INTRODUCTION
Prostate cancer accounts for ∼3.4% of all cancers and ranks eighth in Pakistani males,Reference Bhurgri, Kayani and Pervez1 of whom only 30% of patients are diagnosed with organ confined prostate cancer.Reference Tunio, Rafi and Maqbool2 The external beam radiation therapy (EBRT) with or without androgen deprivation therapy (ADT) is the standard treatment options for such patients along with surgery and brachytherapy. Recent studies have shown increased prostate cancer control rates with radiation dose escalation.Reference Pollack, Zagars and Smith3 The dose escalation by three-dimensional conformal radiotherapy (3D-CRT) and intensity-modulated radiotherapy (IMRT) results in better tumour control rates and reduces the acute and delayed genitourinary (GU) and gastrointestinal (GI) toxicities.Reference Nilsson, Norlen and Widmark4,Reference Zelefsky, Fuks and Happersett5 However, some of issues, for example, uncertainties regarding tumour localization, motion and setup errors may offset the advantages of 3D-CRT and IMRT.Reference Langen and Jones6
High dose brachytherapy (HDR-BT) is alternative method to deliver higher dose to the tumour as with catheters in place only insignificant tumour motion occurs during treatment.Reference Kalkner, Wahlgren and Ryberg7 Recently published trials using EBRT in combination with HDR-BT prostate boost have reported a 4-year biochemical control ranging from 86% to 97.4% and disease free survival rates ranging from 81% to 97%.Reference Chen, Chuang and Hsieh8–Reference Demanes, Rodriguez and Schour10
The authors aimed to evaluate the treatment outcomes, toxicity profile and pathological response achieved by HDR-BT prostate boost adjunct to 3D-CRT in patients with intermediate and high risk prostate cancer.
PATIENTS AND METHODS
During the period between August 2005 and July 2007, total 205 consecutive patients with histologically confirmed prostate cancer were treated with 3D-CRT alone or in combination with 192-iridium (Ir192) HDR-BT. Study protocol was approved by institutional ethical committee before patients’ accrual. All patients gave written consent for the treatment.
Pre-treatment assessment
All patients were evaluated by clinical examination, including digital rectal examination (DRE), transrectal ultrasound (TRUS) of the prostate and biopsy with Gleason score, laboratory studies [haematology, renal functions, baseline prostate-specific antigen (PSA)], bone scan and pelvic computed tomography (CT) scans.
Eligibility criteria
(i) Adenocarcinoma of the prostate stage T2–T4, according to the American Joint Committee on Cancer (AJCC), (ii) Eastern Co-operative Oncology Group (ECOG) performance scale of 0–1 and (iii) intermediate and high risk prostate cancer, according to National cancer Comprehensive Network (NCCN) stratification. Intermediate risk was defined as Stage T2b–T2c or Gleason score 7 or PSA level of 10–20 ng/mL (patients with multiple adverse factors were considered high risk). Finally, high risk was defined as T3a or Gleason score 8–10 or PSA level >20 ng/mL.
Exclusion criteria
Patients with a prior history of other malignancy (excluding basal cell carcinoma), previous pelvic radiotherapy, radical prostatectomy and evidence of metastatic nodal and bone disease were excluded from the study.
The choice of HDR-BT or not was non-randomized but mostly followed the suitability of spinal anaesthesia and patients’ own choice. The previous history of trans-urethral resection of the prostate (TURP) was not considered as contraindication for HDR-BT. The characteristics of the patients are shown in Table 1.
Table 1. The characteristics of the patients
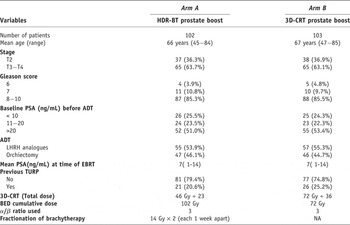
3D-CRT = Three-dimensional conformal radiation therapy, BED = biological equivalent dose, ADT = androgen deprivation therapy, LHRH = luteinizing hormone releasing hormone, EBRT = external beam radiation therapy, TURP = transurethral resection of prostate and HDR-BT = high dose rate brachytherapy.
