Subclinical inflammation processes are closely related to the development of common chronic diseases. Healthy subjects with higher concentrations of the inflammatory marker C-reactive protein (CRP) have a higher risk of developing CVD and type 2 diabetes within the next years( Reference Pearson 1 , Reference Kolb and Mandrup-Poulsen 2 ). Thus, dietary supplements with antioxidative or immune modulating properties might be an effective strategy for the prevention of inflammation-related diseases at the population level. However, results from epidemiological studies on antioxidant intake and CRP levels present conflicting scenarios( Reference Scheurig, Thorand and Fischer 3 , Reference Floegel, Chung and von Ruesten 4 ). Low intake of vitamin C and carotenoids was associated with elevated CRP levels in one study( Reference Floegel, Chung and von Ruesten 4 ), whereas their association was found to be non-significant in another study( Reference Scheurig, Thorand and Fischer 3 ). Furthermore, another study found a significant inverse association of vitamin E and CRP only in women( Reference Scheurig, Thorand and Fischer 3 ), while yet another study reported a such significant association in both men and women( Reference Floegel, Chung and von Ruesten 4 ). Therefore, it is not clear if there are sex specific effects. As intervention studies are usually conducted on diseased or high-risk samples( Reference Bae, Jung and Lee 5 – Reference Biniaz, Sadeghi Shermeh and Ebadi 9 ), conclusions cannot be drawn from them for the general healthy population. Moreover, it is possible that effects of intakes of single nutrients differ from those of combined intakes, because nutrients might interact with each other. Besides, there might be dose–effect relationships, which need close investigation( Reference Scheurig, Thorand and Fischer 3 ). Intervention studies which are most reliable to detect effects of nutrient supplementation should begin with sufficient evidence from observational studies on specific target populations, most effective nutrient combinations, and possible dose–response effects. The aim of the present study was, therefore, to investigate the association between intakes of common antioxidative vitamins and minerals from supplements and medications (vitamin E, vitamin C, carotenoids, Se, and Zn)( Reference Hercberg, Galan and Preziosi 10 ) with levels of high-sensitivity C-reactive protein (hs-CRP) in the general population. In this respect, dose–response relationships, effects of single v. multiple use of antioxidants, and potential sex-specific effects were examined.
Methods
Data are based on the German Cooperative Health Research in the Region of Augsburg (KORA) F4 study (2006–8), a follow-up examination of the population-based KORA S4 survey (1999–2001)( Reference Rathmann, Strassburger and Heier 11 ). Exclusion criteria for the present investigation were hs-CRP levels >10 mg/l( Reference Pearson 1 ), pregnancy and missing values in any exposure, outcome or confounder (n 155). This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the ethics committee of the Bavarian Chamber of Physicians (Munich, Germany). Written informed consent was obtained from all participants.
For assessment of the outcome hs-CRP, fasting blood samples were collected in the morning, kept at 4°C until centrifugation and stored at − 80°C until laboratory measurements. Hs-CRP was measured of EDTA plasma samples with a high-sensitivity latex-enhanced nephelometric assay on a BN II analyzer (Siemens); intra-assay and inter-assay CV were < 5 % and < 10 %, respectively.
For exposure assessment, intake of dietary supplements during the last 7 d was recorded together with medication used through computer-based software( Reference Mühlberger, Behrend and Stark 12 ) in a personal interview, when participants were asked to show product packages of ingested preparations( Reference Schwab, Heier and Schneider 13 ). Vitamins and minerals not only from supplements, but also from medications were taken into consideration for the investigation of the etiological association between nutrients and hs-CRP levels, as the same ingredients (e.g. α-tocopherol) can be marketed either as supplements or as medications in Germany. Relative serum α-tocopherol levels were measured in a subgroup of 1685 subjects (CV: 19·3 %), as part of a non-targeted metabolomics experiment using GC coupled with MS (GC–MS). Serum samples that were collected after overnight fasting during the KORA F4 examinations were analysed at Metabolon, Inc., a commercial supplier of metabolomics measurements( Reference Illig, Gieger and Zhai 14 ). The measurement followed established protocols as described earlier( Reference Dehaven, Evans and Dai 15 , Reference Boudonck, Mitchell and Wulff 16 ). Raw quantification values (ion counts) were normalized to the median of the samples measured at the same run day to account for inter-day variation of the instrument. Normalized values were log-transformed and values above or below four standard deviations from the mean were excluded from further analysis.
