The bioavailability of a lipid nutrient present in food depends on complex physico-chemical and enzymatic processes including digestion, intestinal absorption and delivery to all organs for cell lipid requirements(Reference Embleton and Poutonb1–Reference Kubow3). Several parameters affect the lipid digestion and absorption steps. The influence of the lipid nature(Reference Ikeda, Sasaki and Yasunami4–Reference Bach and Babayan8) results, at least partly, from the pancreatic lipase activity due to the TAG structure(Reference Mu and Porsgaard7), the inherent resistance of very long PUFA to hydrolysis(Reference Ikeda, Sasaki and Yasunami4) and the more efficient hydrolysis of short- and medium-chain TAG(Reference Bach and Babayan8, Reference Marten, Pfeuffer and Schrezenmeir9). Fat digestion also depends on the supramolecular lipid structures ingested(Reference McClements and Li10), i.e. liposomes(Reference Cansell, Nacka and Combe11) and emulsified oils(Reference Armand, Pasquier and André12–Reference Garaiova, Guschina and Plummer16). In this latter case, the impact of interfacial composition of the emulsion(Reference Nishimukai, Hara and Aoyama13, Reference Mun, Decker and McClements17, Reference Sandra, Decker and McClements18), as well as the influence of the droplet size of the emulsified lipid phase(Reference Armand, Pasquier and André12–Reference Michaslki, Soares and Lopez15), should be taken into account. More recently, it has been demonstrated that the physical state of the TAG also influences the rate and extent of lipid digestion(Reference McClements and Li10, Reference Bonnaire, Sandra and Helgason19).
In this context, the aim of the present study was to measure the intestinal absorption of α-linolenic acid (ALA) in rats after the administration of flaxseed oil in both emulsified and non-emulsified states. Flaxseed oil was chosen because of its high ALA content. The current French dietary guidelines recommend a daily intake of at least 1 g of n-3 ALA for adults, while keeping the linoleic acid:ALA ratio lower than 5(Reference Legrand, Astorg and Bougnoux20). By comparison, the WHO advises an n-3 PUFA intake between 0·8 and 1·1 g of ALA/d, and the US Dietary Guidelines recommend 1·1 g for women and 1·6 g for men. Linoleic acid and ALA are usually consumed in a ratio ranging from 10 to 15 in France, thus making ALA supplementation necessary(Reference Astorg, Arnault and Czernichow21, Reference Combe and Boué22). Among various vegetable sources, flaxseed oil nowadays provides the highest amount of ALA (40–45 wt % of total fatty acids). Several studies have shown the potential to increase the ALA plasma level through flaxseed oil ingestion(Reference Cunnane, Ganguli and Menard23–Reference Bloedon, Balikai and Chittams26). As a result, in July 2006, the French agency for food, environmental and occupational health safety authorised the introduction of flaxseed oil in foods(27). Recently, flaxseed oil has been marketed as virgin oil(28). With the aim of probing how lipid bioavailability depends on its dispersion state, rats were fed flaxseed oil either in a bulk phase or an oil-in-water (O/W) emulsion. Soya lecithin was used to stabilise the oil interface. The present study was carried out to determine whether the emulsified state of the lipids influenced ALA lymphatic absorption and, more generally, its bioavailability. In addition, the emulsion stability was characterised under conditions that mimicked those of the gastrointestinal tract in terms of pH variation, enzyme lipolysis and bile salt (BS) solubilisation.
Experimental methods
Materials
Flaxseed oil was supplied by ITERG (Pessac, France). Soya lecithin (Lecimulthin) was kindly provided by Cargill (Baupte, France). The lecithin composition was determined after the separation of different phospholipid (PL) species by two-dimensional TLC using the following solvents: in the first direction, chloroform–acetone–methanol–acetic acid–water (50:20:10:10:5, by vol.), and in the second direction, butanol–acetic acid–water (60:20:20, by vol.)(Reference Wolff, Combe and Entressangles29). Various diacyl PL and lysophospholipid classes were visualised by exposing the plates to iodine vapour. Different spots were scraped and analysed for total P determination according to the method used by Ames(Reference Ames30). PL composition of the lecithin is reported in Table 1. Phospholipase A2 from Naja mossambica mossambica, lipase from porcine pancreas, sodium deoxycholate and porcine bile extract were obtained from Sigma (Sigma, St Louis MO, USA). Porcine bile extract was purified from coloured pigments on activated charcoal, as described previously(Reference Nacka, Cansell and Entressangles31). The solvents were of analytical grade.