Androgen deprivation therapy
In all patients, hormonal therapy was started 2 months prior to radiotherapy then concurrent and adjuvant in form of luteinizing-hormone releasing hormone (LHRH) analogues for 24 months. Alternatively, patients refusing LHRH analogues underwent bilateral sub-capsular orchiectomy (BSO).
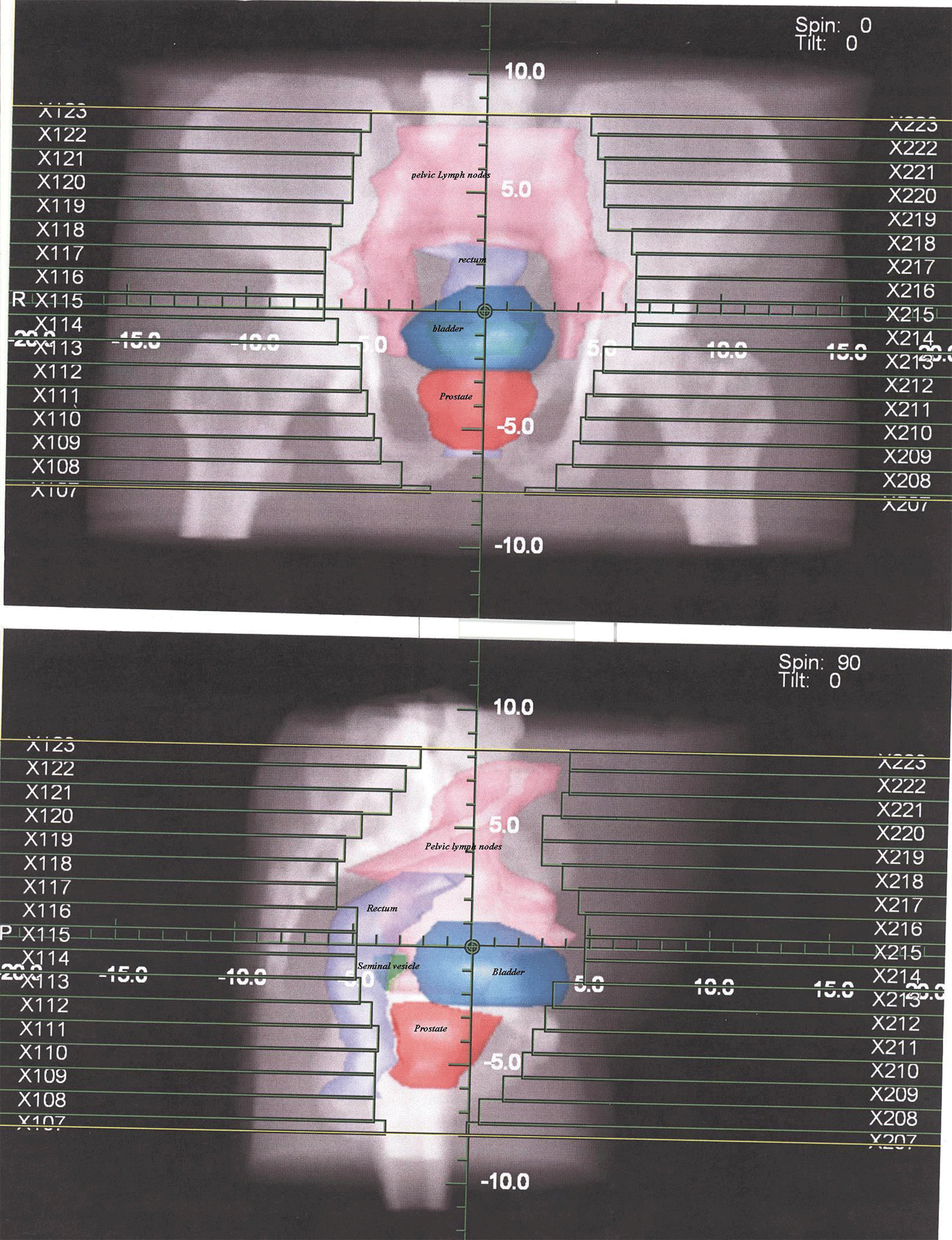
Figure 1. Digital reconstructed radiograph for anterior, posterior and lateral fields to irradiate lymph nodes in first phase.
