The growing epidemic of obesity, which is also observed among the poor in developing countries, such as Brazil, has led researchers to question the true causes of this weight gain for the purpose of implementing effective prevention and treatment strategies( Reference Victora, Adair and Queda 1 – Reference Oddo, Rah and Semba 3 ). Much of the disease burden in adulthood, which implies damage to human development and national economies, can partly be prevented by lifestyle changes, such as diet and physical activity( Reference Gardner and Rhodes 4 – Reference Clemente, Santos and Martins 7 ). Obesity is often associated with poor dietary habits; therefore, recent studies have investigated the relationship between increased consumption of processed foods and weight gain( Reference Pereira 8 – Reference Larsson, Akesson and Wolk 10 ).
Using this rationale, it is likely that the increased incidence of obesity in the primitive populations of the Americas and Canada is associated with changes in their eating habits( Reference Murphy, Schraer and Thiele 11 ). In the past, these people consumed natural foods such as fish, meat, wild plants and fruits. However, in the course of time, they transitioned to the contemporary eating pattern, which is based on processed flour (breads, pasta and cakes), cassava flour and maize (couscous, polenta and crackers), and foods with a high glycaemic index and load( Reference Compher 12 ), which may cause metabolic alterations. These alterations cause the body to remain in a ‘glucocentric’ state (i.e. constantly using glucose as an energy source), leading to frequent lipogenesis and weight gain( Reference Esfahani, Wong and Mirrahimi 13 , Reference Westman, Feinman and Mavropoulos 14 ). Lifestyle changes may also explain the increased incidence of obesity among developing countries in recent decades, such as an increased intake of processed foods with high levels of carbohydrates and fats and a sedentary lifestyle, a process known as nutrition transition( Reference Popkin, Adair and Ng 15 ).
In addition, epigenetic changes due to malnutrition during prenatal growth and early childhood may play a role in the development of obesity in low-income populations( Reference Lillycrop and Burdge 16 ). This condition may induce growth retardation and energy-sparing mechanisms, such as impaired fat oxidation, decreased resting expenditure and low physical activity( Reference Said-Mohamed, Bernard and Ndzana 17 ). The first insights regarding this theory is known as the ‘Barker hypothesis’, which states that fetal undernutrition in middle to late gestation programmes later CHD( Reference Barker 18 ). Thereafter, studies have suggested that early life events, which determine in part the risk of later disease, occur not only in the fetal period specifically, but also throughout the plastic phase of development, a process also known as the Developmental Origins of Health and Disease( Reference Gluckman, Hanson and Buklijas 19 ). More recently, the intergenerational hypothesis has regained space. This hypothesis states that maternal disadvantage leads to worse health at birth through poor health behaviours, such as exposure to harmful environmental factors, worse access to medical care, including family planning, and worse underlying maternal health( Reference Aizer and Currie 20 ).
In Brazil, excess weight can be found in all social classes. In populations similar to that of the present study, it has been observed that the prevalence of excess weight is higher among short-statured women in the lower quintile of income (a possible sequela of malnutrition in early life, especially in a poor socio-economic environment). In this particular group, the prevalence of overweight increased from 35 % in 2001 to 56·5 % in 2009( Reference Florêncio, Ferreira and França 21 – 23 ). The increased prevalence of obesity has been associated with increased risk factors for developing CVD, such as high levels of cholesterol, reduced glucose tolerance and increased prevalence of hypertension( Reference Florêncio, Ferreira and Cavalcante 24 – Reference Cunha, Almeida and Sichieri 27 ). These factors influence the incidence of morbidity and mortality worldwide; approximately two-thirds of global deaths are due to chronic diseases( 28 ). In Brazil, the number of obesity-related deaths has more than tripled in 10 years. Furthermore, the cost of hospitalisation and medicines associated with this condition exceeded half a billion US dollars in 2013( 29 ).
Therefore, we performed a prospective study on poor women living in slums who were followed up for a period of 4 years. The dietary intake, biochemical profile, energy expenditure and physical activity level (PAL) of these women were measured. The objectives of the present study were to assess the changes in the aforementioned parameters in women living in a poor socio-economic environment, and to explore the influence of their dietary intake and physical activity patterns on these changes.