Table 1 Main phospholipid species of the lecithin

Emulsion preparation and characterisation
O/W emulsions were prepared at room temperature. The oil phase contained 1 g of sodium deoxycholate and 20 g of lecithin per 100 g of oil. This oil phase was manually dispersed into the aqueous phase to an oil fraction of 45 g/100 g. The coarse O/W emulsions obtained were then sheared using an Ultraturax apparatus (IKA, Staufen, Germany), equipped with a generator axis (10 mm S25-N-10G; IKA, Staufen, Germany) under a nitrogen flux to prevent lipid oxidation. Direct visualisation of the oil droplets just after preparation and under acid stress was carried out using a phase-contrast microscope (Axiovert 135 with a water immersion, 100 × objective; Zeiss, Germany). Mean particle diameter (as evaluated by the volume-weighted average diameter d 4,3) and particle size distribution were determined by static light scattering using a Coulter LS 230 apparatus (Brea, CA, USA).
O/W emulsions were exposed to acidic conditions (pH 1·5) using 10 m-HCl. The emulsions were incubated at acid pH, from 0 to 3 h, at 37°C and analysed regularly. Neutralisation was carried out by the addition of small volumes of 10 m-NaOH solution in emulsions previously stored 2 h under acidic conditions at 37°C. All experiments were performed under stirring.
Lipid hydrolysis by pancreatic lipase
The time course of lipase-catalysed hydrolysis of both flaxseed oil and O/W emulsions was monitored using a pH-stat (Titroline alpha plus TA10 plus; SCHOTT Instrument, Mainz, Germany) on the basis of protocols described in Hope & Theimer(Reference Hope and Theimer32). Experiments were performed in a thermostated bath at 37°C, and at a pH of 8, using a 0·5 m-NaOH solution for automatic titration. In the case of flaxseed oil, an emulsion was prepared by mixing 40 ml of methocel (3·5 %), 0·6 m-NaCl solution and 25 ml of oil, under stirring. The hydrolysis reaction was initiated by mixing 10 ml of the previous emulsion with 10 mg of pancreatic lipase in the presence of 2 m-CaCl2. In the case of O/W emulsions, the lipase solution was directly added. In another set of experiments, a colipase solution (1 mg/ml) was also added to the reaction medium. Lipase activity was calculated from the initial slope of the titration curve and expressed as milliequivalents of fatty acids released per min and per g of enzyme extract (mEq/min per g of enzyme extract). The amount of enzyme and the oil and emulsion concentrations used were chosen to ensure substrate-saturating conditions. In both cases, assays were performed in triplicate.
Lipid solubilisation by bile salts
Lipid micellisation was performed by BS addition to the hydrolysis products obtained from flaxseed oil in both emulsified and non-emulsified (bulk) states. In the case of bulk flaxseed oil, NEFA and monoacylglycerols (MAG) resulting from pancreatic lipase hydrolysis were mixed with BS. In the case of emulsified flaxseed oil, the PL used to stabilise the emulsion were first subjected to phospholipase A2 hydrolysis (19 units/ml of emulsion) before pancreatic lipase addition. In this instance, NEFA and MAG from lipase hydrolysis, and NEFA and lysophospholipids from phospholipase A2 hydrolysis were solubilised.
The lipid–BS mixtures were prepared by adding 10 ml of a concentrated BS solution (concentrations ranging from 5 to 70 mm, considering an average molecular weight for BS of 480 g/mol) to the hydrolysed lipids (ranging from 3 to 50 mg, i.e. concentrations ranging from 0·5 to 5 mm, considering an average molecular weight for both TAG and PL of 775 g/mol). Micellisation by BS was followed by measuring the turbidity at 400 nm. Turbidity measurements (optical density (OD) at 400 nm) were performed in a thermostated cell support using a Perkin Elmer lambda Bio 20 spectrophotometer (Waltham, MA, USA). After stirring, the mixtures were allowed to equilibrate until stable turbidity values were obtained. For each lipid concentration ([lip]tot), a solubilisation curve was obtained by plotting the evolution of OD as a function of total BS concentration ([BS]tot). The solubilisation point, corresponding to the BS amount required to completely solubilise the lipids into mixed micelles, was determined as the point at which further addition of BS only slightly affected the suspension turbidity. In practice, this point corresponded to an OD approximately equal to zero, as in Lichtenberg(Reference Lichtenberg33). At this solubilisation point, the concentration of BS molecules not associated with the lipids ([BS]bulk) and the molecular ratio of BS to lipid in the mixed micelles ([BS:lip]mic) were determined by the following equation(Reference Paternostre, Roux and Rigaud34):
Using linear regression analysis, [BS]bulk was deduced from the intercept of the extrapolated curve with the ordinate axis, and [BS:lip]mic was deduced from the slope. Solubilisation was performed at 25 and 37°C on hydrolysis products derived from flaxseed oil in the bulk and emulsified states.