Radiotherapy techniques
All patients underwent virtual simulation using CT scanner Emotion6 Siemens®. The prostate, seminal vesicles, bladder, rectum and small bowel were delineated. For lymph nodes mapping, the contrast-filled vessels were contoured as surrogate markers according to Radiation Therapy Oncology Group Genitourinary (RTOG-GU) Consensus.Reference Lawton, Michalski and El-Naqa11 On digital reconstructed radiographs (DRRs), the field borders in anterior–posterior were caudally at the L4–L5 interspace, 1.5 cm margin to contoured vessels laterally and caudally inferior margin of ischial tuberosities and in lateral were anteriorly 1.5 cm margin to contoured vessels and posteriorly pre-sacral lymph nodes were included up to level of S2 vertebra (Figure 1). The dose prescribed at isocenter was 46 Gy (2Gy per fraction per day). After first phase, prostate boost patients were divided into two arms.

Figure 2. The 3D-CRT prostate boost with six fields.
Prostate boost via 3D-CRT
The planning target volume (PTV) for boost included the prostate with seminal vesicles (clinical target volumes [CTVs]), with a margin of 10 mm all around and 8 mm towards the rectum. Four, six and eight conformal fields were used to deliver 26 Gy (2Gy per fraction per day) to PTV (Figure 2).
Prostate boost via HDR-BT
HDR-BT was given in two sessions, each 1 week apart. Keeping the patient in lithotomy position under spinal anaesthesia, urologists inserted catheters transperineally into the prostate guided by transrectal ultrasonography after identifying contrast-filled urethra. After catheter insertion, real-time images were obtained and all organs (prostate, urethra, bladder and rectum) were contoured with help of a radiologist, and treatment planning was done on Flexiplan version 2.2 (Isodose Control, Nucletron®, The Netherlands). Using optimized reverse planning 14 Gy at periphery of prostate in each session was prescribed. The urethral dose constraints were kept low to keep mean urethral doses < 8 Gy by reducing dwell time or zero position of radioactive source adjacent to urethra; however, efforts were made not to under dose the anterior and transition regions below 11 Gy of prescribed dose Figure 3.

Figure 3. High dose rate brachytherapy boost showing (a) isodose distribution and (b) cumulative dose–volume histogram.
Primary and secondary end points
The primary end points were PSA progression free survival (PPFS), distant metastases free survival (DMFS) and overall survival (OS) rates. Biochemical failure was defined as a 2 ng/mL increase greater than a nadir PSA (the Phoenix definition).Reference Thompson, Keyes and Pickles12 DMFS was defined as no evidence of distant metastases (negative bone scans) from end of treatment to time of analysis. Loco-regional failure was defined as positive (prostate or lymph nodes) pelvic CT or magnetic imaging resonance (MRI) in setting of biochemical failure confirmed with positive core biopsies. However, core biopsies were not done in routine fashion.
The secondary endpoints were GU and GI toxicities in both arms, prostate cancer-related mortality and histopathological response in repeat prostate biopsies after the treatment at 2 years. For acute toxicities, all patients were assessed according to Radiation Therapy Oncology Group (RTOG) criteria,Reference Syndikus, Morgan and Sydes13 from date of radiotherapy commencement to 12 weeks after the completion of radiotherapy. For scoring the delayed side effects, Late Effects Normal Tissue Task Force (LENT)-Subjective, Objective, Management, Analytic (SOMA) scale was used.Reference Livsey, Routledge and Burns14 The TRUS-guided biopsies were performed for 3D-CRT alone arm and trans-perineal biopsies were performed for HDR-BT arm to reduce any potential rectal mucosa injury as per study design protocol.
