Introduction
Palms are an iconic feature of the Caribbean landscape and are associated with strong folk and ethnobotanical traditions (Liogier, Reference Liogier1978; Leiva Sánchez, Reference Leiva Sánchez1999). The Caribbean Islands hold 103 endemic species of palms, with Cuba (65) and Hispaniola (20) hosting the majority (Moya & Leiva Sánchez, Reference Moya and Leiva Sánchez2000; Roncal et al., Reference Roncal, Zona and Lewis2008; Freid et al., Reference Freid, Francisco-Ortega and Jestrow2014). The islands have two endemic palm genera: Hemithrinax (four species) from Cuba and the monotypic Zombia from Hispaniola. The most widespread genera with endemic species can be divided into two major groups. The first group (c. 18 endemic species in 10 genera) has few species in the Caribbean Islands but many taxa from the Neotropical mainland. The second group comprises genera with their centre of diversity in these islands (c. 80 species in six genera: Coccothrinax, Copernicia, Gaussia, Pseudophoenix, Roystonea and Thrinax) and with few species occurring on the mainland (Zona et al., Reference Zona, Verdecia, Leiva Sánchez, Lewis and Maunder2007; Roncal et al., Reference Roncal, Zona and Lewis2008). Among them, Coccothrinax is the genus with the highest number of species in the Caribbean Islands.
Coccothrinax is regarded as being taxonomically difficult and in need of further systematic revision (Zona, Reference Zona1997). Two prior taxonomic revisions focused on species from Cuba (León, Reference León1939) and the southern Greater Antilles (Bailey, Reference Bailey1939). Prior to these two works, Burret (Reference Burret1929) provided a taxonomic account of the taxa on Cuba and Hispaniola. However, Burret's (Reference Burret1929) publication was based solely on material collected by the Swedish botanist and plant collector Leonard Ekman (1883–1931). In the 1980s the Hungarian botanist A. Borhidi (1932–) and the Caribbean botanist O. Muñiz (1937–2007) described new species for Cuba, resulting in a palm catalogue for the island (Muñiz & Borhidi, Reference Muñiz and Borhidi1982). Additional attempts were made to clarify the taxonomy of Coccothrinax (Nauman & Sanders, Reference Nauman and Sanders1991a,Reference Nauman and Sandersb), based on phylogenetic analyses of morphological traits mostly observed from cultivated plants.
The current taxonomic uncertainties within this genus arise, in part, from the work of Henderson et al. (Reference Henderson, Galenao and Bernal1995), whose influential field guide for New World palms recognized only 14 species. Nevertheless, most palm taxonomists and botanists working on the Caribbean Islands (e.g. Moya & Leiva Sánchez, Reference Moya and Leiva Sánchez2000; Acevedo-Rodríguez & Strong, Reference Acevedo-Rodríguez and Strong2012; Greuter & Rankin, Reference Greuter and Rankin2016) do not follow that narrow taxonomic view and still recognize many species that were synonymized by Henderson et al. (Reference Henderson, Galenao and Bernal1995). For instance, C. jamaicensis and C. proctorii are currently regarded as distinct species endemic to Jamaica and the Cayman Islands, respectively (Proctor, Reference Proctor2012; Duno de Stefano & Moya, Reference Duno de Stefano and Moya2014). Likewise, botanists from Cuba and Hispaniola still accept Coccothrinax species that Henderson et al. (Reference Henderson, Galenao and Bernal1995) synonomized (e.g. González-Oliva et al., Reference González-Oliva, González-Torres, Palmarola and Barrios2014, Reference González-Oliva, González-Torres, Palmarola, Barrios and Testé2015; Peguero et al., Reference Peguero, Veloz, García, Clase, De Los Santos, Jones and Jiménez2015b; Verdecia, Reference Verdecia2015).
The current available taxonomic framework for Coccothrinax is not the result of a single monographic endeavour, and therefore as a working taxonomy for our contribution we follow the classification system currently accepted by most palm biologists and plant taxonomists working in the region (Fig. 1). Therefore, we consider that the genus has 54 species, with 51 of these endemic to the Caribbean Islands (Fig. 1). These island endemics are restricted to the Bahamas and the Greater Antilles (except Puerto Rico). The taxonomic placement of C. alta, thought to be endemic to Puerto Rico and the Virgin Islands (Acevedo-Rodríguez & Strong, Reference Acevedo-Rodríguez and Strong2005), needs further study. In this review, C. alta is considered a synonym of C. barbadensis following the recent treatment of Acevedo-Rodríguez & Strong (Reference Acevedo-Rodríguez and Strong2012).