Confounders were selected on the basis of practices followed in literature. Fully adjusted models included the following covariables: age (continuous), sex, education (low/high), BMI (continuous), BMI2 (continuous), physical activity (active/inactive), smoking (current/former/never), alcohol consumption (none/moderate/high), cholesterol:HDL ratio (continuous), actual hypertension (yes/no), CVD (yes/no), diabetes (yes/no), non-steroidal anti-inflammatory drug use (yes/no), and statin use (yes/no). In sensitivity analyses, regression models were additionally adjusted for the use of other dietary supplements, and for the use of hormone therapy and oral contraceptives in women.
Data collection and description of covariables
Covariables were collected through a personal interview or measured during an examination at the study centre. For age, the actual age at examination was recdorded. Information about years of education was collected in the baseline S4 study (1999–2001)( Reference Rathmann, Strassburger and Heier 11 ) and summarized as years at school plus professional education. The variable was dichotomized into low (8–10 years) and high (11–17 years) education. For calculating the BMI, participants' weight and height were measured. Categories as per the WHO standard were applied to the classification in Table 1 ( 17 ). Physical activity included frequency and duration of activity in summer and in winter and was divided into active and inactive( Reference Meisinger, Lowel and Thorand 18 ). Smoking was defined as smoking cigarettes and categorized as current (further subdivided into ‘regularly’ and ‘irregularly’), former and never. Irregular smoking was defined as usually smoking less than 1 cigarette/d. Alcohol intake was assessed as daily average intake based on the consumption of last weekend and the last weekday before the examination, following the validated recall method of Döring et al ( Reference Döring, Filipiak and Stieber 19 ). Moderate consumption was defined as more than zero and less than 40 g alcohol/d in men, and more than zero and less than 20 g/d in women; and high consumption was defined as 40 g/d or more in men, and 20 g/d or more in women( Reference Rehm and Room 20 ). For serum cholesterol measurement, blood was drawn from sedentary participants in the morning after an overnight fast of at least 8 h. Total cholesterol was measured using the Boehringer CHOD-PAP method (inter-assay CV: ≤ 2·1 %; Roche Diagnostics). HDL-cholesterol was measured using the phosphotungstic acid method (inter-assay CV: ≤ 3·5 %; Boehringer Mannheim). Cut-off points for total:HDL-cholesterol ratio used for description in Table 1 were defined according to the American Heart Association( 21 ). A participant was classified as hypertensive if the mean of the second and the third blood pressure measurements met the criteria of the WHO for hypertension, i.e. a systolic pressure ≥ 140 mmHg and/or diastolic ≥ 90 mmHg( 22 ), or if the participant was taking antihypertensive medication and was aware of being hypertensive. CVD was defined as a participant's having a history of myocardial infarction or stroke (treated as an inpatient, self-reported), or a history of angina pectoris (self-reported). Diabetes mellitus was defined according to self-report or current use of antidiabetic drugs. Medication intake during the last 7 d was collected from product packages showed by participants, and recorded through a database supported computer software( Reference Mühlberger, Behrend and Stark 12 ). For the definition of non-steroidal anti-inflammatory drugs, regular use was considered, and acetylsalicylic acid use in dosages of 100–300 mg was not included. Hormone therapy was defined as use of systemic estrogens with or without gestagen intake. The adjustment variable dietary supplement intake, that was used for sensitivity analysis, was built including supplementation of any of the following nutrients which have been found to be associated with CRP levels: vitamin A, D, B1, B2, B6, B12, folic acid, pantothenic acid, niacin, biotin, Mg, Fe, DHA, EPA, α-linolenic acid, glucosamine and chondroitin( Reference Scheurig, Thorand and Fischer 3 , Reference Morris, Sakakeeny and Jacques 23 – Reference Chen, Wan and Han 34 ).
