The emerging antibiotic resistance in animals and humans has caused the global media to highlight its importance, to the extent that all meetings of the G20 Summit since 2017 have emphasised combatting the continuing emergence and control of antimicrobial resistance (AMR) in a ‘One Health’ approach (Destoumieux-Garzón et al., Reference Destoumieux-Garzón, Mavingui, Boetsch, Boissier, Darriet, Duboz, Fritsch, Giraudoux, Le Roux, Morand, Paillard, Pontier, Sueur and Voituron2018). In addition, a vocal action group body, the AMR Industry Alliance, engaged with relevant industries to highlight antimicrobial resistance. However, none of these spotlights on AMR address the farming communities which use antibiotics as therapeutic defenses against animal infections.
Mastitis has a long history globally of infection in dairy cows (Ruegg, Reference Ruegg2017). The cow's immune system responds to the infection and sub clinical infections can be recognised by an increase in somatic cell counts (SCC) detected on analysis of the milk (Schukken, et al., Reference Schukken, Wilson, Welcome, Garrison-Tikofsky and Gonzalez2003). Normal milk has a SCC of 100 000 cells/ml, a rise of SCC above 200 000 cells /ml indicates infection, while the European Union (EU) Directive 92/46, (EU, 1992), rules that raw milk with an SCC over 400 000 cells/ml may not be used for human consumption. The EU was the second largest producer of milk in the world in 2019, after India, with the US lagging behind (Statista, 2019).
Both clinical and subclinical mastitis may result in the use of antibiotics at farm level. A review of mastitis treatment can be found by Pyörälä (Reference Pyörälä2009). There are a number of commercial preparations and formulations on the market for use at farm level available under veterinary prescription only (POM-V). Trends in IMM antimicrobial sales data in Ireland from 2003 to 2015 is available (More et al., Reference More, Clegg and McCoy2017). The duration of the antibiotic residues in milk after treatment is a major consideration of dairy milk suppliers, as they want cows to be back in commercial readiness as soon as possible. Monitoring of composite milk samples are taken at farm level for the detection of antibiotics to be tested when the milk arrives at the dairy processing factory. If positive results are obtained, substantial fines are imposed on the dairy farmer. The antibiotic residue tests uses in dairy processing factories include diagnostic kits, generally to test for beta lactam group antibiotics. Different tests kits on the market include Charm©, Delvotest®, Copan, which test for microbial inhibitors, which can be specific for tetracycline, β-lactam and broad-spectrum antibiotics residues. The Charm test is most often used on the composite bulk milk arriving at the milk processing factory. If the composite sample is positive, the sample is further tested on the Delvotest®. If this sample tests positive, the bulk milk is discarded and samples from individual farmers whose milk was contained in the contaminated milk tanker compartment is further investigated to determine which sample failed the processors residue test.
The aim of this study was to determine which intramammary antibiotic treatments are used at dairy farm level.
Material and methods
A direct interview (questionnaire) was used to collect data from dairy farmers (n = 202) attending a national farming event in Ireland in 2017. A list of 10 mastitis intramammary antibiotics, with images of the product box, available from veterinary suppliers was presented. Of the 26 counties in the Republic of Ireland, farmers from 23 of these were interviewed. Two specific questions were addressed: (i) county of residence (ii) mastitis intramammary antibiotic most used product in order of preference. Ethical approval from the University of Limerick ethics committee was obtained for the questionnaire. Personnel at three dairy processing factories were interviewed to determine antibiotic residue testing methods carried out at the milk intake point and what actions were taken if positive results were obtained. An outline of the IMM products and their properties is available in the supplementary material. The data were compiled in Microsoft Office Excel® and analysed.
Results and discussion
The intramammary (IMM) most used and preferences are given in Table 1. The fourth choice Cobactan, was considered to be expensive, but used if conditions were not improving. Some dairy farmers used non-antibiotic treatment including honey and essential oils. The target organisms for all formulations include: Staphylococci spp (including β -lactamase producing strains), Streptococci spp (including S. agalactiae, S. dysgalactiae and S. uberis) and Escherichia coli (including β -lactamase producing strains). The cost of the antibiotic formulations and their milk withdrawal time periods were the main consideration in the dairy farmer's preferences for treatment.
Table 1. Preferential Intermammary (IMM) antibiotics product used by dairy farmers

a Other formulation included alternatives to antibiotics e.g. honey and essential oils.