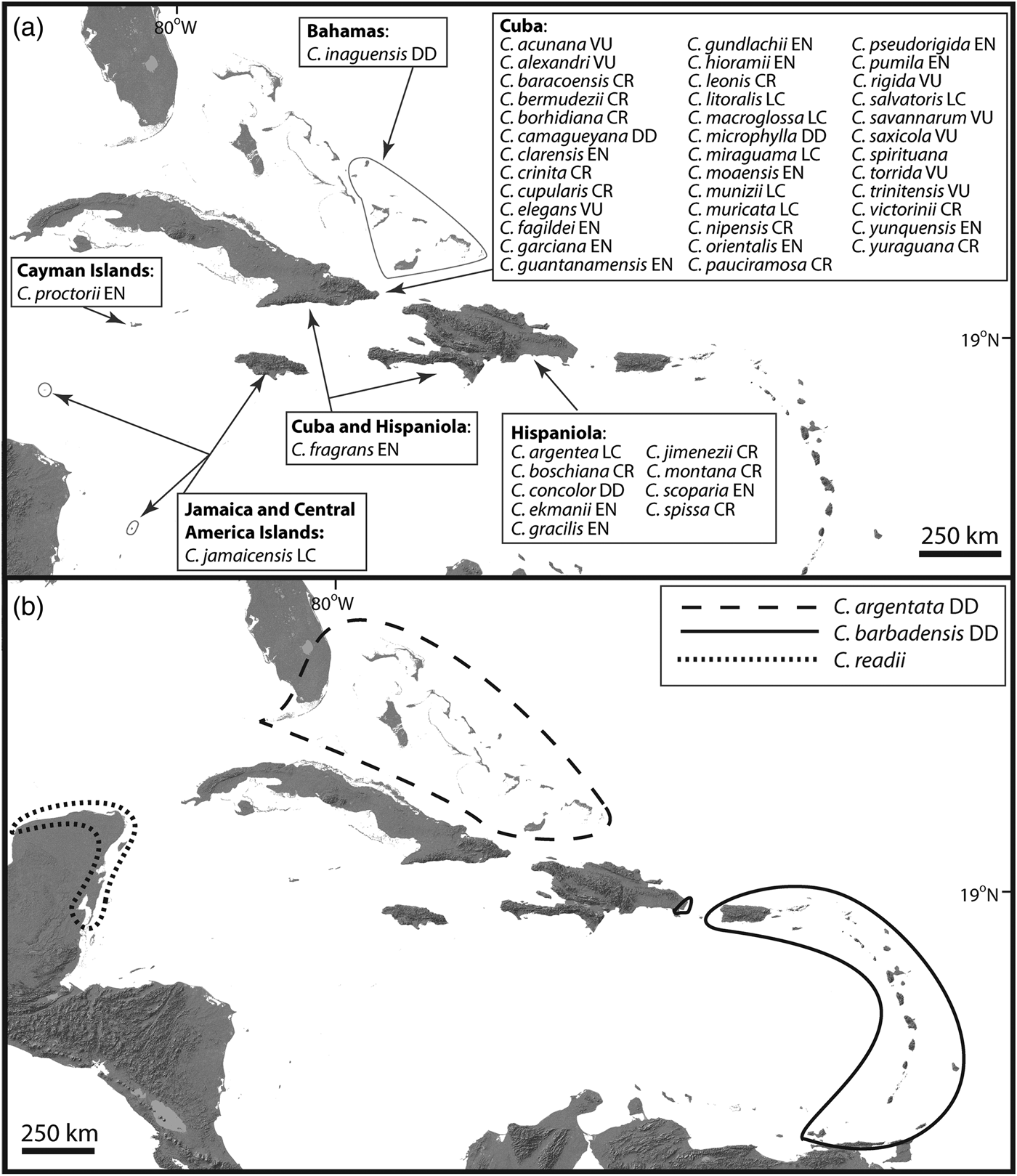
Fig. 1 Geographical distribution and Red List status of Coccothrinax species: (a) Caribbean Island endemics; (b) species found both on the Neotropical mainland and on the islands. Red List categorizations (LC, Least Concern; VU, Vulnerable; EN, Endangered; CR, Critically Endangered; DD, Data Deficient) follow the IUCN (2001, 2014) guidelines and come from Zona et al. (Reference Zona, Verdecia, Leiva Sánchez, Lewis and Maunder2007), Burton (Reference Burton2008), González-Oliva et al. (Reference González-Oliva, González-Torres, Palmarola and Barrios2014, Reference González-Oliva, González-Torres, Palmarola, Barrios and Testé2015) and Peguero et al. (Reference Peguero, Veloz, García, Clase, De Los Santos, Jones and Jiménez2015b). Taxonomy and geographical distribution follow Moya & Leiva Sánchez (Reference Moya and Leiva Sánchez2000), Hoyos Fernández & Braun (Reference Hoyos Fernández and Braun2001), Acevedo-Rodríguez & Strong (Reference Acevedo-Rodríguez and Strong2012), Proctor (Reference Proctor2012), Duno de Stefano & Moya (Reference Duno de Stefano and Moya2014), Freid et al. (Reference Freid, Francisco-Ortega and Jestrow2014), Peguero et al. (Reference Peguero, Jiménez, Joseph, Cinea, Griffith, Francisco-Ortega and Jestrow2015a,Reference Peguero, Veloz, García, Clase, De Los Santos, Jones and Jiménezb), Verdecia (Reference Verdecia2015), Greuter & Rankin (Reference Greuter and Rankin2016), Moya et al. (Reference Moya, Verdecia and Martínez-Pentón2017b) and Jestrow (unpubl. data).
Two Caribbean species (C. argentata and C. barbadensis) are found both on the islands and on the mainland, and only one species (C. readii) is restricted to the mainland (Fig. 1). The vast majority of Caribbean Island endemic species are single-island endemics, with the exception of C. fragrans (found on Hispaniola and Cuba). Reports of the presence of C. jamaicensis on the small islands of Providencia, Colombia (Galeano-Garces, Reference Galeano-Garces1986), and Swan, Honduras (Nelson & Proctor, Reference Nelson and Proctor1994), need to be validated by additional taxonomic studies. For intraspecific taxa, C. alexandri, C. clarensis, C. crinita and C. salvatoris have one subspecies each, and C. miraguama has three subspecies.
Given its high number of species, many with restricted ranges in the Caribbean Islands, Coccothrinax provides a good case to study regional species conservation issues in the Caribbean. We provide a review of conservation challenges and perspectives for the 14 Critically Endangered species of Coccothrinax that are restricted to the Caribbean Islands, covering (1) relationships between taxonomy and conservation, (2) integrative species conservation management, (3) in situ and ex situ conservation, (4) plant exploration, (5) the role of DNA data in understanding phylogenetic relationships and patterns of genetic diversity, and (6) outreach and environmental education.
Based on our own conservation initiatives we assert that international collaboration within the region provides the best possible approach to deal with these six issues. Similar conservation challenges are faced by other threatened endemics of the Caribbean Islands (reviewed by Maunder et al., Reference Maunder, Leiva, Santiago-Valentín, Stevenson, Acevedo-Rodríguez and Meerow2008, Reference Maunder, Abdo, Berazaín, Clubbe, Jiménez, Leiva, Bramwell and Caujapé-Castells2011, and Carey et al., Reference Carey, Gape, Manco, Hepburn, Smith and Knowles2014), and we propose that conservation initiatives centred on Coccothrinax could be applied effectively to other taxa. A main goal of this review is to provide a framework for land managers, conservationists, palm biologists and environmental educators that will offer a regional perspective for what we consider the flagship palm genus for conservation in the Caribbean Islands. This is particularly relevant as this Biodiversity Hotspot comprises several countries with a range of historical and sociological backgrounds (Maunder et al., Reference Maunder, Leiva, Santiago-Valentín, Stevenson, Acevedo-Rodríguez and Meerow2008, Reference Maunder, Abdo, Berazaín, Clubbe, Jiménez, Leiva, Bramwell and Caujapé-Castells2011).
