Introduction
Increasing rates of spinal fusion with evolving indications have been well documented. Reference Adler, Sethi and Yeh1–Reference Rajaee, Bae and Kanim3 Differences in surgical treatment are likely explained by both patient and surgeon factors. Previous attempts at the establishment of guidelines have provided mostly low-quality evidence for surgical indications. Reference Resnick, Watters and Mummaneni4 For example, the addition of interbody fusion has failed to provide any clear benefit in patient outcomes research. Reference Mummaneni, Dhall and Eck5 Furthermore, financial incentive has been shown to be a major confounder in the decision-making of physicians in multiple specialties, but interestingly has not been extensively studied in spine surgery. Reference Adler, Sethi and Yeh1,Reference Nguyen and Derrick6–Reference Schoenfeld, Harris and Liu8 This is surprising given the disparities in surgeon remuneration between decompression alone and interbody fusion, which can be up to fourfold higher. Reference Schoenfeld, Weiner and Smith7,Reference Deyo, Mirza and Martin9,Reference Schoenfeld, Makanji and Jiang10 Canada is one of few countries in the world with a universal health care system. Although there exists regional variability, remuneration for surgical procedures is based on government fee schedules termed fee-for-service (FFS) or a fixed service contract, essentially an annual salary. Other countries have a blend of public and private health care funding. In the United States, payment structure increases in complexity, where fees are often pre-negotiated between providers and insurance companies prior to providing care. Reference Steenhuis, Struijs and Koolman11 Furthermore, the introduction of bundled care payment models has further increased both variability and complexity. Reference Malik, Phillips and Retchin12 For this reason, Canada’s universal health care system provides a unique environment to study the impact of compensation on surgical management as variability in renumeration is markedly reduced.
Through the identification of unnecessary spending, precious health care resources can be saved and value-based care optimized. Therefore, the primary purpose of this study was to examine differences in surgical practice between FFS and salaried physicians for two common degenerative lumbar spine conditions. A secondary goal was to investigate any differences in baseline patient characteristics, treatment characteristics, and patient reported outcome measures based on method of surgeon remuneration. The study alternative hypothesis was that salaried physicians would be more conservative in terms of surgical approach of both conditions.
Methods
Study Design
This was a multicenter, ambispective review of consecutive spine surgery patients enrolled by the Canadian Spine Outcomes and Research Network (CSORN) between October 2012 and July 2018.
A survey was designed and sent to all spine surgeons enrolling patients in CSORN (Appendix 1). Questions included queries pertinent to their mode of remuneration, their practice, and their training.
CSORN is a group of 70 neurosurgical and orthopedic spine surgeons from 18 tertiary care academic and nonacademic hospitals across eight provinces that prospectively collects data on patients with spinal conditions. This database serves as a national registry created to answer research questions and to facilitate the implementation of best practices.
A national database research coordinator audits data quality and performance and sends reports to each contributing hospital site coordinator on a quarterly basis. Reports track data completion and follow-up rates to facilitate internal data validation at each site. A national privacy and security framework was created for CSORN that includes a governance structure, standard operating procedures, training processes, physical and technical security, and privacy impact assessments. This model ensures privacy and security of personal health information. Written informed consent is obtained from all participating patients. Patient identification is anonymized to ensure that patients in the Network cannot be individually identified. All 18 participating sites obtained Research Ethics Board (REB) approval prior to any data collection. Decisions regarding data collection, storage, and analysis are independent of any particular company or commercial interest.
Patient Sample
Local research coordinators enrolled consecutive patients at each site. All patients that provided informed written consent with a diagnosis of: (1) spinal stenosis without instability (n = 2234) or (2) degenerative lumbar spondylolisthesis (n = 1292) who were treated surgically were included in this study. No patients were excluded from the analyses.
Patient Variables
Collected baseline patient characteristics included sociodemographic factors (age, sex, body mass index, smoking status, and marital status), insurance claim status, work status, comorbidities, and symptom duration.