Methods
Participants and study design
The present longitudinal study, with 4 years of follow-up, was performed from June 2009 to July 2013 in women living in the slums of Maceió-AL in northeast Brazil. These women were mothers of malnourished children who attended the Center for Nutritional Recovery and Education/Maceió (CREN), an advanced campus at the Federal University of Alagoas (UFAL). Sample size was determined based on the number of mothers (n 100) with their children in the day-hospital system at the Center (i.e. the physical capacity of the Center). All mothers aged between 18 and 45 years who were affiliated with the CREN and who attended monthly meetings at the Center were included in the study. Pregnant women and women with anatomical deformities and/or prostheses that might interfere with anthropometric measurements were excluded. Initially, a total of eighty-eight women were assessed.
Measurements
All data were collected from the entire cohort on two different occasions, at baseline and at the end of the follow-up (4 years), except for total energy expenditure (TEE) data, measured using the doubly labelled water (DLW) technique, and PAL data, measured with triaxial accelerometers. Participants were subjected to the assessment of the following clinical and anthropometric variables: body weight; height; waist and hip circumference; blood pressure (BP). Blood was collected from the participants for biochemical analyses. Dietary intake data were analysed using the 24 h recall method on three separate days. TEE and PAL were assessed only in a subgroup of women (n 44) at the end of the follow-up period due to the high costs and methodological requirements of these variables. Similarly, dietary intake data were measured only in the same subgroup at the end of the follow-up period. This subgroup had similar anthropometric and socio-economic characteristics (education, income, height, age, weight and BMI) to those of the total sample.
Socio-economic data
Data collection for socio-economic analysis was made at the CREN using an individual protocol that contained structured questions about the participants' housing conditions, number of family members, family incomes, employment and education.
Anthropometric data
Anthropometric measurements were obtained while the women were barefoot and wearing light clothing. Body weight was measured using a Marte® PP180 electronic scale with 180 kg capacity and 100 g precision. Height was measured while the women stood in the upright position, using a portable stadiometer with a non-extensible tape and a sensitivity of 0·1 cm. Waist circumference was obtained during normal expiration at the midpoint between the iliac crest and the last rib. Hip circumference was measured at the point of maximum circumference over the buttocks, with the tape held flat horizontally without pressing the soft tissue. Current nutritional status was defined by the BMI (weight (kg)/height2 (m2)), which was classified according to the recommendations of the World Health Organization( 30 ).
Metabolic and biochemical data
BP was measured at the beginning and end of the study using OMRON digital monitors (model HEM-421-CO) that were calibrated weekly against mercury manometers. The measurements were repeated three consecutive times, with the participants seated after a 5 min rest, according to the Sixth Report of the Joint National Committee (USA)( 31 ). Regarding the biochemical profile, blood samples were taken at the beginning and at the end of the study. The participants fasted for 12 h before the blood sample collection. The samples were collected via venepuncture at the CREN. The following tests were performed by an accredited clinical laboratory: blood glucose and fasting insulin; homeostasis model assessment for insulin resistance (HOMA-IR); HOMA-β for β-cell function; lipid profile (total cholesterol, HDL-cholesterol, LDL-cholesterol and TAG), aspartate transaminase; alanine transaminase; γ-glutamyl transpeptidase (GGT); thyroid hormones (thyroid-stimulating hormone; triiodothyronine).
HOMA-IR was calculated as follows( Reference Matthews, Hosker and Rudenski 32 ):
HOMA-β was derived as follows( Reference Matthews, Hosker and Rudenski 32 ):
LDL-cholesterol values were calculated using a standardised procedure( Reference Friedewald, Levy and Fredrickson 33 ).
To convert insulin in μU/ml to pmol/l, multiply by 6·945; to convert glucose in mg/dl to mmol/l, multiply by 0·0555.