Table 1 C-reactive protein (CRP) levels by categories of covariables in KORA F4 (Cooperative Health Research in the Region of Augsburg) (Geometric mean values and antilog standard deviations, n 2924)

hs-CRP, high sensitivity C-reactive protein; NSAID, non-steroidal anti-inflammatory drugs.
Mean value was significantly different compared with the first category: ** P< 0·01, *** P< 0·001.
† Adjusted for age (continuous) and sex; age was only adjusted for sex and sex was only adjusted for age.
‡ P for age- and sex-adjusted mean difference (ANCOVA).
§ BMI < 18·5 kg/m2 (n 10) and 18·5 ≤ BMI < 25 (n 927) were combined.
∥ Smoking regularly (n 450) and irregularly (n 78) were combined.
¶ Moderate: men >0 to < 40 g/d; women >0 to < 20 g/d; high: men ≥ 40 g/d; women ≥ 20 g/d.
†† Actual hypertension ( ≥ 140/90 mmHg) or intake of antihypertensive medication and awareness of being hypertensive.
‡‡ History of myocardial infarction, stroke or angina pectoris.
§§ Without acetylsalicylic acid in dosages of 100 or 300 mg.
∥∥ In women only.
Statistical analysis
Age- and sex-adjusted mean levels of hs-CRP by categories of covariables and exposure variables were compared using ANCOVA. If the P-value for the group was < 0·05, post hoc analyses were performed with the first category as reference group, to clarify where the differences are. For statistical analyses, hs-CRP was log-transformed to approximate a normal distribution, and back-transformed for presentation of results. These are displayed as ratio (95 % CI) of geometric mean hs-CRP levels among nutrient users v. non-users. The association of vitamin E, vitamin C, carotenoids, Se and Zn intake with hs-CRP was investigated cross-sectionally using linear regression models; initially total regular nutrient intake was treated binary (yes/no), then regularly ingested amounts were divided into quartiles to investigate dose–response effects. The reference group comprised persons with a zero intake of the nutrients of interest from supplements or medications. Assumptions of linear regression models were checked and fulfilled. Effect modification was tested for sex, and intake of at least one of the other four antioxidants, respectively. Through the latter we examined the effect of single v. combined supplementation of antioxidants, i.e., if the association of each nutrient with hs-CRP depends on the intake of at least one of the other four antioxidants, or if supplementation of each nutrient in isolation (without other antioxidants) is associated with hs-CRP. An independent effect of each antioxidant was assessed via adjustment for use of at least one of the other four antioxidants, respectively. We used a spline, i.e. a piecewise polynomial function, to examine a non-linear relationship between regularly ingested vitamin E amounts and hs-CRP levels. The Spearman correlation coefficient was calculated to assess the correlation between average daily intake amounts of vitamin E from supplements, and serum levels of α-tocopherol, in regular vitamin E supplement users. Linear regression analyses were conducted for regular vitamin E supplement users dividing the exposure serum α-tocopherol levels into quartiles with the first quartile as reference group; and effect modification through intake of at least one of the other four antioxidants (vitamin C, carotenoids, Se, and Zn) was investigated by inclusion of the respective interaction terms in the models. The quadratic term of BMI (BMI2) was used in the analyses in addition to BMI, because of a non-linear relationship of BMI with hs-CRP. Statistical analyses were performed using Statistical Analysis Systems version 9.3 (SAS Institute, Inc.).