Outcome Measures
The primary outcome was the difference in surgical procedure type between FFS and salaried physicians for these two diagnoses. Procedure type were categorized as either: (1) decompression alone, (2) decompression and uninstrumented fusion, (3) decompression and instrumented fusion, and (4) decompression, instrumented fusion, and interbody fusion. Secondary outcome measures included number of surgical levels, use of minimally invasive technique, and patient reported outcome measures (PROMs): back and leg pain numeric rating scale, Short Form-12, Oswestry Disability Index, and EuroQOL-5D. Research coordinators, unaware of the study hypothesis, collected baseline questionnaires, and PROMs at 12 months postoperatively. Collection was in person, via post or utilizing an online patient portal.
Statistical Analysis
Binary logistic regression modeling was used to evaluate differences in types of procedures performed, adjusting for surgeon characteristics (differences in specialty training (ortho vs neuro), years in practice, location of fellowship training), and baseline patient characteristics (age, BMI, and smoking status). An a priori level of 5% was used to determine statistical significance. The adjusted analyses were performed to help isolate the mode of remuneration as a main difference between the two groups and were based on factors shown to affect outcomes in spine surgery literature and/or theorized as potential confounders. ANCOVA was utilized to assess differences in baseline questionnaire scores and in PROMs, adjusted for age, BMI, smoking status. Analyses were conducted using SPSS (IBM Corp. Released 2019. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp.)
Results
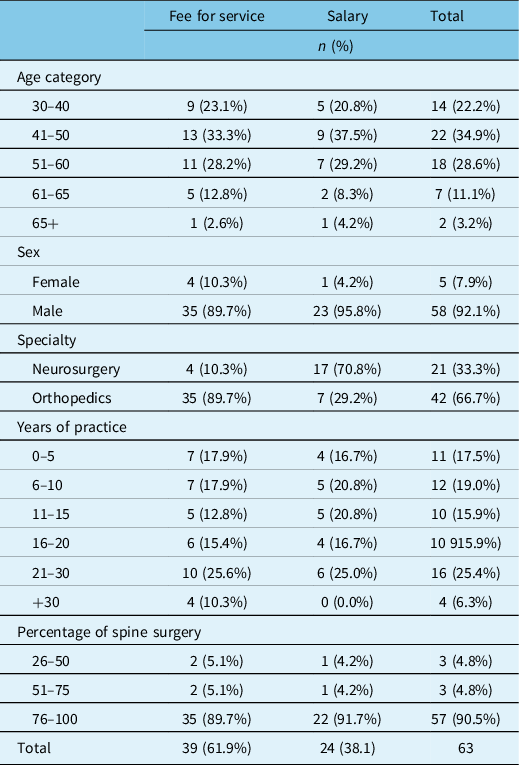
In total, 63 out of 70 surgeons completed the survey. Table 1 summarizes the surgeon demographics. Thirty-nine surgeons (62%) were paid FFS and 24 (38%) were salaried. Totally, 42/63 (66.7%) of surgeons had an orthopedic training background. Of note, 17/24 (70.8%) of salaried surgeons were neurosurgeons. All but three surgeons did their residency training in Canada, and only one surgeon was not fellowship trained in spine surgery. Fellowship training was completed in Canada for 45.8%, 35.6% in the USA, and 18.6% outside North America.
Table 1: Surgeon demographics
Stable Lumbar Spinal Stenosis
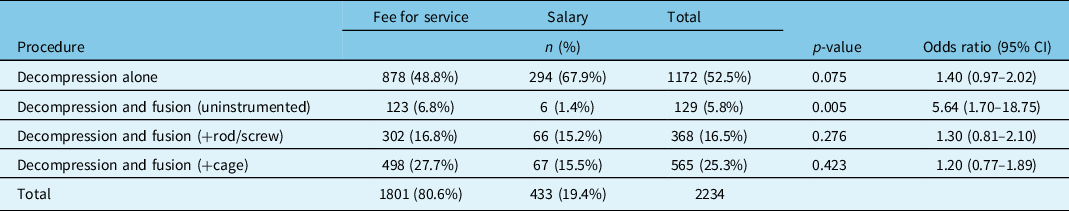
Controlling for baseline patient and surgeon characteristics, FFS physicians performed significantly more decompression with uninstrumented fusions than salaried physicians (6.8% vs 1.4%, odds ratio (OR) = 5.6, p < 0.005). There were no significant differences between groups with regard to decompression alone, instrumented, or interbody fusions (Table 2).