Animals, surgical procedures and lymph analysis
Male Wistar rats (8 weeks old, body weight 300–350 g) were obtained from Elevage Janvier (St-Berthevin, France) and were randomly assigned to one of the dietary groups. The study was conducted in accordance with European Community Council Directives (861609/EEC). All experiments conformed to the Guidelines for the Handling and Training of Laboratory Animals. Rats were housed for at least 3 d before the experiment in a controlled environment, with constant temperature and humidity, and with free water and food access. Two lipid formulas based on flaxseed oil were used for the experiments (Table 2). Rats were fed a fat-free diet (Epinay, France) and had free access to water 24 h before the surgery. For collecting the lymph, a polyethylene catheter (inner diameter 0·86 mm, outer diameter 1·27 mm; Biotrol, Paris, France) was inserted into the main mesenteric lymph duct of each rat placed under ketamine/xylazine anaesthesia (100 and 10 μg/g of body weight), as described in Bollman et al. (Reference Bollman, Cain and Grindlay35) and Combe et al. (Reference Combe, Constantin and Entressangles36). After the surgery, rats were placed in individual restraining cages, in a warm environment, with tap water freely available. A few hours later, 0·3 g of flaxseed oil, either as bulk oil or O/W emulsion, were administered through a gastric feeding tube, followed by 0·5 ml of water. The amounts of total fatty acids and ALA per rat were, for the bulk-phase group, 230 and 103 mg, respectively, and, for the emulsion group, 260 and 104 mg, respectively. Lymph was collected for 24 h with fractionation in tared tubes maintained in an ice bath. During the collection period, the lymph flow averaged 0·30 (sem 0·03) ml/h. The total lipid contents of chylomicrons were immediately analysed. At least eight cannulated rats were used for each studied lipid ingestion condition.
Table 2 Main fatty acid profile of the dietary lipids and their intramolecular distribution in TAG
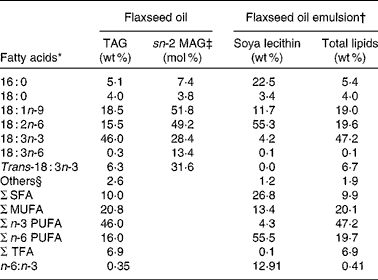
sn-2 MAG, sn-2 monoacylglycerol; TFA, trans-fatty acid.
* Fatty acid composition represents the mean of two measurements.
† Flaxseed oil was emulsified using 8 % soya lecithin.
‡ The fatty acid composition of sn-2 MAG of flaxseed oil TAG was determined after pancreatic lipase hydrolysis followed by isolation and analysis of monoacylglycerols. Results are expressed in mol% corresponding to the distribution of each fatty acid in the internal position of TAG.
§ Others represent the sum of the fatty acids that each contributes to < 1 g/100 g.
Lipids from lymph samples were transmethylated according to the method of Lepage & Roy(Reference Lepage and Roy37). Trimyristolein was added as an internal standard for TAG fatty acid quantification. Fatty acid methyl esters were analysed by GC on a BPX 70 capillary column (60 m long, 0·25 μm film, 0·25 mm inner diameter (SGE, Victoria, Australia), H2 as a carrier gas and split ratio of 1:80). The GC system consisted of a gas chromatograph (HP 4890; Hewlett Packard, Palo Alto, CA, USA) equipped with a flame ionisation detector maintained at 280°C. The injector temperature was 250°C. The column temperature was increased from 150 to 200°C (1·3°C/min), maintained at 200°C for 20 min, increased from 200 to 235°C (10°C/min) and held at 235°C for 20 min. Data were collected and integrated by a Chromjet SP 4400 integration system (Spectra-Physics, Irvine, CA, USA). Fatty acids from Sigma France (St Quentin Fallavier, France) and natural extracts of known composition were used as standards for column calibration. The variation in peak area between injections was less than 2 %.