Data collection pertinent to ex situ conservation of these threatened species in botanic gardens, as well as literature reviews, were conducted during January 2016–March 2017. Field observations were conducted during 2015–2016.
Ecology, biology, and conservation biology of the Critically Endangered species of Coccothrinax
Red List assessments of Coccothrinax species
Zona et al. (Reference Zona, Verdecia, Leiva Sánchez, Lewis and Maunder2007) assessed the conservation status of species of Coccothrinax of the Caribbean Islands using the IUCN Red List categories and criteria (IUCN, 2001, 2014). Other Red List assessments have targeted Coccothrinax and other endemic plant species of the Cayman Islands (Burton, Reference Burton2008), Cuba (Rankin Rodríguez & Areces Berazaín, Reference Rankin Rodríguez and Areces Berazaín2003; Berazaín Iturralde et al., Reference Berazaín Iturralde, Areces Berazaín, Lazcano Lara and González-Torres2005; González-Oliva et al., Reference González-Oliva, González-Torres, Palmarola and Barrios2014, Reference González-Oliva, González-Torres, Palmarola, Barrios and Testé2015; González-Torres et al., Reference González-Torres, Palmarola, González Oliva, Bécquer, Testé and Barrios2016) and Hispaniola (Peguero & Jiménez, Reference Peguero and Jiménez2011; Peguero et al., Reference Peguero, Veloz, García, Clase, De Los Santos, Jones and Jiménez2015b). Of the 51 species of Coccothrinax endemic to the Caribbean Islands, 16 are categorized as Endangered and 14 as Critically Endangered, with 10 of the latter restricted to Cuba (Fig. 2).
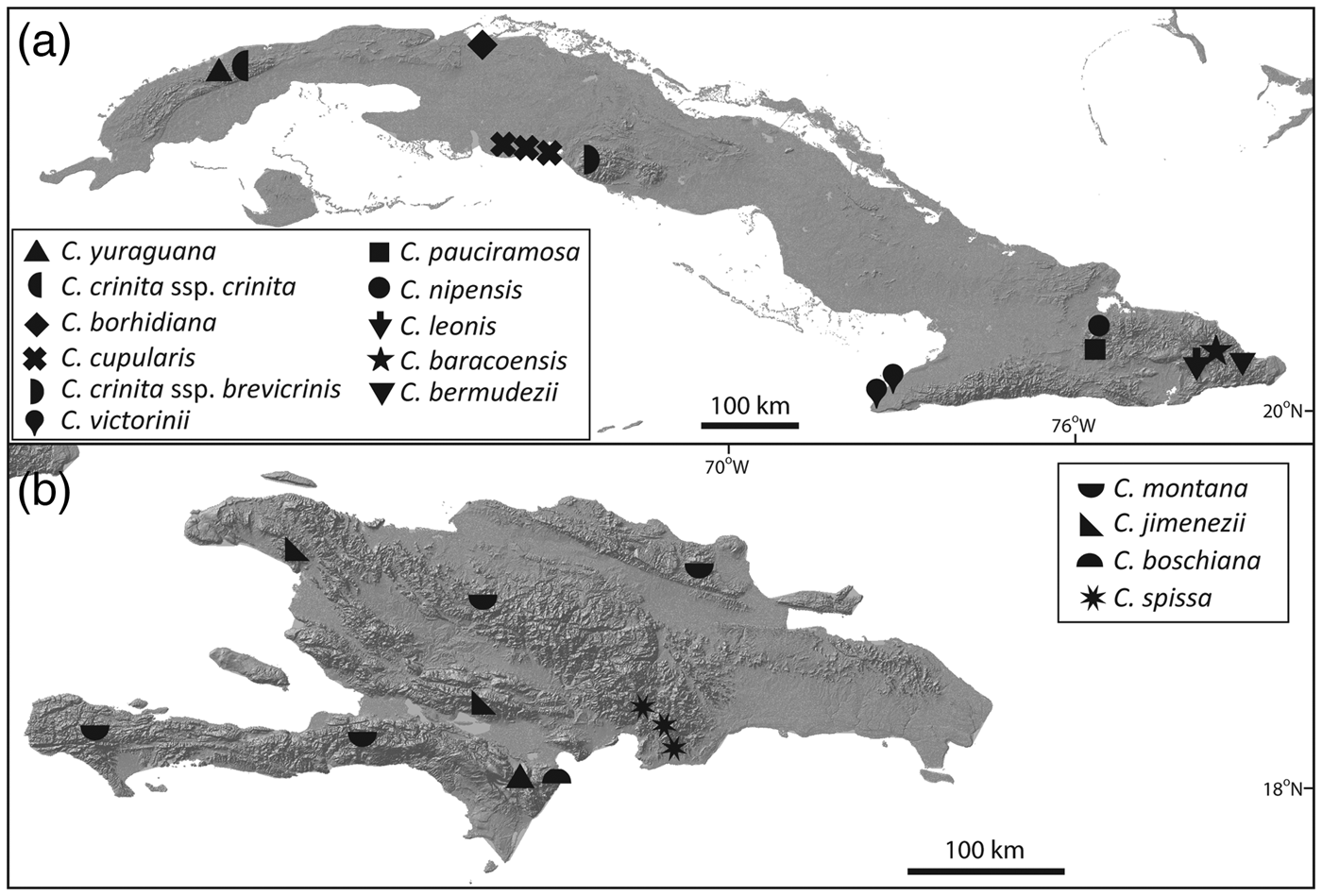
Fig. 2 Distribution of the 14 Critically Endangered species of Coccothrinax. (a) Cuban taxa; the three sites for C. cupularis represent the distribution range of the highly fragmented population of this species. (b) Hispaniolan taxa.