Table 2: Types of operative procedures for lumbar spinal stenosis without instability by surgeon remuneration; adjusted for patient variables (age, BMI, smoking status) and surgeon differences (specialty, years in practice, location of fellowship training)
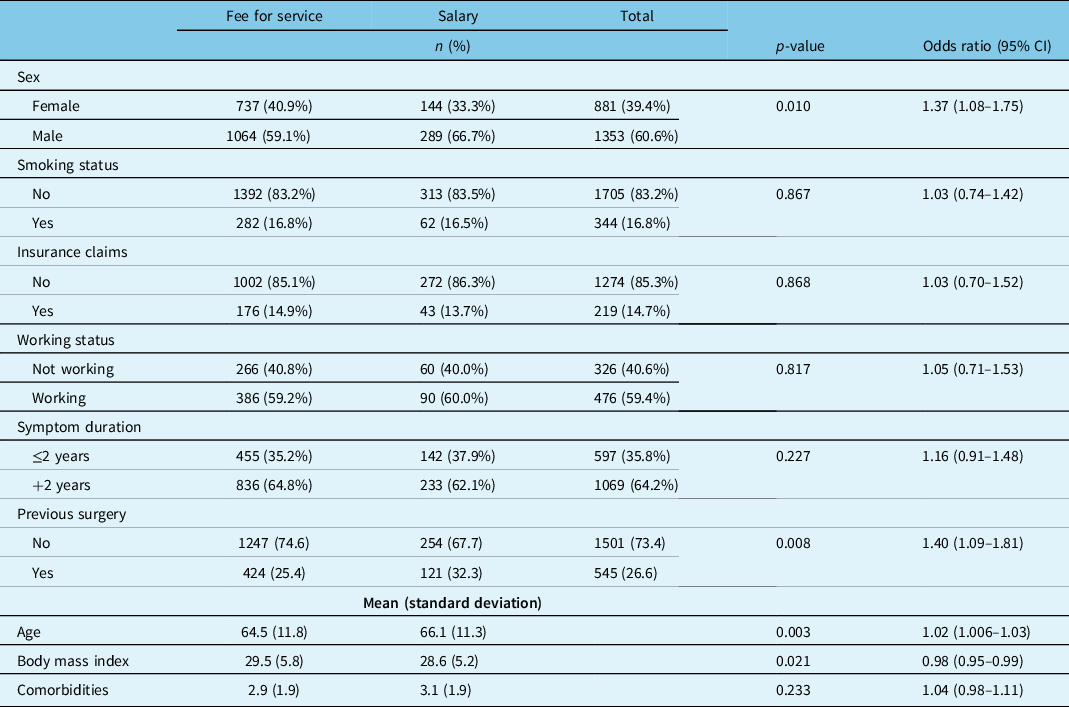
Examining baseline characteristics, patients treated by salaried physicians were significantly older (p < 0.003), more likely to be male (p < 0.01), and had a lower average BMI (p < 0.02). Salaried physicians saw more patients with previous surgery (32.3% vs 25.4%, p < 0.008). No other statistical differences were observed (Table 3).
Table 3: Lumbar spinal stenosis patient demographics by surgeon remuneration
Salaried surgeons operated on significantly fewer spinal levels (1.77 vs 2.01, OR = 1.153, p < 0.006). Salaried physicians performed significantly fewer minimally invasive (MIS) procedures (25.6% vs 36.9%, OR = 4.074, p < 0.001).
Table 4 reveals that there was no statistically significant difference in baseline patients reported questionnaire scores between groups. Of the 2234 stenosis patients, 1748 were eligible for follow-up; the remaining had not yet reached the 12-month post-surgical mark. Follow-up data were obtained from 1335 (76%). Using ANCOVA to account for possible confounders, those treated by salary physicians had significantly higher ODI scores at 12-month follow-up (p < 0.026). There were no other statistically significant differences in PROMs at 12 months between remuneration groups.