The intramolecular fatty acid distribution in TAG of dietary flaxseed oil and lymph chylomicrons was determined through lipase hydrolysis according to Desnuelle(Reference Desnuelle38) and Entressangles et al. (Reference Entressangles, Sari and Desnuelle39). The resulting 2-MAG and 1,3-diacylglycerols were separated by TLC using hexane–diethyl ether–formic acid (70:30:1, by vol.) as a developing solvent(Reference Entressangles, Sari and Desnuelle39). Respective fractions were transmethylated, and fatty acid methyl esters were analysed by GC, as described previously. The proportion of ALA in the sn-2 position of TAG was obtained by the following equation:
Statistical analysis
Data are expressed as means with their standard errors. When only two independent groups of data were compared (solubilisation data and kinetic study), the parametric Student's t test was used. The area under the curve was calculated according to the trapezoidal method, and the data were compared with the Mann–Whitney test. Differences were considered significant at P < 0·05. The statistical significance of differences in the fatty acid compositions of chylomicron TAG between the three dietary conditions (fasted, bulk flaxseed oil and emulsion) was analysed by one-way ANOVA. V max values obtained with different hydrolysis conditions were analysed by two-way ANOVA. These analyses included Dunnett's multiple comparison procedure and Tukey's honestly significant difference procedure. Only when two of the above tests showed significance at the P < 0·05 level were the differences judged to be significant.
Results
Emulsion behaviour under acid conditions
The O/W emulsions were characterised in terms of droplet size distribution. The mean diameter of the droplets was found to be 4·9 μm, with a distribution ranging from 0·8 to 15 μm as confirmed by optical microscopy (Fig. 1(a)). When the medium was acidified (pH 1·5) in order to mimic gastrointestinal tract conditions, the mean diameter increased up to 12·2 μm because of droplet coalescence (Fig. 1(b)). Some phase separation was observed under this acidic condition during a 3 h storage period. In the physiological digestion process, emulsions stayed for approximately 2–3 h under acidic conditions (comparable to the human stomach) before returning to a neutral environment (comparable to the human intestine). To mimic this shift in pH, an emulsion sample previously stored at pH 1·5 for 2 h at 37°C was further neutralised (pH 7·3). This resulted in a slight increase in the mean diameter, although no phase separation was observed in the sample (Fig. 1(c)). The size distribution did not vary for at least 24 h (results not shown).

Fig. 1 Microscopy observation (100 × ) of flaxseed emulsions stabilised by soya lecithin: (a) just after preparation; (b) in acid conditions (pH 1·5, 10 min, 37°C); (c) in neutral conditions (pH 7·3) after an incubation of 2 h at pH 1·5 (37°C).
Lipid solubilisation by bile salts
To mimic lipid transfer from the stomach to the intestine, bulk oil and emulsions were exposed to pancreatic lipase with or without phospholipase A2 before BS addition. The solubilisation process was followed by variations in turbidity measured as a function of BS addition. Almost zero turbidity levels corresponding to mixed micelles at equilibrium were obtained after 36 h (results not shown), indicating that equilibrium was slowly reached when BS were added to the digestion products of either bulk oil or emulsions. Fig. 2 presents typical solubilisation curves obtained for various lipid concentrations in emulsions. Lipid micellisation was achieved when a drastic decrease in OD was observed. The complex evolutions of the OD suggested that the lipid–surfactant structures underwent size and/or shape variations, as already reported for the solubilisation of other colloidal structures such as liposomes(Reference Paternostre, Roux and Rigaud34, Reference Lichtenberg, Zilberman and Greenzaid40, Reference Walter, Vinson and Kaplun41). Varying the lipid concentration made it possible to calculate two parameters that fully describe the solubilisation process, i.e. [BS]bulk and [BS:lip]mic. The results for mixed micelles at equilibrium are reported in Table 3. Irrespective of the initial lipid system and/or of the temperature, [BS]bulk values were well above the critical micellar concentration of the main bile acids present in bile (11 mm for cholic acid and 3 mm for deoxycholic acid)(Reference Hofmann and Mysels42), suggesting that, at the micellisation point and thereafter, mixed lipid–BS micelles and pure BS micelles coexisted. The composition of mixed micelles consisting of fatty acids, MAG and BS was hardly influenced by an increase in temperature up to 37°C. This may be related to the fact that, at 25°C, acyl chains were already in the liquid state. In the present study, increasing the temperature from 25 to 37°C may only modify the solubility of BS in the aqueous phase and/or the partition of the surfactant between the aqueous phase and the micelles. In the case of flaxseed emulsions, the resulting mixed micelles contained lysophospholipids in addition to fatty acids, MAG and BS. At 25°C, lysophospholipids significantly decreased the [BS:lip]mic value due to their surface-active properties (Table 3). Increasing the temperature from 25 to 37°C led to a significant increase in the [BS:lip]mic value (Table 3). This suggested a lower solubility of BS monomers at the physiological temperature. No phase transition of lysophospholipids occurred because they were already in a liquid-crystalline phase at 25°C. Increasing the temperature may lead to the coexistence of different types of micelles. Indeed, with regard to lecithin/BS-mixed micelles, simple BS micelles may coexist in varying proportions with mixed micelles, depending on the type of BS, the [lecithin]:[BS] ratio and the temperature(Reference Mazer, Benedek and Carey43).