Follow-up schedule
Follow up was done till July 2010. After completion of radiotherapy, patients were planned for follow up at every 3 months for the first 2 years, then every 6 months for the next 5 years and annually thereafter. At each visit, in addition to PSA test and DRE, patients were also evaluated for any treatment-related toxicity.
Statistical analysis
PPFS, DMFS and OS rates were calculated by the Kaplan–Meier method. The association of clinical, pathological, and treatment-related variables with any given event was analysed using Cox regression for continuous variables and Fisher’s exact test (two-tailed) for categorical variables. The student’s unpaired t test was used to determine the significance of the difference between two arms. The statistical significance of differences between PPFS, DMFS and OS curves in two arms was calculated with the log-rank test. Multivariate analysis was performed using the Cox proportional hazards model. A p value of 0.05 was considered statistically significant. All time intervals were calculated from the date of completion of radiation treatment. Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS version 17.0, SPSS Inc, Chicago, IL, USA).
RESULTS
Median follow up was 3.5 years (3–5). Eight patients in 3D-CRT and HDR-BT arm (total 102 patients) and 10 patients in 3D-CRT alone arm (total 103 patients) did not come for outpatient re-assessment and were not analysed. The analysis was based on remaining 187 patients: 94 patients in 3D-CRT and HDR-BT arm and 93 patients in 3D-CRT alone arm.
Biochemical control and PPFS and histopathological response
At the median follow up of 3.5 years, total 12 patients experienced rise in PSA levels (biochemical failure): 2 in 3D-CRT with HDR-BT arm and 10 in the 3D-CRT alone arm (hazard ratio, 0.48; 95% Confidence interval [CI], 0.19–0.83; p value 0.009). The overall PPFS over time is given in Figure 4. Further, clinical local failure was seen in 2/94 patients in 3D-CRT plus HDR-BT arm and 3/93 patients in 3D-CRT alone arm. These patients were offered salvage prostatectomy (1 patient), high intensity-focused ultrasound (HIFU) (1 patient) and re-irradiation via HDR-BT (1 patient) along with second line hormonal therapy.
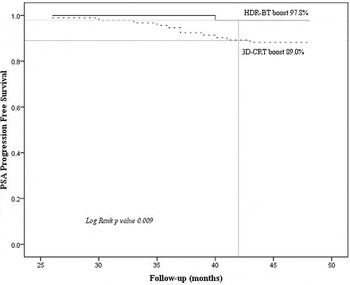
Figure 4. (a) Overall PPFS rate in both arms (3D-CRT plus HDR-BT versus 3D-CRT alone).
At follow-up period of 2 years (1.8–3), post radiotherapy, TRUS-guided or trans-perineal prostate biopsies were performed in total 32 patients. Majority of patients refused repeated prostate biopsies due to their fears for per rectal bleeding, procedure-related pain or procedure-induced dissemination of cancer. Of 32 patients who underwent repeat prostate biopsies, 17 patients were in 3D-CRT plus HDR-BT arm and 15 patients were in 3D-CRT alone arm. The negative biopsies were 14 (82.3%) and 9 (60%) in 3D-CRT plus HDR-BT arm and 3D-CRT alone arm, respectively (Odds ratio 0.4; 95% CI, 0.10–0.80 with p value 0.01) Table 2. No interventions were carried out for the patients with positive prostate biopsies as these patients were already on ADT and were without any biochemical or metastatic evident disease.
Table 2. Post-radiation prostate biopsies results according to type of prostate boost

3D-CRT = Three-dimensional conformal therapy, HDR-BT = high dose rate brachytherapy and PSA = prostate specific antigen.
Distant metastases free survival
At the median follow up of 3.5 years, total seven patients experienced bone metastasis: two in 3D-CRT with HDR-BT arm and five in the 3D-CRT alone arm (hazard ratio, 1.46; 95% CI, 0.6–2.60; p value 0.139). The DMFS rate is given in Figure 5. The earliest metastatic disease was manifested at 2.5 and 3 years in Arm A and Arm B, respectively. One patient in 3D-CRT plus HDR arm and two patients in 3D-CRT alone arm had positive CT/MRI scan for pelvic lymphadenopathy. These all patients were treated with second-line hormonal therapy and docetaxel-based chemotherapy (2 patients) along with bisphosphonates. Palliative radiotherapy was given to three patients during the course of second-line hormonal and chemotherapy (one patient for spinal cord compression at T11 level and two for pain relief (lumbar spine and sacroiliac joints).