Table 4: Adjusted* baseline and 12-month follow-up PROMS for stable spinal stenosis patients by surgeon remuneration
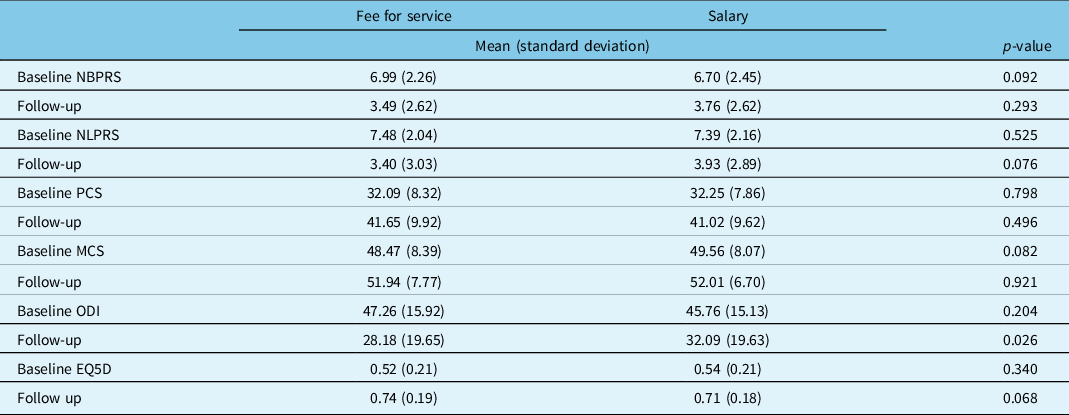
NBPRS=Numeric Back Pain Rating Scale; NLPRS=Numeric Leg Pain Rating Scale; PCS=Physical Component Score of the SF12; MCS=Mental Component Score of the SF12; ODI=Oswestry Disability Index; EQ5D=European Quality of Life-5D.
* Adjusted for age, BMI, smoking status, specialty, years in practice, location of fellowship training; follow up results additionally adjusted for baseline questionnaire score.
Degenerative Spondylolisthesis
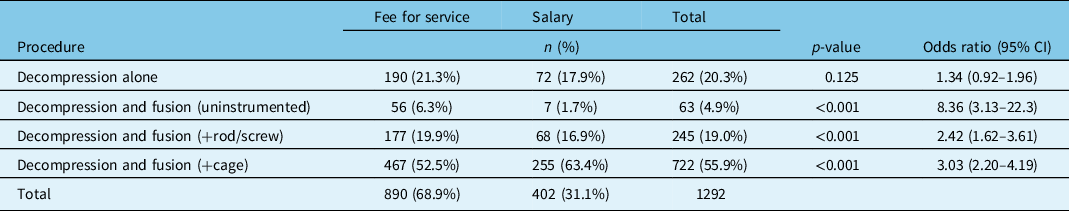
After adjusting for patient and surgeon factors, there were no statistically significant differences in the rates of decompression-alone procedures between groups; however, FFS physicians performed significantly more uninstrumented (OR = 8.36, p < 0.001) and instrumented fusions (OR = 2.42, p < 0.001), but salaried physicians performed more interbody fusions than their FFS colleagues (OR = 3.03, p < 0.001) (Table 5).
