < 0.05). Ribe et al. found that associations were stable in subgroups relating to medical comorbidities, with no evidence of differences in relation to diabetes, ischaemic heart disease, congestive heart failure, atrial fibrillation, or peripheral vascular disease. Schizophrenia showed stronger associations with dementia in those without cerebrovascular disease; IRR = 2.23, 95% CI (2.08–2.39) v. IRR = 1.71, 95% CI (1.47–2.00), p = 0.002. In contrast to the Lin et al. study, the association between schizophrenia and dementia was stronger in those without comorbid substance abuse; IRR = 1.96, 95% CI (1.82–2.11) v. IRR = 1.09, 95% CI (0.95–1.24), p < 0.001.
Anti-psychotic medication
Two studies stratified analyses by anti-psychotic medications (Kodesh et al., Reference Kodesh, Goldberg, Rotstein, Weinstein, Reichenberg, Sandin and Levine2020; Lin et al., Reference Lin, Chung, Chen and Chi2018). One study found that both first-generation anti-psychotics (FGAs) and second-generation anti-psychotics (SGAs) were associated with a lower risk of dementia in individuals with psychotic disorders compared to those not taking anti-psychotic medication (Lin et al., Reference Lin, Chung, Chen and Chi2018). However, in the second study FGAs were associated with a decreased risk of dementia across the whole sample, whereas SGAs were associated with an increased risk (Kodesh et al., Reference Kodesh, Goldberg, Rotstein, Weinstein, Reichenberg, Sandin and Levine2020).
Discussion
Summary of the evidence
To the best of our knowledge, this is the first high-quality systematic review and meta-analysis to synthesise worldwide evidence on the association between non-affective psychotic disorders and future dementia risk. Our comprehensive search identified over 9000 records and included a separate search of the grey literature. Our meta-analysis has shown that the risk of dementia in individuals with non-affective psychotic disorders is 2.52 the risk of those without a non-affective psychotic disorder. This is an important finding indicating that the risk of future dementia attributed to non-affective psychotic disorders is higher than the relative risk associated with other psychiatric disorders and future dementia, such as depression, anxiety, and PTSD (Günak et al., Reference Günak, Billings, Carratu, Marchant, Favarato and Orgeta2020; Kuring, Mathias, & Ward, Reference Kuring, Mathias and Ward2020). Importantly, our results remained robust after excluding lower-quality studies and studies that did not adjust for confounders.
A significant contribution of our review is the finding that the association between psychotic disorders and dementia was present in individuals with a range of non-affective psychotic disorders such as LODD and LOATP, and in those with typical-onset (onset <40 years) as well as late-onset psychotic disorders (onset >40 years), and VLOSLP (onset >60 years). The association was stronger, however, in those with typical and late-onset psychotic disorders, in studies following individuals for <10 years, and in studies conducted in non-European countries. Associations were also stronger in studies with a higher proportion of females and a younger minimum age at baseline. Our narrative synthesis provided some evidence that people with psychotic disorders tended to be younger at dementia diagnosis and that the association between psychotic disorders and dementia was stronger when the interval between the two diagnoses was shorter. Our findings highlight a need for further research examining whether findings vary across different dementia subtypes, for example Alzheimer's disease relative to vascular dementia, and to formally test whether the age-at-onset of dementia differs between those with and without psychotic disorders.
Mechanisms
There are several potential causal mechanisms that may explain the association between psychotic disorders and increased dementia risk. Schizophrenia has been posited to be a disorder of accelerated ageing, whereby the physiological changes of ageing associated with the disorder may occur earlier than normal, predisposing individuals to cognitive decline (Kirkpatrick, Messias, Harvey, Fernandez-Egea, & Bowie, Reference Kirkpatrick, Messias, Harvey, Fernandez-Egea and Bowie2008; Okusaga, Reference Okusaga2014). Lower educational attainment and impairments in cognitive functioning which are important markers of psychotic disorders could also lead to increased risk by limiting individuals' cognitive reserve, thereby increasing vulnerability to exhibiting dementia symptoms (Stern, Reference Stern2012). Psychotic disorders may also confer greater risk via several underlying disease mechanisms such as cardiovascular disease, and obesity, which are known risk factors for both dementia and non-affective psychotic disorders (Cohn, Prud'homme, Streiner, Kameh, & Remington, Reference Cohn, Prud'homme, Streiner, Kameh and Remington2004; Jeste, Gladsjo, Lindamer, & Lacro, Reference Jeste, Gladsjo, Lindamer and Lacro1996; Stahl, Mignon, & Meyer, Reference Stahl, Mignon and Meyer2009). People with psychotic disorders are also more likely to have a poor diet, smoke and abuse substances, which are health behaviours that may accelerate neuropathology associated with dementia (Lambert et al., Reference Lambert, Conus, Lubman, Wade, Yuen, Moritz and Schimmelmann2005; Mamakou, Thanopoulou, Gonidakis, Tentolouris, & Kontaxakis, Reference Mamakou, Thanopoulou, Gonidakis, Tentolouris and Kontaxakis2018). This remains an important area for future investigation, given that only a few of the identified studies investigated the role of comorbidities in the association between psychotic disorders and dementia, and findings were mixed overall. Examination of psychiatric comorbidities, such as depression and PTSD, was particularly lacking.