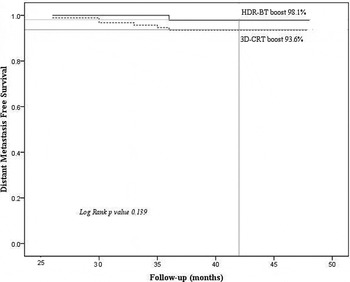
Figure 5. Distant metastasis free survival rate in both arms (3D-CRT plus HDR-BT versus 3D-CRT alone).
Overall survival
At the median follow up of 3.5years, total 24 patients died. Fourteen patients died secondary to their prostate cancer: five in 3D-CRT with HDR-BT arm and nine in the 3D-CRT alone arm (hazard ratio, 1.20; 95% CI, 0.54–2.60; p value 0.24). Ten patients died due to non-prostate cancer-related problems (six cardiac events, two cerebrovascular accident [CVA], one suicidal bomb blast and one cause not known). The OS rate is given in Figure 6.

Figure 6. The OS rate in both arms (3D-CRT plus HDR-BT versus 3D-CRT alone).
Further multivariate analysis results showed that pre-treatment risk factors (T stage, baseline PSA, Gleason score and treatment modality) were important prognostic factors on PPFS, DMFS and OS (Table 3).
Table 3. Multivariate analysis of endpoints using Cox proportional hazard model
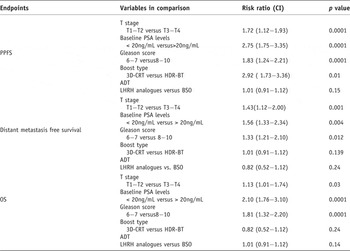
CI = confidence intervals, 3D-CRT = three-dimensional conformal radiotherapy, HDR-BT = high dose rate brachytherapy, ADT = androgen deprivation therapy, LHRH = luteinizing hormone releasing hormone, BSO = bilateral sub-capsular orchiectomy.
Toxicity profile
Overall, both treatment modalities, that is, 3D-CRT alone and 3D-CRT plus HDR-BT were well tolerated as shown in Table 4. No treatment-related death was observed.
Table 4. Acute GI and GU toxicity profile according RTOG scoring in both arms

At 3 years, the rectal symptoms were 2% and 3.9% in Arm A and Arm B, respectively (p = 0.01). Bladder-related symptoms were slightly higher in 3D-CRT alone arm (2% versus 4.8% with p value of 0.001). However, two patients (2.1%) in 3D-CRT plus HDR-BT arm developed stricture in urethra at 1 year (1–2) after completion of radiation. These patients recovered successfully after dilatation. Mean urethral volume was 0.34 cc (0.1–0.8 cc). Mean urethral D90 was 7.5 Gy (5–11). No correlation was seen between very close positions of catheters to urethral doses, as dwell position and time were kept low or nil during treatment. However there was strong correlation between acute GU toxicity grading and urethral V10, V30, V50, V80, V100, V130 and V150 (p value < 0.001).
The sexual dysfunction scoring was done only for three patients for their self-reported erectile dysfunction (one in Arm A and two in Arm B). Main reason was their religious and social issues as majority believed it is sin to discuss sexual issues to the physician (Table 5).