Dietary intake data
To assess dietary intake, three 24 h recall questionnaires were used on random days, including a weekend day, by means of a photographic food manual( Reference Zabotto, Vianna and Gil 34 ), widely used in Brazilian research. To calculate energy and macronutrient intake, the Nutwin (Federal University of São Paulo) software was used. To assess macronutrient intake adequacy, the estimated average requirement method from the National Research Council was employed( 35 ).
To classify foods according to their processing level, the parameters described by Monteiro et al. ( Reference Monteiro, Levy and Claro 36 ) were used. Foods were classified into three groups as follows: group 1, in natura or minimally processed foods; group 2, processed culinary ingredients; group 3, ready-to-consume, ultra-processed foods.
To determine the glycaemic index of the consumed meals, the Food and Agriculture Organization/WHO Expert Consultation Protocol was followed. The meals were given a low ( ≤ 55), moderate (56–69) or high-glycaemic index ( ≥ 70) rating( 37 ).
In addition, we calculated the dietary diversity score, according to the Food and Agriculture Organization guidelines( 38 ). Briefly, this tool summarises all food products consumed by the individuals into twelve different groups (cereals; roots and tubers; vegetables; fruits; meats; eggs; fish and seafood; legumes, nuts and seeds; milk and milk products; oils and fats; sweets; spices and condiments), and yields a value ranging from 0 to 12, where 0 represents absence of diversity and 12 represents plenty of diversity.
Total energy expenditure
To measure TEE, the DLW technique (2H2 18O) was used. On the first day of the study, we collected a urine sample from each woman in the morning after a 12 h fast. Then, the volunteers were orally administered a mixture of 0·12 g 2H2O (99·8 % excess atoms) per kg body water and 2 g 2H2 18O standard (10 % excess atoms) per kg body water. After 30 min, they received a standard breakfast. Urine samples were collected once per d for a period of 14 d in the homes of the volunteers. These samples were collected at the same time each day. The samples were frozen ( − 20°C) and then transported to the Department of Human Nutrition/Clinical Medicine, University of São Paulo – Ribeirão Preto for analysis.
For the analysis, 2H2 18O was prepared for isotopic weighing (2H/1H and 18O2/16O2)( Reference Wong, Lee and Klein 39 ) and then analysed with extreme-precision MS (Hydra System HIP 20-20; Europa Scientific). The collected data were analysed with DLW Software using the standard calculation procedures of the International Dietary Energy Consulting Group.
Physical activity level
To measure PAL, triaxial accelerometers (activPAL®) were used. The accelerometers were fixed on the volunteers' thighs, where the stickers remained for seven consecutive days. The devices monitored all activities that were performed during the day, and registered the intensity and duration of each category of activity, yielding a coefficient of the individual's PAL. The categories of PAL were defined as sedentary (PAL ≥ 1·0 < 1·4), low active (PAL ≥ 1·4 < 1·6), active (PAL ≥ 1·6 < 1·9) and very active (PAL ≥ 1·9 < 2·5) according to the Institute of Medicine( 35 ).
Ethical aspects
Data were collected only after the participants provided written consent. The present study was approved by the Ethics Research Committee of the Federal University of Alagoas (no. 20090132001-6).
Statistical analyses
Categorical data are presented as absolute and relative frequencies, and continuous data are presented as means and 95 % CI. McNemar's test was used to assess the differences between the baseline and follow-up measurements of categorical variables, and the paired-samples t test was used for continuous variables. Homogeneity of variance for these variables was tested using Levene's test.
To assess the influence of independent variables on weight gain during the 4 years of follow-up, multivariate linear regression analyses were used, controlling for socio-economic variables. For all analyses, an α value of 5 % was used. All statistical analyses were performed using SPSS version 20.0 (IBM Statistics).