Fig. 2 Dependence of turbidity on the bile salt concentration in equilibrated NEFA–lysophospholipid–monoacylglycerol–bile salt-mixed dispersions containing constant lipid levels and varying levels of bile salts. The samples were made by a series of dilutions of the lipid aggregates after phospholipase A2 followed by pancreatic lipase hydrolysis with various bile salt solutions ([lip]tot = 4·2 (♦), 2·1 (□), 1·0 (▲) and 0·5 (○) mm). [lip]tot, lipid concentration. OD, optical density.
Table 3 Bile salt:lipid molecular ratio in mixed micelles ([BS:lip]mic) at equilibrium and the corresponding bile salt concentration in the continuum medium ([BS]bulk) as a function of temperature and the initial lipid system (oil and emulsion)
(Mean values with their standard errors)*
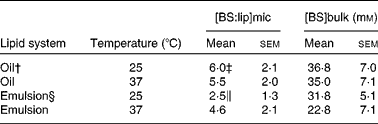
* Values represent the mean of at least five independent experiments for each lipid system (oil and emulsion) and temperature (25 and 37°C).
† Oil was subjected to pancreatic lipase before bile salt addition.
‡ Mean values were significantly different from those of the emulsion system (P < 0·05).
§ Emulsion was subjected to phospholipase A2 and pancreatic lipase before bile salt addition.
∥ Mean values were significantly different from the experiment performed at 37°C (P < 0·05).
Oil and emulsion hydrolysis by pancreatic lipase
Pancreatic lipase catalyses the hydrolysis of sn-1 and sn-3 fatty acyl ester bonds of TAG to produce 2-MAG and fatty acids. The catalytic behaviour was studied on bulk oil and emulsions stabilised by lecithin. All experiments were performed with a lipid:enzyme ratio greater than 105 that ensured a saturating concentration of substrate (results not shown). Under these conditions, the initial rate per g of enzyme extract corresponding to the maximal activity of the enzyme (V max) was calculated from curves (Fig. 3, inset). The V max value obtained with bulk oil (2·2 (sem 0·7) mEq/min per g of enzyme extract) was significantly higher than that measured in the emulsion (0·7 (sem 0·2) mEq/min per g of enzyme extract; P < 0·05). The addition of colipase to this emulsified system did not increase lipase activity, suggesting that, in vitro, colipase could not prevent pancreatic lipase inhibition by PL (Fig. 3).

Fig. 3 Time course for a typical hydrolysis of oil by pancreatic lipase (—), of oil-in-water emulsion stabilised by lecithin by pancreatic lipase (– –) and by pancreatic lipase and colipase (……). Inset: the total velocity (V max) was determined as extrapolation of the linear line to zero abscissa. All experiments were performed at least five times. * Mean values were significantly different at a time point (P < 0·05).
Lymphatic recovery of α-linolenic acid
Because lymph flow did not show any significant difference between rats throughout the kinetic study, the two treated groups were compared with regard to fatty acid concentration in the lymph. At 24 h after feeding, the total fatty acid concentration in the lymph was twice as high in the emulsion group as in the oil group (32·5 v. 14·5 mg/ml of lymph; P < 0·001). This tendency was also observed for ALA (8·0 mg/ml of lymph in the emulsion group, compared with 3·5 mg/ml of lymph in the oil group; P < 0·05). The rate and total extent of ALA absorption at the intestinal site are illustrated in Fig. 4 for up to 6 h after feeding. The rate of ALA absorption was similar for both groups in the first 2 h. After 3 h, ALA recovery in the lymph of the emulsion group was significantly higher than that of the bulk-phase group (14·0 v. 6·5 mg/ml, respectively). Moreover, the maximum ALA concentration (C max) was obtained sooner, and to a significantly greater extent in the emulsion group compared with the bulk-phase group (Table 4). Likewise, the area under the curve that estimates the intestinal bioavailability of ALA was significantly higher for rats fed emulsified flaxseed oil (Fig. 4, inset; Table 4).