Table 5: Types of operative procedures for degenerative spondylolisthesis patients by surgeon remuneration adjusted for patient variables (age, BMI, smoking status) and surgeon differences (specialty, years in practice, location of fellowship training)
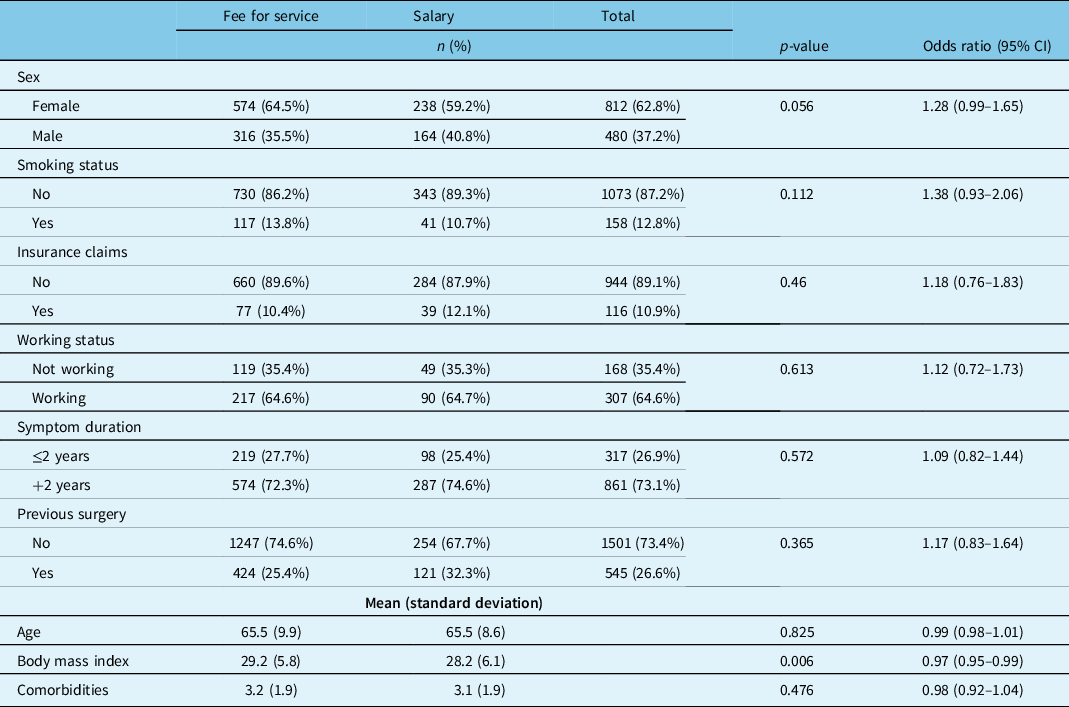
Patients treated by salaried physicians had a lower average BMI at baseline (28.2 vs 29.2, p < 0.007) but were otherwise very similar to the FFS group (Table 6).
Table 6: Degenerative spondylolisthesis patient demographics by surgeon remuneration
There was no statistically significant difference in the number of surgical levels (1.49 vs 1.47) between surgeon groups. Salaried physicians utilized fewer minimally invasive procedures (13.9% vs 44.6%, OR = 9.95, p < 0.001).
Of the 1292 spondylolisthesis patients, 1023 were eligible for follow-up. Post-surgical follow-up data were obtained from 819 (80%). At both baseline and 12 month follow-up, there were no statistically significant differences between patient PROM scores for salary vs FFS physicians (Table 7). Both groups demonstrated significant improvement.
Table 7: Adjusted* baseline and 12-month follow up PROMS for degenerative lumbar spondylolisthesis patients by surgeon remuneration
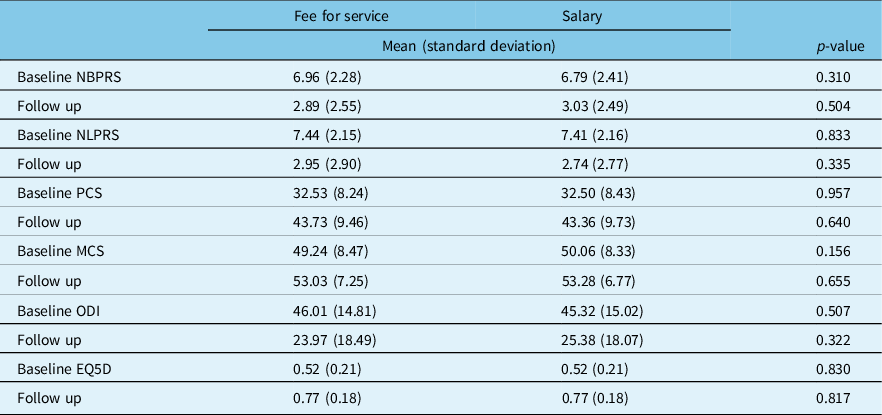
NBPRS=Numeric Back Pain Rating Scale; NLPRS=Numeric Leg Pain Rating Scale; PCS=Physical Component Score of the SF12; MCS=Mental Component Score of the SF12; ODI=Oswestry Disability Index; EQ5D=European Quality of Life-5D.