Results
The final study population comprised 2924 participants (1413 men, 1511 women) with a mean age of 56 years, ranging from 31 to 82 years. Frequency of regular antioxidant intake was 9·1 % for vitamin E, 9·2 % for vitamin C, 4·0 % for carotenoids, 5·0 % for Se, and 6·9 % for Zn. Frequencies of sex-specific intakes and intakes as needed are presented in Table 2. The geometric mean hs-CRP concentration in the study population was 1·13 (antilog sd 2·74) mg/l. Unadjusted and adjusted geometric means by categories of covariables are provided in Table 1, and by categories of exposure variables in Table 3.
Table 2 Use of antioxidative supplements in KORA F4 (Cooperative Health Research in the Region of Augsburg) (Number of subjects and percentages, n 2924)
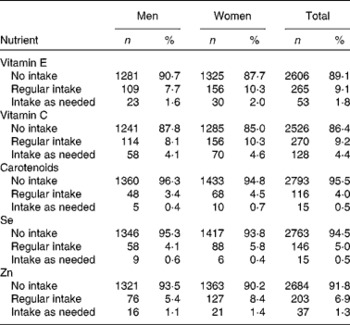
Table 3 C-reactive protein (CRP) levels by categories of exposure variables in KORA F4 (Cooperative Health Research in the Region of Augsburg) (Geometric mean values and antilog standard deviations, n 2924)

hs-CRP, high sensitivity C-reactive protein.
* Adjusted for age (continuous) and sex.
† P for age- and sex-adjusted mean difference (ANCOVA).
‡ Division into quartiles was not possible due to distribution, because 40 % of persons with regular intakes ingested 5 mg Zn/d.
Regular intake of any of the five investigated antioxidative nutrients per se was not associated with hs-CRP (Table 4). However, dose–effect analyses revealed that regular intake of more than 78 mg vitamin E, which corresponds to the top quartile, was significantly associated with lower hs-CRP levels. In fully adjusted analyses hs-CRP levels of persons who ingested more than 78 mg vitamin E/d were 22 % lower compared to those of participants who did not ingest any vitamin E from supplements or medications (Table 4). This association persisted after additional adjustment for the use of at least one of the other four antioxidants (Table 4) and other nutrient supplements (ratio 0·79 (95 % CI 0·63, 0·99)). A spline (P= 0·25, χ2 test) did not improve significantly the description of the association between regularly ingested vitamin E amounts and hs-CRP compared to the linear model.
Table 4 Dose–response relationship between regular nutrient intake with high sensitivity C-reactive protein (hs-CRP) levels (linear regression) (Ratios and 95 % confidence intervals of geometric means)

* Reference group: no intake of nutrient of interest from supplements or medications.
† Binary coded as yes/no.
‡ Adjusted for age (continuous), sex, education (low/high), BMI (continuous), BMI2 (continuous), physical activity (active/inactive), smoking (current/former/never), alcohol consumption (none/moderate/high), cholesterol:HDL ratio (continuous), actual hypertension (yes/no), CVD (yes/no), diabetes (yes/no), non-steroidal anti-inflammatory drug use (yes/no), and statin use (yes/no).
§ Division into quartiles was not possible due to distribution, because 40 % of persons with regular intakes ingested 5 mg Zn/d.
The interaction term of regular vitamin E use in the top quartile and use of any of the other four antioxidants was significant with a P-value of 0·028. Stratification according to single vitamin E supplementation (without the use of any of the other four antioxidants) and supplementation of vitamin E in combination with at least one of the other antioxidants showed that associations were confined to those, who ingested vitamin E in combination with at least one other antioxidant (ratio 0·66 (95 % CI 0·48, 0·90)), but the same could not be seen in those with supplementation of vitamin E alone (ratio 1·09 (95 % CI 0·77, 1·56)) (see Fig. 1).