Results
Of the eighty-eight women initially assessed at baseline, eighty-five were reassessed at the end of the follow-up. Therefore, data are presented for these eighty-five women. TEE, PAL and dietary intake data were measured only in a subgroup of women (n 44) at the end of the follow-up. The mean age of the participants was 27·8 (26·5–29·05) years, and 43 % of the women were born in rural areas in the state. The mean years of education of the women was < 6 years, and 82 % were functionally illiterate or had only a primary education. Over 80 % of the women were housewives. The average height of the cohort was 1·54 m, ranking them in the seventh percentile of the WHO's growth curves. The mean PAL was classified as ‘low activity’, according to the Institute of Medicine, with more than 3 h spent watching television (TV) daily, and a TEE of 8798·1 kJ/d was recorded (Table 1). Regarding housing, 40 % of the participants lived in brick houses without floors and/or wall coverings, with an average of four rooms housing five people. There were no significant changes in these data during the 4 years of follow-up. The significant increase in the per capita income (US$1·73/d per capita at baseline) was accompanied by the increased coverage of the Bolsa Família Program, a federal government's cash transfer programme, which covered 72 % in 2009 and 95 % in 2013 (Table 1). Although the coverage and the mean value of the benefit increased, the percentage of the benefit in the total income decreased during the follow-up period.
Table 1 Socio-economic data of the cohort at the beginning and at the end of the follow-up period (Number of participants and percentages; mean values and 95 % confidence intervals)

* P value was obtained using the paired-samples t test or McNemar's test.
† Analysis included only the sixty-one individuals who received benefit at the baseline and at the end of the follow-up period.
The analyses of dietary intake data showed a non-significant increase in energy intake (approximately 753·4 kJ) during the 4 years of follow-up (Table 2). There were no differences between the mean daily energy intake at baseline and the intake at the end of the follow-up. These values were lower than those of TEE estimated by the DLW technique. However, the paired-samples t test conducted between the mean final energy intake and TEE including only the subgroup (n 44) subjected to the DLW technique did not show significant differences (7990·3 (7173·7–8806·8) v. 8798·1 (8169·0–9432·4) kJ, respectively; P= 0·084). In addition, the variety of foods consumed was low, as shown by the dietary diversity score analysis.
Table 2 Dietary intake data for the entire cohort at the beginning and for the subgroup at the beginning and at the end of the follow-up period (Mean values and 95 % confidence intervals)

* P value was obtained using the paired-samples t test (n 44).
Table 3 presents the anthropometric and biochemical characteristics at baseline and after 4 years of follow-up. Between the beginning and the end of the study, significant increases were observed in weight, BMI, and waist and hip circumference. Weight gain did not differ between women with excess weight (BMI>25 kg/m2) at baseline and those without excess weight (4·7 (3·16–6·41) kg, n 43 v. 2·8 (0·98–4·77) kg, n 42, respectively; P= 0·126). Regarding the biochemical profile, a significant increase in the levels of glucose, TAG, total cholesterol and aspartate transaminase and a decreased sensitivity of β-cell were observed. Furthermore, thyroid hormone levels (triiodothyronine) decreased significantly and systolic BP increased.
Table 3 Anthropometric and biochemical data of the cohort at the beginning and at the end of follow-up period (Mean values and 95 % confidence intervals)

HOMA-IR, homeostasis model assessment for insulin resistance; HOMA-β, homeostasis model assessment for β-cell function.
* P value was obtained using the paired-samples t test.
† To convert to μkat/l, multiply by 0·0167.
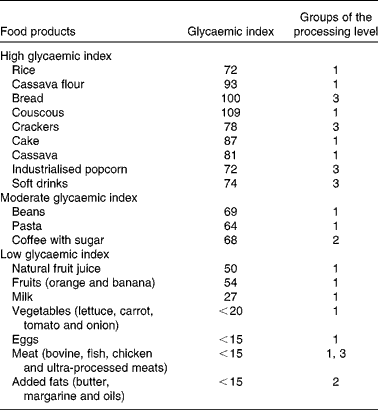
Using weight gain as the dependent variable and dietary intake and physical activity data as independent variables, controlling for socio-economic characteristics, the multivariate linear regression analysis showed that only the variables time spent watching TV and dietary diversity score exhibited a significant effect on weight gain (Table 4) . As a sensitivity analysis, we performed a multivariate regression analysis of PAL and TEE against weight gain within the subgroup of women (n 44), controlling for socio-economic factors. The analysis revealed that only PAL exhibited a significant effect (β = − 24·79 ( − 48·12 to − 1·467); P= 0·038). Table 5 presents the glycaemic index and the processing level of the main foods that the study population consumed, indicating a monotonous food intake pattern. The hypothesis that the intake of high-glycaemic index and ultra-processed foods was associated with weight gain was not confirmed.