The Cuban endemic C. camagueyana was originally described by Muñiz & Borhidi (Reference Muñiz and Borhidi1981), and is reported from the south of Sierra de Cubitas, Camagüey province (Méndez Santos et al., Reference Méndez Santos, Gueorguievich Elemevsky, Risco Villalobos, Martínez Jiménez and Trujillo Sánchez1989). It was categorized as Critically Endangered by Zona et al. (Reference Zona, Verdecia, Leiva Sánchez, Lewis and Maunder2007); however, its taxonomic status is unclear, and more recently it was categorized as Data Deficient (González-Oliva et al., Reference González-Oliva, González-Torres, Palmarola, Barrios and Testé2015). Field exploration led by CM in 2016 failed to find this species in the location from which it was originally reported (Moya et al., Reference Moya, Morales Pérez, Méndez Santos and Martínez-Pentón2017a).
All of the Critically Endangered species have been assigned to this category based on the criteria B1ab or B2ab (IUCN, 2001, 2014; Table 1); i.e. they have an extent of occurrence < 100 km2 or an area of occupancy < 10 km2. They are restricted to a single locality or to a fragmented site. In addition, they show continuing decline in their extent of occurrence, area of occupancy, habitat quality, number of sites, or number of mature individuals (IUCN, 2001, 2014). Eight of these species are shown in Plates 1 and 2.

Plate 1 Critically Endangered species of Coccothrinax: (a) C. jimenezii, (b) C. borhidiana (in ex situ collection of Montgomery Botanical Center), (c) C. spissa, (d) C. victorinii (in ex situ collection of Jardín Botánico de Cupainicú). Photograph credits: (a) Francisco Jiménez, (b) Patrick Griffith, (c) Scott Zona, and (d) Raúl Verdecia.

Plate 2 Critically Endangered species of Coccothrinax (all in habitat): (a) C. yuraguana, (b) C. montana, (c) C. boschiana, (d) C. jimenezii (heavily harvested to make brooms). Photograph credits: (a) Lisbet González-Oliva, (b–d) Brett Jestrow.
Table 1 Red List assessment criteria (sensu IUCN, 2001, 2014), ecology, number of populations, demographics, and the in situ and ex situ conservation status of the 14 Critically Endangered species of Coccothrinax.
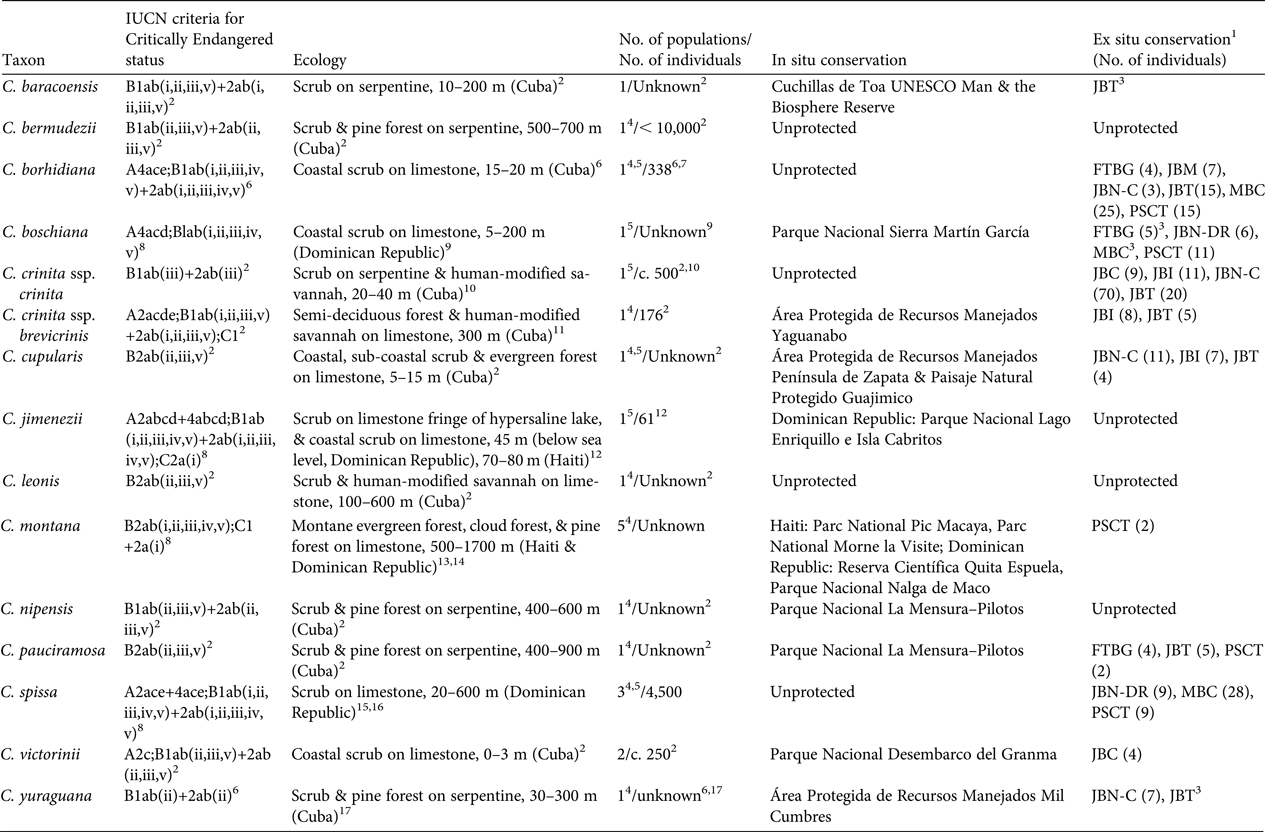
1 Botanic gardens with ex situ collections based on wild collected individuals are as follows: FTBG, Fairchild Tropical Botanic Garden, USA; JBC, Jardín Botánico de Cupainicú, Cuba; JBI, Jardín Botánico de Cienfuegos; JBN-C, Jardín Botánico Nacional, Cuba; JBN-DR, Jardín Botánico Nacional, Dominican Republic; JBM, Jardín Botánico de Matanzas, Cuba; JBT, Jardín Botánico de las Tunas, Cuba; MBC, Montgomery Botanical Center, USA; PSCT, Palmetum de Santa Cruz de Tenerife, Spain
2 González-Oliva et al. (Reference González-Oliva, González-Torres, Palmarola, Barrios and Testé2015)
3 Ex situ collection has seedlings that have not yet been transplanted to the field
4 Highly fragmented population(s)
5 Population(s) exhibit(s) recruitment
6 González-Oliva et al. (Reference González-Oliva, González-Torres, Palmarola and Barrios2014)
7 Enríquez Rodríguez et al. (Reference Enríquez Rodríguez, Robledo Ortega and Cruz Nardo2006)
9 Mejía & García (Reference Mejía and García1997)
10 Leiva Sánchez et al. (Reference Leiva Sánchez, Verdecia, Franco Flores, Ojeda and Urquiola2008)
11 Suárez Oropesa (Reference Suárez Oropesa2015)
12 Peguero et al. (Reference Peguero, Veloz, García, Clase, De Los Santos, Jones and Jiménez2015b)
13 Burret (Reference Burret1929)
14 Judd (Reference Judd1987)
15 Peguero et al. (Reference Peguero, Veloz, García, Clase, De Los Santos, Jones, Jiménez, Lagos-Witte, Sanabria Diago, Chacón and García2011)
16 Veloz et al. (Reference Veloz, Peguero and Clase2011)
17 Urquiola Cruz et al. (Reference Urquiola Cruz, González-Oliva, Novo Carbó and Acosta Ramos2010)
Ecology, distribution and demography
Of the 14 Critically Endangered species of Coccothrinax, most occur on limestone soils, with six Cuban taxa (five species and one subspecies) occurring on serpentine soils (Table 1). Only five species occur in coastal environments (Table 1). One of the two populations of C. jimenezii occurs on the fringes of Lago Enriquillo in the Dominican Republic, a hypersaline lake 45 m below sea level, which is currently expanding (Romero Luna & Poteau, Reference Romero Luna and Poteau2011), and where the wild populations are threatened by saline intrusions (Peguero et al., Reference Peguero, Jiménez, Joseph, Cinea, Griffith, Francisco-Ortega and Jestrow2015a).