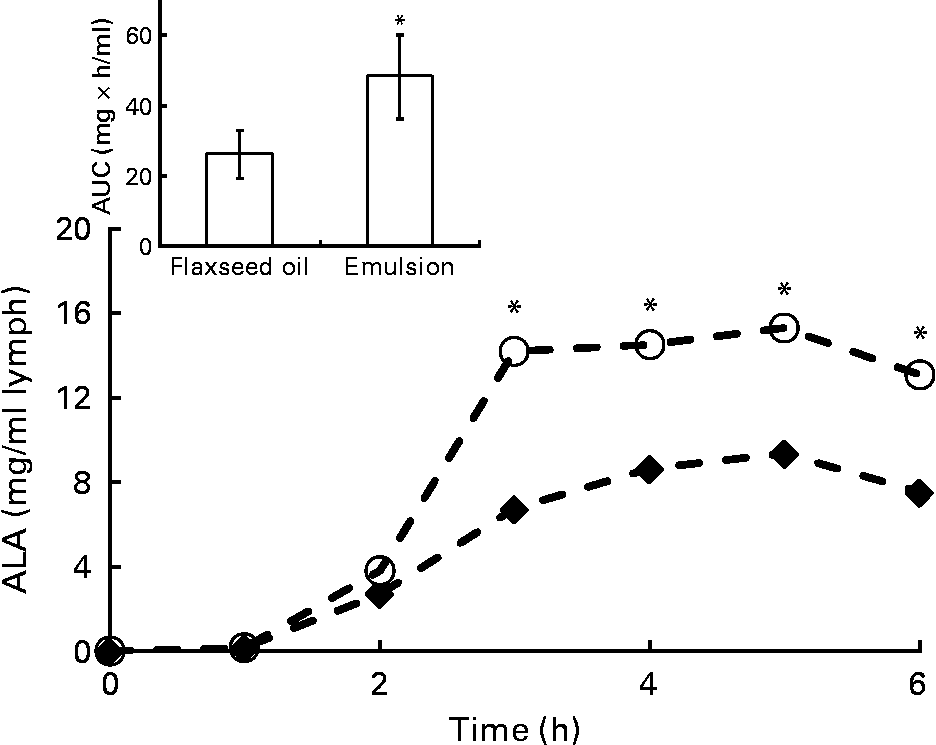
Fig. 4 Time-course concentration of α-linolenic acid (ALA) in chylomicrons for the oil (♦) and emulsion (○) rat groups. Values are means of at least eight rats for each lipid system, at each time point. * Mean values were significantly different at a time point (P < 0·05). Inset represents the area under the curve (AUC) for the two rat groups.
Table 4 Maximum α-linolenic acid concentration (C max), time required to reach C max (T max) and area under the curve (AUC) in the lymph of rats fed flaxseed oil in the bulk phase or in the oil-in-water emulsion
(Mean values with their standard errors, n 8)
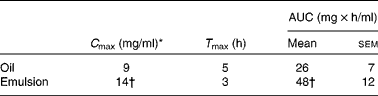
* Lipid ingestion by rats corresponded to 0·3 g in the form of flaxseed oil in the bulk phase or emulsified. Chylomicrons were collected for 24 h from eight animals, and the lipid fractions were extracted. Following separation by TLC, TAG was analysed for fatty acid composition by GC.
† Mean values were significantly different (P < 0·05).
Fatty acid composition of flaxseed oil and the intramolecular fatty acid distribution in TAG are shown in Table 2. In bulk flaxseed oil, ALA, the major fatty acid, was evenly distributed between the three positions of the TAG molecules. The two other main fatty acids, oleic acid (18 : 1) and linoleic acid (18 : 2), were mainly esterified in the internal position. The fatty acid composition of lymphatic chylomicron TAG and their intramolecular fatty acid distribution following administration of flaxseed oil as a bulk phase or as an emulsion were examined (Table 5). The fatty acid composition of chylomicron TAG of both rat groups mainly reflected the fatty acid profile of the dietary lipid source. As expected, feeding rats with bulk flaxseed oil or with an emulsion increased the proportion of ALA in the lymph compared with fasted rats. A significant decrease in the proportion of endogenous fatty acids, palmitic acid (16 : 0) and arachidonic acid (20 : 4), was observed in the lymph, mainly due to a dilution of these fatty acids in TAG as a consequence of their low proportions of dietary lipids. The proportion of ALA in the sn-2 position of chylomicron TAG was similar in the three dietary groups (Table 5).
Table 5 Main fatty acid composition and distribution of chylomicron TAG in the rat lymph resulting from oil in the bulk phase or oil-in-water (O/W) emulsion ingestion 24 h after feeding
(Mean values with their standard errors, n 8)

sn-2 MAG, sn-2 monoacylglycerol; TFA, trans-fatty acid.
a,b Mean values within a row with unlike superscript letters were significantly different for lymphatic fatty acid wt % (P < 0·05).