* Adjusted for age, BMI, smoking status, specialty, years in practice, location of fellowship training; follow up results additionally adjusted for baseline questionnaire score.
Discussion
After controlling for patient’s baseline differences and surgeon factors, we observed that surgical approach with regard to common conditions of the lumbar spine is different between salaried and FFS spine surgeons in Canada. Salaried surgeons performed fewer uninstrumented fusion procedures, operated on fewer levels, and used fewer minimally invasive techniques when treating stable lumbar spine stenosis. Salaried surgeons, however, showed the opposite trend in the treatment of degenerative spondylolisthesis for which they performed more interbody fusions. Baseline patient characteristics were similar in both groups. Outcome measures reflected no differences in patients treated by either FFs or salaried groups for either diagnosis. Our results support the finding that factors other than patient demographics appear to influence the surgical decision making in this population. Although remuneration likely does play a role, its impact is likely overshadowed by surgeon training, experience, and regional practices.
One hypothesis to explain our results stems from a crude analysis of compensation/time ratio. If one assumes that renumeration is a driver of procedure choice, doing more extensive surgery may not produce acceptable monetary benefit for the FFS surgeon, while reflecting an indifference to the salaried surgeon. As previously mentioned, reimbursement in Canada is dictated by individual province fee schedules. For example, under the Ontario Health Insurance Plan (OHIP), a single-level decompression in the lumbar spine is valued at $1004.70CAD, with a posterior uninstrumented fusion yielding an additional $408.00CAD, $867.00CAD for a posterior instrumented fusion, and an interbody device an additional $510.00CAD. 13 Similarly, in British Columbia, these same procedures are valued at $789.13CAD, $490.15CAD, $1769.22CAD, and $403.00CAD, respectively. 14 In practice, the additional time required to do an interbody fusion is not reflected in the fee schedule, which may be a disincentive for the FFS surgeon, especially considering the paucity of proven clinical benefits. On the other hand, the addition of a posterior uninstrumented fusion (higher in the FFS group for both diagnoses), which remunerates approximately the same as an interbody fusion with less hassle, may provide a better cost-effective basis for the FFS surgeon.
Salaried surgeons receive the same remuneration regardless of the listed fee for a particular procedure. This fact should remove remuneration incentive from their operative decisions. One would expect that this may translate in less exhaustive surgery across the board. This was seen for stable spinal stenosis, where the FFS group performed more uninstrumented fusion than the salaried group. The proportion of patients undergoing uninstrumented fusion was, however, small in both groups and thus cannot be considered a big driver. On the other end, despite the lack of financial incentives, salaried surgeons performed three times more interbody fusions than FFS surgeons when treating degenerative spondylolisthesis, which cannot be explained by monetary incentives. Without the noncompelling financial incentives of adding an interbody fusion, a hypothesis is that the potential minor clinical benefit of interbody fusion may be seen as valuable by the salaried surgeons, but not worth it by the FFS surgeons.