Among the inland species, the Hispaniolan endemic C. montana occurs at the highest elevations, to 1,700 m; it was described from the Cordillera Central in the Dominican Republic and the Massif de la Selle in Haiti (Burret, Reference Burret1929). However, its distribution is poorly understood, with populations reported from three disjunct sites in the Dominican Republic (Cordillera Septentrional, Cordillera Central, and Sierra de Bahoruco) and two sites in southern Haiti (Massif de la Hotte and Massif de la Selle) (Burret, Reference Burret1929; Judd, Reference Judd1987; B. Peguero, unpubl. data). We have no recent data on population size and distribution ranges for C. montana except from the Massif de la Hotte, where there are two populations of fewer than seven individuals each (B. Jestrow, unpubl. data), and from Bahoruco, where a single population with very few individuals is known to exist. Fieldwork is needed to determine the taxonomic and distribution status of this species both in Haiti and the Dominican Republic. Reports of the Hispaniola endemic C. spissa in northern Haiti (Bailey, Reference Bailey1939) need to be confirmed (Henderson et al., Reference Henderson, Aubry, Timyan and Balick1990).
We have population estimates for only five of the 14 Critically Endangered species of Coccothrinax (Table 1). Coccothrinax cupularis, C. leonis, C. nipensis and C. pauciramosa each have a single but dispersed population comprising scattered individuals; fieldwork is required to confirm their distribution range, population sizes and demographic status.
Population recruitment has been recorded for six taxa (Table 1). Based on preliminary field observations, both C. yuraguana and C. pauciramosa also appear to have recruitment in their single locations. Coccothrinax jimenezii has low levels of recruitment, with seedlings found only in the Dominican Republic population (Peguero et al., Reference Peguero, Jiménez, Joseph, Cinea, Griffith, Francisco-Ortega and Jestrow2015a). There are no population size estimates for eight species.
Conservation challenges
Our review identified six major threats (Table 2). Two of these threats (population decline; urban, agricultural and forest development) affect 11 of the Critically Endangered species. Threats from invasive alien plant species, unsustainable ethnobotanical exploitation, and uncontrolled fires are relevant to seven taxa. Poaching for the horticulture trade has been reported only for C. victorinii. Four species are at risk because of nickel mining or oil drilling. One species (C. crinita) has been detected to hybridize with at least another species of the genus, and one is facing the threat of saline intrusion in soils. Based on our experience in botanic gardens, interspecific hybrids are produced easily in Coccothrinax. These threats are likely to intensify in the near future; for example, nickel mining and oil drilling are major development priorities for the Cuban economy (Wacaster et al., Reference Wacaster, Baker, Soto-Viruet and Textoris2015). Furthermore, invasive alien plant species are regarded as a major challenge for plant biodiversity management in the Caribbean (Maunder et al., Reference Maunder, Leiva, Santiago-Valentín, Stevenson, Acevedo-Rodríguez and Meerow2008, Reference Maunder, Abdo, Berazaín, Clubbe, Jiménez, Leiva, Bramwell and Caujapé-Castells2011; reviewed for Cuba by Oviedo Prieto & González-Oliva, Reference Oviedo Prieto and González-Oliva2015, and González-Torres et al., Reference González-Torres, Palmarola, González Oliva, Bécquer, Testé and Barrios2016; reviewed for the Dominican Republic by Ministerio de Medio Ambiente y Recursos Naturales, 2012) and will be a major concern for in situ conservation of threatened Coccothrinax species. Tourism is an important economic activity in the Greater Antilles (Gayle & Goodrich, Reference Gayle and Goodrich1993), and is a growing industry particularly in Cuba (Hingtgen et al., Reference Hingtgen, Kline, Fernandes and McGehee2015), where the sector is likely to undergo significant development following the easing of U.S. embargo restrictions and the establishment of formal diplomatic relationships in July 2015 (Hershberg & LeoGrande, Reference Hershberg and LeoGrande2016). This may result in an intensification of human pressure on Cuban ecosystems, with a potential negative impact on endemic palms (González-Torres et al., Reference González-Torres, Palmarola, González Oliva, Bécquer, Testé and Barrios2016).