Furthermore, there is a substantial mortality gap between people with psychotic disorders and the general population (Saha, Chant, & McGrath, Reference Saha, Chant and McGrath2007). In support of our findings, a recent study which did not meet our inclusion criteria due to ascertaining dementia via cause-of-death data only, demonstrated substantial premature mortality among people with serious mental illness using primary care data, with particularly high standardised mortality ratios identified for conditions including dementia (John et al., Reference John, McGregor, Jones, Lee, Walters, Owen and Lloyd2018).
In addition, it has been posited that antipsychotic medications could contribute to an increased risk of dementia (Jonas, Abi-Dargham, & Kotov, Reference Jonas, Abi-Dargham and Kotov2021). A nationwide population-based study in South Korea found that, among older people with depressive disorders, users of atypical antipsychotics were at an increased risk of dementia (Kim et al., Reference Kim, Ha, Kim, Lee, Kim, Kim and Myung2021), and a small trial of first episode psychosis patients found a potential negative role of second-generation antipsychotics (Faber, Smid, Van Gool, Wiersma, & Van Den Bosch, Reference Faber, Smid, Van Gool, Wiersma and Van Den Bosch2012). In contrast, we identified only two studies examining the role of antipsychotic medications, which yielded mixed findings, with one study reporting a protective effect on dementia risk in people with schizophrenia, while another study found a protective effect for FGAs, but an increased risk of dementia in relation to SGAs. Given these mixed findings, this remains an important area for future research.
Although our data indicate an increased risk of dementia in non-affective psychotic disorders, it remains possible that this association could be partly explained by reverse causation, whereby psychotic symptoms could represent markers of prodromal dementia rather than causal risk factors (Ismail et al., Reference Ismail, Smith, Geda, Sultzer, Brodaty, Smith and Lyketsos2016). An important finding of our review is that, although subgroup analyses indicated stronger associations in studies with shorter follow-up periods, associations remained substantial in studies with follow-up periods longer than 10 years, and in studies including individuals diagnosed with psychotic disorders in early adulthood and mid-life. This therefore suggests that psychotic disorders could represent a causal risk factor for dementia, given that associations found with longer follow-up periods are less likely to be explained by reverse causation.
In line with this hypothesis, our subgroup analysis suggested that individuals with an earlier onset of psychotic disorders had a greater risk of dementia than individuals diagnosed with VLOSLP. People with typical and late-onset psychotic disorders are likely to have been diagnosed with a psychotic disorder many years before the symptoms of dementia begin, consistent with the possibility that psychotic disorders are a risk factor for dementia. A possible explanation for this finding is that individuals with an earlier onset have a longer exposure to the adverse effects of living with a psychotic disorder, resulting in accumulation of several negative health behaviours and medical comorbidities increasing dementia risk.
Further longitudinal studies with long follow-up periods and studies that model trajectories of association over time are required to elucidate possible mechanisms underlying the relationship between psychotic disorders and increased dementia risk (Singh-Manoux et al., Reference Singh-Manoux, Dugravot, Fournier, Abell, Ebmeier, Kivimäki and Sabia2017). In addition, triangulation across multiple study designs, including studies involving twin- and sibling-difference designs, would provide further insight into the likelihood of a causal relationship between psychotic disorders and dementia. These studies should also investigate whether factors such as medical comorbidities, health behaviours, substance abuse, educational attainment, and anti-psychotic medication may explain the association between psychotic disorders and future dementia. Understanding which lifestyle risk factors or comorbidities impact the risk of dementia for people with psychotic disorders could help develop treatments better tailored to people living with serious mental illness, helping to reduce or delay number of years living with a neurodegenerative disease.