Table 4 Multivariate linear regression analyses of changes in body weight (final−baseline values) as the dependent variable (β Coefficients and 95 % confidence intervals)

TV television.
* All analyses were controlled for baseline values of age, years of schooling, family income per capita, receiving government benefits, BMI and the metabolic syndrome.
† Analysis included only the subgroup (n 44) who were subjected to the doubly labelled water technique and triaxial accelerometer testing procedures.
Table 5 Glycaemic index and groups of the processing level of the reported food products consumed
Discussion
The average stature of the study cohort ranged from the 1·5th percentile (1·49 m) to the 25th percentile (1·59 m), according to the WHO. It is known that adult height is the result of a combination of genetic and environmental factors. In a study conducted in 473 Brazilian women aged 19 years residing in the city of Pelotas (South Brazil), early life factors such as family income, birth weight and maternal height have been found to be the determinants of adult height( Reference Gigante, Horta and Lima 40 ). The influence of the environment on adult height may be even higher in poor socio-economic settings( Reference Silventoinen 41 ). The mean height of the Pelotas study population was 161 cm( Reference Gigante, Horta and Lima 40 ), and the median height of the Brazilian women was 160 cm( 29 ). It must be considered that the women in the present study were born and currently live in a very poor socio-economic environment. Unlike the city of Pelotas, Maceió is a very poor Brazilian city. A probabilistic survey conducted in 1992 showed that stunting levels among children were 22·1 %( Reference Ferreira Hda, Cesar and Assunção 42 ), and a survey conducted in the countryside population of the Alagoas state in 1995 found this level to be 39·8 %( Reference Ferreira, Albuquerque and Ataide 43 ). Considering that all women in the present study were born in the Alagoas state before 1995, these data might suggest that these women possibly lived in adverse health and nutritional conditions in early life and, perhaps, failed to reach their full growth potential and development, typically defined as the 50th height percentile of the WHO growth curves( 44 ).
Poverty persisted in the lives of these women, who were semi-illiterate mothers of malnourished children, lived in homes (with an average of five people) without sanitation and had limited financial resources (2·56 US$/capita per d at the end of the follow-up period). This stratum of income classifies this population as relatively poor, considering the cost of living in Brazil. It is noteworthy that most of the families participated in the federal government's cash transfer programme, with the value of the benefit representing almost 20 % of the family income at the end of the follow-up period. This condition, which links current poverty with possible long-lasting effects of malnutrition, reflected by the short stature of the women, is supported by the fact that the intergenerational transmission of inequality in Brazil is estimated to be among the highest( Reference Dunn 45 ).
The women in the present study worsened their metabolic profile during the follow-up period, showing increased body weight, total cholesterol, TAG, fasting blood glucose and systolic BP. A lower socio-economic status increases the risk of developing chronic diseases prematurely, as demonstrated previously in our studies of low-income populations. These studies found a high prevalence of hypertension, dyslipidaemia, insulin resistance and obesity, particularly in women and female adolescents( Reference Ferreira, Moura and Cabral-Júnior 22 , Reference Florêncio, Ferreira and Cavalcante 46 , Reference Clemente, Santos and Silva 47 ). During the 4 years of follow-up, the mean income of families increased, together with the coverage of the federal government's cash transfer programme. However, the percentage of the benefit in the total income decreased, indicating that the programme is not solely responsible for the increased income. It is possible that new national policies on minimum wage annual increase could also be responsible for this factor. Studies that investigated similar cash transfer programmes on adult health have shown a positive association between the programme and higher BMI and systolic BP( Reference Fernald, Gertler and Hou 48 ).