Table 2 Main conservation challenges and conservation biology research conducted for the 14 Critically Endangered species of Coccothrinax (Table 1).
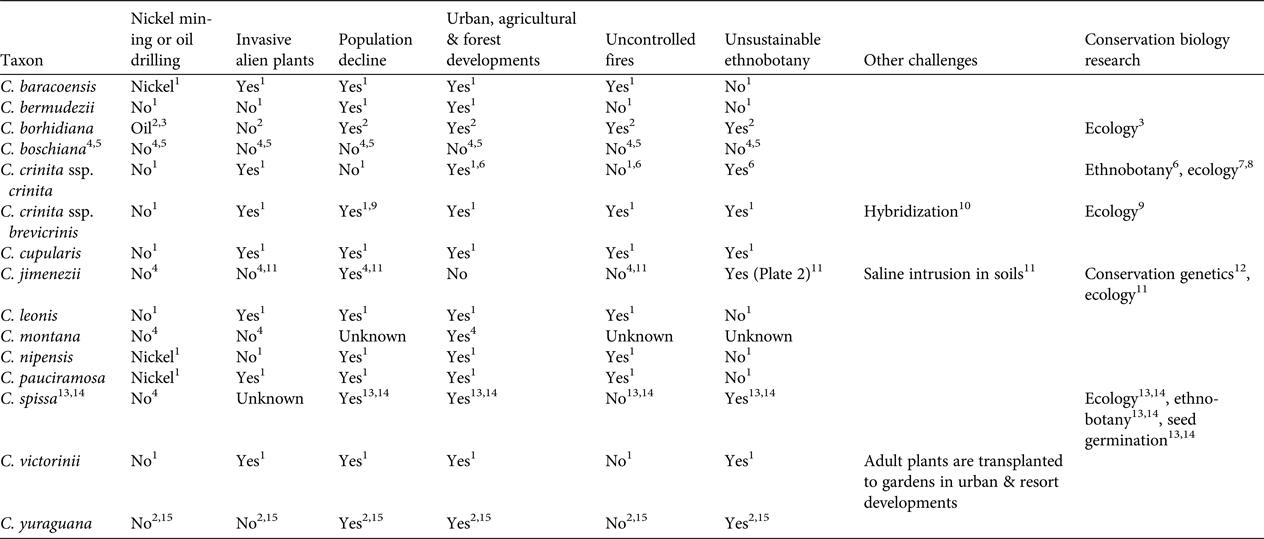
1 González-Oliva et al. (Reference González-Oliva, González-Torres, Palmarola, Barrios and Testé2015)
2 González-Oliva et al. (Reference González-Oliva, González-Torres, Palmarola and Barrios2014)
3 Enríquez Rodríguez et al. (Reference Enríquez Rodríguez, Robledo Ortega and Cruz Nardo2006)
5 Mejía & García (Reference Mejía and García1997)
6 Martínez Betancourt & Miranda (Reference Martínez Betancourt and Miranda2009–Reference Martínez Betancourt and Miranda2010)
7 Leiva Sánchez et al. (Reference Leiva Sánchez, Verdecia, Franco Flores, Ojeda and Urquiola2008)
8 Martínez Betancourt (Reference Martínez Betancourt2016)
9 Suárez Oropesa et al. (Reference Suárez Oropesa, Sosa Rodríguez, Vega Marrero, Gómez Brito and Panfet Valdés2013)
10 Suárez Oropesa (Reference Suárez Oropesa2015)
11 Peguero et al. (Reference Peguero, Jiménez, Joseph, Cinea, Griffith, Francisco-Ortega and Jestrow2015a)
12 Jestrow et al. (Reference Jestrow, Peguero, Joseph, Cinea and Francisco-Ortega2016b)
13 Peguero et al. (Reference Peguero, Veloz, García, Clase, De Los Santos, Jones, Jiménez, Lagos-Witte, Sanabria Diago, Chacón and García2011)
14 Veloz et al. (Reference Veloz, Peguero and Clase2011)
15 Urquiola Cruz et al. (Reference Urquiola Cruz, González-Oliva, Novo Carbó and Acosta Ramos2010)
Five of the Critically Endangered taxa are not recorded from protected areas (Table 1), and those found within protected areas of the Dominican Republic are also of major conservation concern. From our field observations it appears that most of these protected areas (with the exception of Parque Nacional Nalga de Maco and Reserva Científica Quita Espuela) have poor conservation enforcement (Powell & Inchaustegui, Reference Powell and Inchaustegui2009; Pasachnik et al., Reference Pasachnik, Carreras De León and León2016).
Eleven of the Critically Endangered taxa are cultivated in botanic gardens (Table 1). These collections are located in Cuba (five botanic gardens), the Dominican Republic (one), Spain (one), and USA (two) and they are based on wild collected germplasm. Four of the Critically Endangered species of Coccothrinax are not held in ex situ conservation collections (Table 1).
Conservation biology research
Only four taxa have been the focus of conservation research that included population size studies, ethnobotanical surveys, ecological studies or seed germination protocols (Table 2). Coccothrinax jimenezii is the only Critically Endangered species that has been the subject of conservation genetics studies (Jestrow et al., Reference Jestrow, Peguero, Jiménez, Cinea, Hass and Reeve2016a). This work was based on microsatellite markers and included the two known populations of the species, with the majority of individuals being sampled. Levels of genetic differentiation between the two populations of C. jimenezii are unusually high compared to those reported among populations of other palm species. This suggests that species delimitation within Coccothrinax needs to be re-evaluated, and a clear taxonomic framework for conservation initiatives is needed. Despite their small size, both populations of C. jimenezii have a relatively large number of unique alleles and there is no evidence of genetic bottlenecks. Only the population in Haiti exhibits evidence of inbreeding, with a moderately high positive inbreeding coefficient value (Fis = 0.232 vs Fis = 0.093 for the Dominican Republic population). Recent habitat fragmentation coupled with long generation times could explain the unusually high levels of genetic diversity in these Caribbean species (Jestrow et al., Reference Jestrow, Peguero, Jiménez, Cinea, Hass and Reeve2016a). Future genetic studies may find that at least some of the remaining Critically Endangered species of Coccothrinax for which there are currently no population genetic data available still harbour high genetic diversity.