Strengths and limitations
We used a comprehensive and highly sensitive search strategy, including a grey literature search of the worldwide evidence. Our meta-analysis included studies from several countries, most of which reported data on large sample sizes and long follow-up periods. Most of our studies were also assessed to be of high quality, which increases our confidence in the conclusions of our review. Our meta-analytic estimates are also similar to those reported in an earlier review examining the association between schizophrenia and risk of dementia (Cai & Huang, Reference Cai and Huang2018). Despite these strengths however, our study has several limitations. First, diagnosing dementia in individuals with psychotic disorders is particularly challenging. Cognitive deficits and behavioural symptoms in psychotic disorders may be misdiagnosed as dementia for some individuals, or in some cases, dementia symptoms could be misattributed as being symptoms of psychosis (Ribe et al., Reference Ribe, Laursen, Charles, Katon, Fenger-Grøn, Davydow and Vestergaard2015). It is possible therefore, that the over or underestimation of dementia in individual studies could have affected our pooled estimate. Despite our subgroup and sensitivity analyses, heterogeneity remained high, and we could not detect its source. Nonetheless, almost all included studies found evidence of an association between psychotic disorders and dementia, with variation mainly in the magnitude of observed associations.
In addition, there were insufficient data available to conduct formal subgroup analyses for all factors of interest, including interval between psychotic disorder onset and dementia diagnosis, and age-at-onset of dementia, highlighting the need for further longitudinal evidence in this area. Most studies included in our meta-analysis were retrospective register-based studies, which often lack detailed information on socio-demographic confounders (Talari & Goyal, Reference Talari and Goyal2020), and may be more prone to detection bias whereby comorbidities could be more frequently diagnosed in people with psychotic disorders due to higher levels of contact with mental health services (Viswanathan, Berkman, Dryden, & Hartling, Reference Viswanathan, Berkman, Dryden and Hartling2013). However, in contrast, we found slightly stronger associations between psychotic disorders and dementia in prospective studies, relative to retrospective studies. One possibility is that those with psychotic disorders may have poorer access to healthcare services; hence retrospective studies involving medical records may represent a healthier subset of the population with psychotic disorders who may be less likely to have comorbidities, including dementia, than those in prospective studies.
Conclusion
This systematic review and meta-analysis provides an up-to-date estimate and narrative synthesis of the association between non-affective psychotic disorders and future dementia risk. Our findings indicate that non-affective psychotic disorders constitute an important and potentially modifiable risk factor for all-cause dementia and highlight the need to revise current models of dementia prevention across the life span (Livingston et al., Reference Livingston, Huntley, Sommerlad, Ames, Ballard, Banerjee and Mukadam2020). Individuals with psychotic disorders and people who develop psychotic symptoms later in life require careful monitoring for symptoms of cognitive and functional decline. Given the robust association observed between psychotic disorders and future dementia risk, our findings should be reflected in future clinical guidelines for the treatment and care of people living with non-affective psychotic disorders.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291722002781
Data
Data contributing to the findings of this study are available on request from the study authors (SEM and JS); contact at e-mail: j.stafford@ucl.ac.uk.
Author contributions
Sara El Miniawi: conceptualisation of the review; selection of studies; data extraction; data entry; data analysis; data quality; writing, review and editing of manuscript and final draft. Vasiliki Orgeta: conceptualisation of the review, review and editing of manuscript and final draft. Jean Stafford: conceptualisation of the review; selection of studies; data extraction; data quality, review and editing of manuscript and final draft.
Financial support
This review did not receive any funding. Jean Stafford was supported by an NIHR-UCLH BRC Postdoctoral Bridging Fellowship. Jean Stafford and Vasiliki Orgeta are supported by the National Institute for Health Research Biomedical Research Centre at University College London Hospitals NHS Foundation Trust.
Conflict of interest
Jean Stafford was the principal investigator in one of the studies meeting the inclusion criteria of the review. Sara El Miniawi and Vasiliki Orgeta have nothing to disclose. There are no other known conflicts of interest.