Table 5. Late side effects according to LENT/SOMA scale

DISCUSSION
The interim results of this study of males with prostate cancer suggest that dose escalation by 3D-CRT plus HDR-BT (102 Gy) at 3.5 years improves the biochemical control (97.8%) as compared to 3D-CRT alone (dose 72 Gy). Further HDR-BT adjunct to 3D-CRT resulted in high rates of negative prostate biopsies at 2 years and few delayed side effects. The results are similar to the findings of Western randomized trials of dose escalation of 3D-CRT with or without use of HDR-BT.Reference Dearnaley, Hall and Lawrence15–Reference Zwahlen, Andrianopoulos and Matheson18
At median follow up of 3.5 years, the authors did not see any difference in DMFS and OS (p values 0.13 and 0.24, respectively) between HDR-BT and 3D-CRT alone arms. However, DMFS and OS difference may be expected with longer follow-up time. A number of trials have reported that local failure is highly associated with distant metastasis in prostate cancer patients treated with radiotherapy.Reference Zagars, Eschenbach and Ayala19,Reference Fuks, Leibel and Wallner20 However, minimal absolute difference in reduction of distant metastasis due to dose escalation by HDR-BT was seen in the study. Multivariate subgroups analysis of this study showed that dose escalation by HDR-BT is an important prognostic factor for PPFS along with T stage, Gleason score and baseline PSA levels.
Due to lack of screening in region of study, most of the study patients were with advanced T stage and were intermediate and high risk groups according to NCCN risk stratification and were offered neoadjuvant, concomitant and adjuvant ADT to achieve local control improvement and to reduce radiation treatment volumes. Orchiectomy was kept an option for the patients with poor socio-economic status. Long-term use of LHRH analogues and their associated side effects in intermediate risk patients in the study may be criticized.
Post-radiation biopsies were performed at 2 years in the study patients to minimize the rectal injuries similar to other studies.Reference Crook, Malone and Perry21 Positive biopsies after external beam radiotherapy in patients free of any evidence of treatment failure is not synonymous with eventual recurrence but are considered strong predictive factors of subsequent disease free survival in prostate cancer patients.Reference Merrick, Butler and Galbreath22 In the study, only 17.1% patients underwent post-radiation TRUS-guided/trans-perineal prostate biopsies. Limitation to gather information on repeat prostate biopsies and methodology for obtaining the biopsies may be criticized for potential selection bias and sampling bias.
In the study, there was no significant difference in grades 3 and 4 acute rectal toxicity profile between two arms. The twofold explanation for this no difference was, first, both arms in first phase of radiation were treated with similar field sizes and radiation, and second, the PTV margins were kept 8 mm posteriorly during initial and boost phases in 3D-CRT alone arm.
On the other hand, the possible explanation for more acute GU toxicities in 3D-CRT alone arm was the position of bladder neck within PTV during boost phase. The pre-treatment and post-treatment IPSS were not collected, which could be criticized. There is need to develop comparative radiobiological models to predict normal tissue complication probabilities (NCTP) between 3D-CRT alone 3D-CRT plus HDR-BT.Reference Robertson, Sohn and Yan23
The 3D-CRT plus HDR-BT resulted in fewer Grades 3 and 4 acute and delayed GU toxicities. The urethral stricture in one patient in 3D-CRT plus HDR-BT arm was related with higher urethral dose during HDR-BT and previous TURP. The history of TURP is considered a contra-indication for HDR-BT due to higher incidence of bladder incontinence.Reference Zelefsky, Lyass and Fuks24 However, recent few trials have rejected this hypothesis.Reference Peddada, Jennings and Faricy25 No significant difference was observed in bladder toxicities, due to previous TURP. In the sexual domain, no written questionnaire was used to ask the sexual issues. During the follow up, only three patients (one in HDR-BT arm and two in 3DCRT arm) self reported erectile dysfunction at 20 months. The sildenafil (Viagra) improved the symptoms by 40–50% after 12 months its use.
Limitation of the study was the lack of randomization, shorter follow-up period and in HDR-BT group only prostate boost was given; seminal vesicles were not treated due to technical problems. However, results of this study showed encouraging results of 3D-CRT plus HDR-BT. Future studies incorporating IMRT and image guided radiation therapy plus HDR-BT are warranted.Reference Stephans, Xia and Tendulkar26
Acknowledgements
Authors are thankful to Professor Adibul Hasan Rizvi for the constant support and Razvan M Galalae from Keil, Germany for teaching the HDR-BT techniques.