Conservation and research agenda
The conservation management of threatened plants faces particular challenges in the Caribbean Islands (Brown et al., Reference Brown, Geoghegan and Renard2007; Maunder et al., Reference Maunder, Leiva, Santiago-Valentín, Stevenson, Acevedo-Rodríguez and Meerow2008, Reference Maunder, Abdo, Berazaín, Clubbe, Jiménez, Leiva, Bramwell and Caujapé-Castells2011; Carey et al., Reference Carey, Gape, Manco, Hepburn, Smith and Knowles2014), most notably the diversity of political, cultural and socio-economic systems. We propose the following conservation and research framework for Coccothrinax.
Establish a regional conservation approach
Regional cooperation is required to utilize and coordinate the existing skills and resources of the various Caribbean nations more effectively. With endemic Critically Endangered species in three countries, a comprehensive regional approach to the conservation of Coccothrinax will require collaboration between managers and plant biologists from Cuba, the Dominican Republic and Haiti. Conservation efforts for C. jimenezii have been structured within the context of broad international cooperation led by the Jardín Botánico Nacional (Dominican Republic) and Jardin Botanique des Cayes (Haiti) in association with botanists from South Florida (Fairchild Tropical Botanic Garden, Montgomery Botanical Center, U.S. Department of Agriculture, and Florida International University). There have been extensive plant exploration expeditions in both Haiti and the Dominican Republic, including demographic studies and conservation assessments (Peguero et al., Reference Peguero, Jiménez, Joseph, Cinea, Griffith, Francisco-Ortega and Jestrow2015a). Outreach outputs included the production of printed educational material presented during the VI Simposio Flora de La Española in June 2015 at Santo Domingo, Dominican Republic. Furthermore, we have established joint initiatives for professional progression of young Caribbean botanists, including two workshops on plant systematics in Haiti, training of one graduate student from the Dominican Republic at Florida International University (Rodríguez, Reference Rodríguez2014), and developing grant-supported projects for palm conservation through the Prince Bernhard Nature Fund, the International Palm Society, and the Mohamed bin Zayed Species Conservation Fund.
Botanists from the Dominican Republic have developed successful protocols for seed germination of C. spissa (Peguero et al., Reference Peguero, Veloz, García, Clase, De Los Santos, Jones, Jiménez, Lagos-Witte, Sanabria Diago, Chacón and García2011; Veloz et al., Reference Veloz, Peguero and Clase2011), which may also be applicable for the Critically Endangered species from Cuba. Across the distribution range of Coccothrinax there are field botanists and palm taxonomists who are familiar with its ecology and morphology. Their preliminary surveys suggest that hybridization detected in botanic gardens is also common in areas where species overlap (Suárez Oropesa, Reference Suárez Oropesa2015). Caribbean island conservation biologists working with this genus have also gained experience regarding unsustainable ethnobotanical practices, and are familiar with the challenges faced by land managers and administrators regarding implementation of effective protection in the protected areas of Cuba, the Dominican Republic and Haiti. Preliminary molecular results suggest that the microsatellite loci used for C. jimenezii (Jestrow et al., Reference Jestrow, Peguero, Jiménez, Cinea, Hass and Reeve2016a) are also applicable to other species of this genus. The experiences already gained by individual teams provide a framework for development of a biodiversity strategy for Coccothrinax in the region.
Establish a scientific framework for conservation
A phylogenetic analysis and taxonomic review would clarify the conservation status of Coccothrinax taxa. For instance, C. jimenezii was described as a new species in 2013 (Mejía & García, Reference Mejía and García2013) but molecular studies revealed uncertainties in the taxonomy of the two populations of this species (Jestrow et al., Reference Jestrow, Peguero, Jiménez, Cinea, Hass and Reeve2016a), highlighting the need for a comprehensive taxonomic revision for Coccothrinax. The morphological phylogenetic study of the genus undertaken by Nauman & Sanders (Reference Nauman and Sanders1991a,Reference Nauman and Sandersb) identified three major groups, each one including Critically Endangered species, with high levels of homoplasy for the morphological characters. Molecular phylogenetic reconstructions for Coccothrinax (Roncal et al., Reference Roncal, Zona and Lewis2008) did not resolve major clades. However, the conservation genetics study of C. jimenezii (Jestrow et al., Reference Jestrow, Peguero, Jiménez, Cinea, Hass and Reeve2016a) demonstrated the utility of microsatellite DNA markers for insights into both genetic diversity and taxonomy. There are still many gaps in knowledge of the biology of the species, and there have been no studies focused on breeding systems, plant–animal interactions, competition from invasive alien plant species, or population viability analyses.
Assess and monitor wild populations
There is a need to expand botanical field work in the range nations, particularly in Hispaniola, where there are still under-collected areas, mostly in Haiti. Although Cuba and the Dominican Republic have maintained a tradition of botanical fieldwork and research, Haiti has not had sustained support for field botany. Both the Dominican Republic and Haiti have a single botanic garden, and are key to developing a framework for future conservation initiatives. Through our inter-institutional collaborations (mostly supported by the Mohamed bin Zayed Species Conservation Fund), members of our team have been exploring areas of Haiti seldom visited since Ekman was there in the 1920s (Jestrow et al., Reference Jestrow, Peguero, Joseph, Cinea and Francisco-Ortega2016b). Fieldwork led by RV resulted in the rediscovery of C. rigida on limestone cliffs near Sagua de Tánamo (Holguín province) in 2014 (Verdecia et al., Reference Verdecia, Gómez, Leyva Bermúdez and Hernández Montero2014). This species had not been seen since it was described by Grisebach (Reference Grisebach1866) based on material collected by the American plant explorer Charles Wright (1811–1885). Recent plant exploration activities led to the discovery of a new species, Coccothrinax spirituana, in Cuba by CM and RV (Moya et al., Reference Moya, Verdecia and Martínez-Pentón2017b). This new species is currently known in a single locality in the province of Sancti Spiritus in an area of serpentine soils. Studies focusing on its demography and distribution range are in progress.