Testing for antibiotic residues at three milk processing factories showed that once raw bulk milk is delivered to the plant, the Charm MRLBL3 is used. The method is applicable to raw milk, heat-treated milk and reconstituted dried milk. The Charm MRLBL3 tests for penicillin, while the commercial formulations first preference, Synulox (34%) contains amoxicillin/clavulanic acid, (a penicillin and beta lactamase inhibitor) combination which is a critically important antibiotic (CIA) for human and animal use. Therefore the practice of the Charm test at milk intake is valid. If a negative Charm test is obtained then further tests are carried out. The Delvo SPNT is used by the three milk processing factories surveyed and has an extensive listing of antibiotics with breakpoints below the EU- MRL requirements. The other IMM formulations identified as used at farm level contain a selection of antibiotics, especially the aminoglycoside family of antibiotics, and these if present are not initially detected by the Charm test. Therefore a more comprehensive testing methodology should be included to test for the antibiotics most used by the dairy farmers. The pharmacological active substance and maximum residue limits (MRL) allowed in milk in each of the preferred IMM formulations are given in Table 2, together with their World Organization for Animal Health (OIE, 2018) categorisation and World Health Organization classification (WHO, 2019).
Table 2. Intramammary formulation active pharmaceutical ingredient
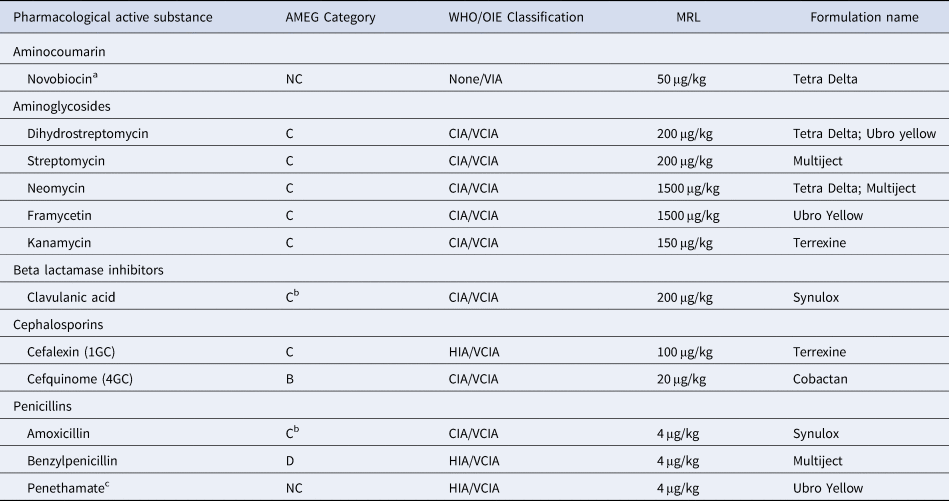
AMEG, Antimicrobial Expert Group categories, A, Avoid; B, Restrict; C, Caution and D, Prudence, use (source EMA, 2019); WHO, World Health Organisation (source WHO, 2019). CIA, critically important antimicrobial; HIA, highly important antimicrobial; OIE, World Organisation for Animal Health, VIA, veterinary important antimicrobial; VCIA, Veterinary critically important antimicrobial (source OIE, 2018); MRL, maximum residue limits, (source EU, 2010); NC, Not categorised by AMEG; 1GC, 4GC first and fourth generation cephalosporin.
a Antibiotic not used in human medicine; therefor it does not have a WHO classification.
b in AMEG Category C when used together as amoxicillin/clavulanic acid, otherwise ampicillin as D.
c Only used in bovine preparations (WHO, 2019).
The OIE (2018), have deemed that antibiotics that are critically important in veterinary medicine should be identified, to complement the identification of antibiotics used in human medicine. Most of the antibiotics used in the IMM formulations identified in this study (Table 2) have been classified by the WHO as critically important antimicrobials (CIA) (WHO, 2019). The EU, Antimicrobial Expert Group (AMEG), has refined its ranking of antibiotics for veterinary use by adding additional risk categories (A-Avoid; B- Restrict; C-Caution and D- Prudence) (EMA, 2019). A focus was on the cephalosporin (3rd and higher generations) as they are known to select for cephalosporin-resistant Salmonella spp., and E. coli in animals, and are the only antibiotics available for these infections in humans and children (WHO, 2019). Of the antibiotics used in the IMM preparations studied, only penethamate (in Ubro Yellow) is used in bovine animal only formulations. The IMM formulations (Table 2) have antibiotics that are considered veterinary critically important antibiotics (VCIA) with only novobiocin considered a veterinary important antibiotic agent (VIA) (OIE, 2018).
The EU is undertaking new regulations on veterinary medicine/medicated feed to be enforced by the Member States by 2022, and include new measures to manage antibiotic resistance, recently reviewed by More (Reference More2020).
In conclusion, this study provided data on dairy farmer's preferences for IMM formulations products in Ireland and their associated antibiotics for the management of mastitis infections. These data have relevance to efforts to combat further increases in antimicrobial resistance.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0022029921000431
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.