* Lipid ingestion by rats corresponded to 0·3 g in the form of flaxseed oil in the bulk phase or emulsified. Chylomicrons were collected for 24 h from eight animals, and the lipid fractions were extracted. Following separation by TLC, TAG was analysed for fatty acid composition by GC.
† The data represent the average of three different determinations.
‡ The fatty acid composition of sn-2 MAG of flaxseed oil and chylomicron TAG was determined after pancreatic lipase hydrolysis followed by isolation and analysis of MAG. The sn-2 MAG analysis was performed on pooled samples of the lymph of eight rats. Results are expressed in mol % corresponding to the distribution of each fatty acid in the internal position of TAG.
Discussion
Among various vegetable oils, flaxseed oil is one of the richest sources of ALA. Several studies in human subjects have already demonstrated that flaxseed oil intake leads to an increase in ALA levels in the plasma(Reference Cunnane, Ganguli and Menard23, Reference Austria, Richard and Chahine25) and an enrichment in ALA of erythrocyte total PL(Reference Cunnane, Ganguli and Menard23, Reference Barcelo-Coblijn, Murphy and Othman44). Moreover, flaxseed oil consumption has been associated with significant health benefits thanks to an improvement of biomarkers of cardiovascular risk(Reference Cunnane, Ganguli and Menard23, Reference Bloedon, Balikai and Chittams26, Reference Harper, Edward and Jacobson45) and of inflammation(Reference Wallace, Miles and Calder24, Reference Bloedon, Balikai and Chittams26). On the one hand, basic information about ALA bioavailability from flaxseed oil is still unavailable in order to understand its biological efficiency. On the other hand, improving the bioaccessibility of ALA from the food matrix is a potential strategy for providing additional n-3 PUFA.
In the present study, we compared the metabolic fate of flaxseed oil delivered either as a bulk phase or as an emulsion on lymphatic absorption. We clearly demonstrated that TAG were more efficiently absorbed when provided as an emulsion rather than as a bulk phase. As a result, fatty acid and ALA enrichment in chylomicrons was greater (C max) and faster (T max) in rats fed emulsified oil than in rats fed bulk oil. These results are in agreement with others studies performed either on animal models(Reference Nishimukai, Hara and Aoyama13, Reference Laugerette, Vors and Géloën46) or on human subjects(Reference Garaiova, Guschina and Plummer16). These studies have suggested that PL from egg phosphatidylcholine(Reference Nishimukai, Hara and Aoyama13, Reference Jiang, Noh and Koo47) or soya phosphatidylcholine(Reference Nishimukai, Hara and Aoyama13) could enhance lipid intestinal absorption in rats. Nishimukai et al. (Reference Nishimukai, Hara and Aoyama13) have also shown that the enhancement of lipid output in the lymph was highly related to the TAG:PL ratio used in the dietary formulation.
The digestion process implies a hydrolysis step by lipolytic enzymes including pancreatic lipase that operates at the oil–water interface. Both the nature of the lipids present at this interface and the curvature of the interface could modulate enzyme activity. Indeed, studies based on in vitro digestion of emulsified lipids coated with various emulsifiers have shown that PL facilitated access to emulsified fats compared with other non-ionic surfactants(Reference Singh, Ye and Horne2, Reference Mun, Decker and McClements17). Above and beyond the interfacial composition, the average oil droplet size also influences lipase activity. Studies have demonstrated that fine emulsions ( < 1 μm) were hydrolysed by pancreatic lipase faster than coarse emulsions (>20 μm) in vitro (Reference Borel, Armand and Ythier48) in both animals(Reference Borel, Armand and Ythier49) and human subjects(Reference Armand, Pasquier and André12, Reference Armand50, Reference Carrière, Renou and Lopez51). In the present in vitro study, we first observed the behaviour of the emulsion provided to rats in gastrointestinal-like conditions. Results showed that the emulsified state was maintained even with large variations in pH (Fig. 1), suggesting that in vivo, the lipid–water interface may be preserved up to the intestine level. Thus, TAG of the emulsified oil may be hydrolysed faster by pancreatic lipase, resulting in an increased absorption of hydrolysed products. In contrast, in the case of flaxseed oil in the bulk phase, the interface must be created by the mechanical mixing in the stomach and intestine. Nevertheless, the in vitro hydrolysis of emulsified oil by pancreatic lipase showed that lipase activity was lower than that in the oil as a bulk phase (Fig. 3). This is in agreement with previous in vitro studies showing that long-chain TAG emulsified with PL were not hydrolysed by pancreatic lipase, even in the presence of BS and colipase(Reference Borgström52–Reference Larsson and