The weight gain exhibited by the women in the present study is difficult to explain because despite following a monotonous food intake pattern with an average variation of only twenty food products, these women consumed adequate amounts of energy and macronutrients, with 16·5 % of energy derived from protein, 58·6 % from carbohydrate and 24·9 % from fat, in accordance with the current Brazilian recommendations( 49 ). In addition, there was no significant difference in energy and macronutrient intakes between the beginning and the end of the follow-up period. The trend of significantly increased lipid intake at the end of the follow-up period was somewhat expected because dietary choices are strongly influenced by the taste, cost and convenience, and to a lesser extent by health and variety. The preference for high-fat foods appears to be a universal human trait, and in the absence of efficient physiological mechanisms regulating fat intake, fat consumption appears to be determined simply by the amount of fat available in the food supply( Reference Drewnowski 50 ). In addition, there is evidence that obesity and socio-economic position may be associated with dietary energy density and energy cost, which means that the choice of more energy-dense diets, usually rich in fat, by low-income populations may be an effective way to save money( Reference Drewnowski and Darmon 51 ). This fact may also explain the low dietary diversity presented by this sample. A systematic review investigating the impact of socio-economic inequalities in food intake among European adults has found that lower socio-economic groups are less likely to consume fruit and vegetables, which have a higher cost and lower energy density( Reference Giskes, Avendano and Brug 52 ). Therefore, this dietary intake pattern could contribute to weight gain.
There was also a trend between meal frequency and weight gain. However, there is controversy regarding this issue; for instance, randomised controlled trials have shown a lack of the effect between the two factors( Reference Kulovitz, Kravitz and Mermier 53 ). Unlike reports in the literature, the intake of foods with high glycaemic index did not appear to influence weight gain( Reference Westman, Feinman and Mavropoulos 14 ). Interestingly, these women did not consume significant quantities of ready-to-eat, ultra-processed foods. On the contrary, they followed the pattern of the traditional Brazilian meal, such as beans with rice and meat. This finding was also observed by Monteiro et al. ( Reference Monteiro, Levy and Claro 36 ), who investigated the intake of ultra-processed foods in a sample from the Brazilian Household Budget Survey and found a decreased intake of these foods in the lower-income quintiles of the population.
The weight gain exhibited by the women in the present study may also be associated with their low level of physical activity. Time spent watching TV was significantly associated with weight gain, as revealed by the multivariate analysis. This variable is a common measure of sedentary behaviour, and a systematic review has pointed out that excess TV viewing is associated with reduced physical and psychosocial health, and that reductions in BMI could be achieved with lowering of sedentary time( Reference Tremblay, LeBlanc and Kho 54 ). According to the accelerometer analysis, the population had a light PAL, which included domestic chores necessary for activities of daily living. Approximately half of the women resided in rural areas, where these daily and labour activities require greater energy expenditure. Thus, it is likely that physical activity was reduced in these women, thereby allowing increased expressions of the mechanisms of energy conservation, which may partly explain the prevalence of overweight. Also, even after the multivariate analysis, the variable time spent watching TV was associated with weight gain. The sensitivity analyses within the subgroup of forty-four women showed that PAL was significantly associated with weight gain, indicating that sedentarism play an important role in the weight gain of this cohort. Similarly, in a study of stunted preschool children, the higher prevalence of overweight was only associated with their reduced PAL in the context of the nutrition transition( Reference Said-Mohamed, Bernard and Ndzana 17 ).