Conflicting results for these two common interventions may in fact show that remuneration does not play a significant role in the management of these patients. Our results clearly highlight the preference sensitive nature of elective spine surgery. Reference Lurie, Bell and Weinstein15–Reference Cook, Weiner and Schallmo17 This is particularly true for elective spinal fusion, which due to its high cost and varied outcomes is the source of much concern and controversy not only in the USA but also most industrialized nations. Reference Martin, Franklin and Deyo18,Reference Deyo, Mirza and Martin19 While it has been postulated that gross increasing rate of spinal fusion is in part due to both regional payer policies, institutional, and provider financial incentives, Reference Martin, Franklin and Deyo18,Reference Deyo, Mirza and Martin19 a recent study suggested that surgeon incentives (or conflicts of interest) do not materially drive fusion rates. Reference Lurie, Bell and Weinstein15 While variable access (so called “supply-sensitive care”) to more costly and remunerative treatment alternatives certainly cannot and should not be directly or indirectly discounted, the issue of financial incentive is not that simple. It is generally thought that variation is more fundamentally driven by clinical uncertainty, patient and provider preference, and regional surgical signatures, an issue that is not by any means unique to the USA. Reference Lurie, Bell and Weinstein15,Reference Bederman, Kreder and Weller20–Reference Hussain, Nasir and Moed23 Traditionally, surgeon preference is believed to be a primary driver; however, this has been shown to be a multifactorial issue where patient, primary care providers and surgeon attitudes play varying, nonlinear, aligned, and mal-aligned roles that requires further study Reference Bederman, Mahomed and Kreder22,Reference Bono, Harris and Warholic24 from a variety of perspectives.
There is little supporting evidence in the literature on this topic. Shoenfeld et al. Reference Schoenfeld, Makanji and Jiang10 postulated that “provider inducement” is a strong confounder in the surgical treatment of degenerative lumbar spine disorders. This group reviewed 28,344 patients undergoing surgical treatment for spinal stenosis, degenerative spondylolisthesis, and disc herniation; 21,290 patients treated by FFS physicians were compared to 7054 patients treated by salaried physicians at Department of Defense facilities. In this study, FFS physicians were at 1.25 (1.20–1.30) times odds of offering an interbody fusion as compared to patients treated in facilities employing salaried surgeons. When looking specifically at a diagnosis, patients with lumbar spine stenosis were at 1.39 (1.15–1.69) times greater odds of receiving an interbody fusion by FFS surgeons. Similarly, patients with a disc herniation were at 2.61 (2.36–2.89) times the odds of interbody fusion by the FFS group. There was no difference between fusion for spondylolisthesis for the two pay structures [OR = 0.99 (0.84–1.16)]. These findings may be explained by their more favorable monetary benefit of adding an interbody fusion than the Canadian one.
Patient selection seemed equivalent between both groups. Salaried surgeons treated older patients and performed more revision cases in the stable stenosis diagnosis but for both diagnoses, FFS surgeons were treating patients with higher BMIs, making both groups comparable from a technical difficulty perspective. FFS surgeons performed more MIS surgeries for both diagnoses. There is no direct additional renumeration for performing MIS procedures in Canada. One explanation could be that once over the initial learning curve, MIS decompression and fusions may be quicker to perform than open surgical approaches, and thus increase potential revenues. Reference Singh, Nandyala and Marquez-Lara25,Reference Price, Dawson and Schwender26
The strengths of this study include its prospective data collection, robust methodology, and sample size. This is also a novel topic which, to the authors’ knowledge, has not been studied in a standardized payer setting. The authors do, however, acknowledge multiple limitations. This study lacks the ability to describe surgical practices outside of the CSORN network. This work is a thus a reflection of practice at academic centers in Canada and may be confounded by referral patterns to these centers. Participation of trainees, which could significantly increase surgical time and thus impact remuneration, was not accounted for in this study.
Similarly, payment structure is seen to vary between provinces in Canada. Although the primary purpose of this study was not a regional analysis of surgical practices in Canada, the authors acknowledge that this may be a confounding factor.
A thorough attempt was made at controlling for as many surgeons and patient factors as possible to isolate remuneration mode. The unequal distribution of neurosurgeons and orthopedic surgeons in salaried versus fee-for-service surgeons likely plays an important role in surgical decision making, as experience and location of fellowship training. As such, we controlled for these three important potential confounders; however, it is likely that other factors pertinent to health care systems play a role in this complex decision-making process and were not controlled for or measured.