Erlanson-Albertsson55). However, it has been reported that this inhibition by PL disappeared due to the presence of NEFA generated by TAG hydrolysis(Reference Patton and Carey54, Reference Larsson and Erlanson-Albertsson55) that modified the interface properties. The efficiency of intestinal absorption is also determined by the solubilisation of hydrolysis products into BS-mixed micelles. In vitro micellisation experiments involving flaxseed oil (both emulsified and non-emulsified) demonstrated that a lower amount of surfactants was required to produce mixed micelles with the emulsion system (Table 3). In the case of rats that were fed emulsion, the additional amount of lysophospholipids indirectly provided by the dietary lipid formulation may facilitate the transport of hydrolysis products through the unstirred water layer of enterocytes. The present in vivo results suggested that the emulsification of flaxseed oil enhances its digestibility, due to the faster hydrolysis of TAG because of the pre-existing oil–water interface and a better solubilisation of hydrolysis products in mixed micelles. Consequently, emulsions may be less prone to oxidative degradation and may reside less long in the intestinal lumen, leading to a reduction of the extent to which they are conveyed to faeces. Besides parameters influencing lipid bioavailability in the intestinal lumen, it is worth noting that lipid recovery in the lymph may also be affected by the processes occurring in the enterocytes, i.e. uptake into the mucosal cells, as well as the packaging and secretion of chylomicrons. The supply of dietary phosphatidylcholine may favour the formation of chylomicrons(Reference Nishimukai and Hara56) and/or be involved in the regulation of jejuna apo A-I synthesis in animals(Reference Wang, Du and Lu57). Indeed, Nishimukai & Hara(Reference Nishimukai and Hara56) have demonstrated that the amount of TAG in the rat lymph increased twofold in the presence of soya lecithin.
Several studies have pointed out that the fatty acid composition of chylomicrons and fatty acid distribution in TAG reflected that of the dietary oil(Reference Lambert, Botham and Mayes58–Reference Yoshida, Mawatari and Ikeda60). It is well established that fatty acids esterified at the sn-2 position of dietary TAG are mainly retained during the absorption process(Reference Hunter61) due to the positional specificity of pancreatic lipase. After incorporation of these hydrolysis products into the mucosal pool, most of the 2-MAG are reacylated to TAG that are incorporated into chylomicrons secreted into the lymph. To our knowledge, no similar studies have been performed with flaxseed oil. ALA enrichment in chylomicrons was observed irrespective of the dietary form of flaxseed oil (Table 5). However, the percentage of ALA esterified at the sn-2 position of chylomicron TAG was slightly lower (18 and 23 % for oil and emulsion, respectively) compared with that in dietary flaxseed oil (28 %). This may be attributed to a degradation of some 2-monolinolenate glycerols(Reference Christensen and Hoy62). Indeed, chylomicron TAG contained a high percentage of endogenous fatty acids, which were supplied by bile lipids, especially 16 : 0, 18 : 1 and 18 : 2 fatty acids(Reference Shrivastava, Redgrave and Simmonds63, Reference Baxter64).
On the whole, the present results showed that the extent of fatty acid absorption, and especially of ALA, was significantly higher in the rat group ingesting emulsified oil compared with the group given oil in the non-emulsified state. Moreover, the results of the in vitro studies dealing with emulsion stability, lipid hydrolysis and solubilisation were used to interpret, at least partly, the increased lymphatic concentration in ALA in the newly synthesised TAG. Nevertheless, basic information on ALA bioavailability from flaxseed oil is still necessary to understand its biological efficiency. In particular, because the intramolecular fatty acid distribution in chylomicron TAG did not exactly reflect that of the dietary oil, the metabolic pathway of ALA during the digestion process remains to be further explored.
Acknowledgements
The authors acknowledge the National Association of Technical Research and the Aquitaine Regional Council for their financial support through a PhD research grant for L. C. The authors state that there are no conflicts of interest. Contribution made by each author to the research is as follows: L. C. is a PhD student who contributed to the design of the in vitro and in vivo experiments. C. B.-V. is the industrial PhD supervisor, specialist in lipid metabolism. L. F., E. M. and S. D. provided technical assistance for the lipid analysis. N. C. is an expert in lipid metabolism who helped to interpret the in vivo results. M. C. is the institutional PhD supervisor and is an expert in the formulation of colloidal systems for nutritional applications and in lipid bioavailability.