The average energy intake of the women did not change significantly during the follow-up period. Energy intake was lower than TEE measured by the DLW technique. Notably, DLW is the ‘gold’ standard biomarker used to measure TEE in this type of evaluation. Studies using the DLW technique have shown the limitations of investigating food intake through dietary surveys, such as under-reporting of dietary intake( Reference Scagliusi, Ferrioli and Pfrimer 55 ). Thus, it is possible that the present study population exhibited this same pattern. The mean TEE of 8798·1 kJ/d is low compared with that reported in previous studies with similar populations. In a study conducted in the USA, adult women exhibited a mean TEE of 11 056·7 kJ/d and a total energy intake of 9181·8 kJ/d. In a study conducted in Brazilian students, teachers and female technicians from a university, the mean TEE was 10 969·1 kJ/d and energy intake was 8551 kJ/d( Reference Scagliusi, Ferrioli and Pfrimer 55 – Reference Wong, Roberts and Racette 57 ). Even after adjusting for BMI, the women in the present study expended less energy than the women of those studies who were from higher socio-economic levels (present study, 8798·1 kJ/27·5 kg/m2= 319·9 kJ × m2/kg v. 626 kJ/27·9 kg/m2= 393·1 kJ × m2/kg)( Reference Sawaya, Tucker and Tsay 56 ). These data show that short-statured women have lower TEE, which corroborates the results found in malnourished children( Reference Said-Mohamed, Bernard and Ndzana 17 , Reference Wilson, Dickinson and Hoffman 58 ). This may be attributed to reduced energy intake (i.e. 7990·3 kJ/d). Also, given the poor socio-economic environment of the population, it is possible to consider some kind of metabolic adaptation to early malnutrition that led to a decrease in TEE.
This finding of low TEE may also be associated with the significant decrease in triiodothyronine levels that was observed in the present study. Lower triiodothyronine levels decrease thermogenesis and oxygen consumption, which allow for greater energy conservation( Reference Kok, Roelfsema and Langendonk 59 ). In addition, Hoffman et al. ( Reference Hoffman, Roberts and Verreschi 60 , Reference Hoffman, Sawaya and Verreschi 61 ) investigated the RMR, TEE, respiratory quotient and substrate oxidation in children with and without short stature, and found that children with short stature had a lower RMR, significantly higher respiratory quotient and, consequently, lower fat oxidation and increased abdominal waist circumference. Changes in waist circumference, particularly in short-statured individuals, are accompanied by metabolic changes in glucose and lipid profiles( Reference Clemente, Santos and Martins 7 , Reference Florêncio, Ferreira and Cavalcante 24 ). Finally, an increase in systolic BP was also observed in the present study. The likely explanation of this finding may be an increased production of angiotensinogen by abdominal adipose tissue, which is locally converted to angiotensin II, inducing an increase in peripheral vascular resistance and BP( Reference Wajchenberg 62 ).
The present study has some limitations. First, the sample size was small; however, it is noteworthy that the cohort consisted of low-income women, mothers of malnourished children, which was a very specific group. The small sample size may have reduced the statistical power of the analyses. In some cases, such as the difference between baseline and final energy intake and the association between the number of meals and weight gain, clinically important findings may have been missed, possibly due to the lack of power. Second, we assessed TEE and PAL only at the end of the follow-up period, and we could not assess the entire cohort, affecting the statistical analyses. Third, dietary intake data were assessed using only the 24 h recall method, which is known to underestimate energy intake due to under-reporting.
In conclusion, the present study shows that even when eating in a reasonably balanced manner (i.e. without high consumption of energy or high-glycaemic index and ultra-processed foods), low-income short-statured women, mothers of malnourished children, gain weight and develop obesity-related co-morbidities. Sedentary behaviour and reduced PAL exhibited by these women partially explained their weight gain. In addition, they exhibited a low TEE when compared with similar cohorts of adult women from better socio-economic conditions. It is possible that the women in the present study exhibited physiological adaptive mechanisms (e.g. low energy expenditure and decreased thyroid hormone levels), resulting from insults in early life. However, the observational nature of the present study prevents the deduction of any direction of causality. Future research should focus on metabolic mechanisms that are involved in weight gain in low-income individuals.
Acknowledgements
The present study was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, grant no. 552194/2011-5). The sponsor had no role in the design and analysis of the study or in the writing of this article.
The authors' contributions are as follows: T. M. M. T. F., A. L. S. and E. F. conceived the idea; N. B. B. and A. P. G. C. conducted the statistical analyses; R. P. A. B. and F. C. A. A. collected the data. All authors wrote and approved the final version of the manuscript.
The authors declare that they have no conflicts of interest.