Conclusion
The treatment of degenerative diseases of the lumbar spine remains controversial. In this study, surgical practices for treatment of spinal stenosis and degenerative spondylolisthesis were seen to vary in opposite directions based on method of compensation. Salaried surgeons treated spinal stenosis with more conservative surgical approaches, while treating spondylolisthesis with more aggressive approaches. These results support the notion that remuneration does not, on its’ own, impact surgical decision making in a consistent fashion in Canada and that factors such as experience, training, and regional variation likely play a significant role.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2022.259
Acknowledgments
The authors thanks all of the subjects who participated in the study, surgeons who completed the survey and the support/research coordinator staff and investigators from the Canadian Spine Outcomes and Research Network (CSORN) contributing sites:
Canada East Spine Centre: Saint John, NB; Foothills Medical Centre: Calgary, AB; Hopital de L’enfant Jesus: Quebec City, QC; McGill University Health Centre: Montreal, QC; Queen Elizabeth II – Health Sciences Centre: Halifax, NS; Sault Area Hospital, Sault Ste Marie, ON; Toronto Western Hospital: Toronto ON, University of Alberta Hospital site: Edmonton, AB; Vancouver General Hospital: Vancouver, BC; Victoria Hospital – London Health Sciences Centre: London, ON; Winnipeg Health Sciences Centre: Winnipeg, MB.
There was no one overall study Research Ethics Board (REB) approval. Each participating site sought individual REB approval:
Saint John: Horizon Health Network Research Ethics Board
Neuro: REB approval number: 22983
Ortho: REB approval number: 18700
University of Calgary: Conjoint Health Research Ethics Board
REB approval number: REB15-1332_REN7
L’enfant Jesus (Quebec), Montreal: Comite d'ethique de la recherche, CHU de Quebec – Universite Laval
REB approval number: F9-50062
McGill University Montreal: MUHC Centre for Applied Ethics
REB approval number: 2013-2106, 12-121-SDR
QEII Halifax: Nova Scotia Health Authority Research Ethics Board
Neuro: REB approval number: 1012957
Ortho: REB approval number: 1012049
Sault Ste Marie: Joint Group Health Centre/Sault Area Hospital Research Ethics Board
REB approval number: 2017-04-01
Toronto Western Hospital: University Health Network Research Ethics Board
REB approval number: 12-5190
University of Alberta (Edmonton): Health Research Ethics Board – Health Panel
REB approval number: Pro00036987_REN7
Vancouver General Hospital: UBC Clinical Research Ethics Board
REB approval number: H12-01627
Victoria Hospital, London: University of Western Ontario Research Ethics Board for Health Sciences Research Involving Human Subjects (HSREB)
REB approval number: 103079
Winnipeg Health Sciences: University of Manitoba Health Research Ethics Board
REB approval number: HS15352 (H2012:176)
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper. RCM reports grants AOSpine, Canadian Orthopaedic Foundation, Cancer Research Society; NM reports a grant from Medtronic Canada; MGJ reports other financial or non-financial interests from Stryker Canada; YRR reports royalties, consulting from Medtronic Canada, stock options from Arthur Health; CF reports grant from OREF, royalties, consulting from Medtronic Canada, consulting from Nuvasive; ND reports consulting from Medtronic Canada, Stryker Canada, Baxter, meeting attendance support from AOSpine, stock options from Medtronic Canada, unpaid leadership role with Canadian Spine Society, Journal of Current Clinical Care.
Statement of Authorship
DB, ND were involved in conception and design. DB, GM performed the acquisition of data. DB, GM, RCM, EA, NM, MGJ, CSB, YRR, RAG, JP, AN, MHW, SC, NA, AS, AK, HH, KCT, CGF, ND participated in analysis and interpretation. DB, GM, RCM, EA, NM, MGJ, CSB, YRR, RAG, JP, AN, MHW, SC, NA, AS, AK, HH, KCT, CGF, ND played a role in drafting the manuscript. DB, GM, RCM, EA, NM, MGJ, CSB, YRR, RAG, JP, AN, MHW, SC, NA, AS, AK, HH, KCT, CGF, ND were involved in critical revision of the manuscript. GM performed the statistical analysis. ND supervised the project.