There are four Coccothrinax species that have not been located since they were originally discovered. The first, C. acunana, was described by León (Reference León1939), and plants of this species were reported to occur only in the highest mountains of Cuba, near Pico Turquino, in the province of Santiago de Cuba. The species is categorized as Vulnerable on the Red List for the Cuban flora (González-Torres et al., Reference González-Torres, Palmarola, González Oliva, Bécquer, Testé and Barrios2016); however, its conservation status needs to be reassessed. The second species, C. microphylla, is another Cuban endemic, described by Muñiz & Borhidi (Reference Muñiz and Borhidi1981). It appears to be restricted to limestone cliffs of Abra de Mariana in the lowlands of Guantanamo province. The third species, C. concolor, is known from only one herbarium collection, by Ekman in 1926. The species was described by Burret (Reference Burret1929) and reported from low-elevation areas with volcanic soils near Jacmel, southern Haiti (Bailey, Reference Bailey1939). The fourth species, C. camagueyana, not only has an uncertain taxonomic status but has not been found since it was described (Moya et al., Reference Moya, Verdecia and Martínez-Pentón2017b).
Ensure wild populations are protected in situ, with supporting ex situ collections
The Critically Endangered species of Coccothrinax are facing a variety of threats. Five of the species are not present in protected areas, and four are not included in ex situ germplasm collections. An integrative approach to conservation is the most immediate priority (Esposito, Reference Esposito2002; Petriello & Wallen, Reference Petriello and Wallen2015). We are aware that in many cases official protected areas are not sufficiently resourced to ensure viability of wild populations; however, both Cuba and the Dominican Republic have protected areas with effective conservation management. Ex situ conservation is necessary in some cases; for instance, the Dominican Republic population of C. jimenezii is facing an immediate threat from the increasing water level of the hypersaline lake Lago Enriquillo, and the small population in Haiti is overharvested.
Establishing an ex situ resource should be part of an integral approach for conservation planning that involves all stakeholders (Pritchard et al., Reference Pritchard, Fa, Oldfield and Harrop2012; McGowan et al., Reference McGowan, Traylor-Holzer and Leus2017). Ideally this resource would be held primarily in the range state, and should ensure adequate genetic representation (Oldfield, Reference Oldfield2009). There are botanic gardens located outside the Caribbean Islands that have ex situ conservation of palms as a mission priority (Maunder et al., Reference Maunder, Lyte, Dransfield and Baker2001). Several of these (e.g. Fairchild Tropical Botanic Garden, Montgomery Botanical Center, and Palmetum of Tenerife) are already collaborating with botanists from Hispaniola and Cuba to develop representative living collections that hold duplicates of living germplasm collections and can mitigate issues regarding limited space and resources for ex situ conservation.
Conclusions
This review is derived from a 15-year collaboration focused on the palm diversity of the Greater Antilles. Based on the results of our research of the conservation biology of palms, we have proposed specific initiatives for the conservation of the most threatened species of Coccothrinax. Our collaborative approach to integrative plant conservation provides a model for other groups of conservation concern. This model is based on regional collaboration, building a framework for genetic conservation, and ensuring the professional progression of new Caribbean botanists. We acknowledge the need for increased conservation awareness of these species, particularly in the communities where they occur.
Acknowledgements
We dedicate this study to the memory of Angela T. Leiva Sánchez (1948–2014) in recognition of her legacy of supporting palm conservation research in the Caribbean Islands. This study was supported with a grant from the Mohamed bin Zayed Conservation Fund (grant number 14258589, through the International Center for Tropical Botany, Florida International University). Fairchild Tropical Botanic Garden provided matching funds to conduct molecular research. The Consejo Nacional de Investigaciones Agropecuarias y Forestales of the Dominican Republic supported in-country conservation assessments of Critically Endangered palms (project number CAD/014-05/RN, through the Consorcio Ambiental Dominicano and the Jardín Botánico Nacional). We thank Carlo Morici for providing data pertinent to the ex situ collections of Palmetum de Santa Cruz de Tenerife, and Scott Zona for sharing insights concerning taxonomic challenges associated with Coccothrinax and allied genera. This is contribution number 338 from the Tropical Biology Program of Florida International University.
Author contributions
BJ, BP, FJ and WC led field work in Hispaniola and reviewed conservation challenges for Haiti and the Dominican Republic. CEM, RV and LGO led field work in Cuba and reviewed conservation challenges for Cuba. MPG made significant contributions pertinent to ex situ conservation. AWM provided insights regarding conservation genetics. BJ, JFO and MM developed the proposed conservation and research agenda and wrote the first draft of the article. BJ and JFO led grant proposals to undertake this project, and designed and developed the structure of this study.
Biographical sketches
Brett Jestrow is a herbarium curator and is specialized in tropical plant systematics. Brígido Peguero leads plant exploration activities in the Dominican Republic. Francisco Jiménez is the head of the botany programme at Jardín Botánico Nacional in the Dominican Republic. Raúl Verdecia leads projects focused on the biodiversity of Cuban palms. Lisbet González-Oliva is a conservation biology researcher interested in Cuban threatened species. Celio Moya is a palm biologist who has worked for the Jardín Botánico de Sancti Spíritus, Cuba and for Gemini Botanic Garden, Florida. William Cinea has an interest in plant conservation and botanic garden management. M. Patrick Griffith is specialized in ex situ conservation in botanic gardens. Alan Meerow is a senior research geneticist and systematist. Mike Maunder is a conservationist with a focus on tropical plant species. Javier Francisco-Ortega has an interest in Caribbean plant biodiversity.