Fig. 1 Interaction between regularly ingested vitamin E amounts in the upper quartile and intake of other antioxidants. Values are ratios of geometric mean hs-CRP, with their 95 % confidence intervals represented by horizontal bars. * Intake of at least one of the other investigated antioxidants (vitamin C, carotenoids, selenium, and zinc). † Estimates result from fully adjusted linear regression models (adjusted for age (continuous), sex, education (low/high), BMI (continuous), BMI2 (continuous), physical activity (active/inactive), smoking (current/former/never), alcohol consumption (none/moderate/high), cholesterol:HDL ratio (continuous), actual hypertension (yes/no), CVD (yes/no), diabetes (yes/no), non-steroidal anti-inflammatory drug use (yes/no), and statin use (yes/no)). hs-CRP, high sensitivity C-reactive protein.
Participants in the highest vitamin E quartile (n 66) were 54·6 % female and ingested mean vitamin E amounts of 256 (sd 141) mg/d. In persons who ingested vitamin E in combination with other antioxidants (n 42), the most frequent combination was use of all five antioxidants (n 14), followed by vitamin E with vitamin C (n 7). Only five participants of the forty-two did not supplement vitamin E together with vitamin C.
Effect modification by sex using quartiles of vitamin E as exposure was not seen (P= 0·80). However, stratified analyses revealed a stronger association in women, whereas the association was weaker and non-significant in men (ratios for highest vitamin E quartiles: 0·72 (95 % CI 0·52, 0·98) and 0·88 (95 % CI 0·63, 1·23), respectively). Further adjustment for hormone therapy and use of oral contraceptives in women did not alter results substantially (data not shown).
As a sensitivity analysis, the relationship between serum levels of α-tocopherol and hs-CRP concentrations in persons who regularly ingested vitamin E supplements was investigated (n 165) (see online Supplementary Table S1). The correlation coefficient between average intake amounts of vitamin E and serum levels of α-tocopherol was 0·53 (P< 0·001). Ratios for the association of serum α-tocopherol concentrations with hs-CRP from linear regression analyses decreased non-significantly for α-tocopherol quartile numbers two, three and four v. one as follows respectively: 1·03 (95 % CI 0·70, 1·52), P= 0·88; 0·83 (95 % CI 0·56, 1·23), P= 0·36; 0·79 (95 % CI 0·53, 1·17), P= 0·23 (see online Supplementary Table S1). The P-value for the interaction with intake of at least one of the other four antioxidants was 0·0260. In persons who ingested other antioxidants as well, higher α-tocopherol levels compared to lower values showed a tendency to be associated with lower hs-CRP levels (ratios for quartiles three and four v. one, respectively: 0·85 (95 % CI 0·56, 1·29), P= 0·45; 0·76 (95 % CI 0·49, 1·17), P= 0·21) (see online Supplementary Table S1). On the contrary, in persons who ingested vitamin E supplements only without any other antioxidants, higher α-tocopherol levels tended to be associated with higher hs-CRP levels (ratios for quartiles three and four v. one, respectively: 1·62 (95 % CI 0·33, 8·01), P= 0·54; 1·47 (95 % CI 0·34, 6·39), P= 0·59) (see online Supplementary Table S1). Overall, associations were not significant possibly due to small numbers especially in the stratified analyses (n ≤ 36 in the group of subjects who did ingest other antioxidants besides vitamin E, and n≤ 11 in the group of subjects who did not).
Discussion
Regular vitamin E intake in amounts above 78 mg/d was significantly associated with lower hs-CRP levels in the present study. Stratified analyses showed this association was found only in persons who used vitamin E in combination with other antioxidants. The interaction term for sex was not significant; however, associations were stronger in women and non-significant in men after stratification.
Vitamin E is a potent antioxidant that can reduce oxidative stress and influence inflammatory processes( Reference Calder, Albers and Antoine 35 ). Direct anti-inflammatory effects are triggered through several mechanisms including down regulation of the proinflammatory transcription factor NF-κB( Reference Islam, Devaraj and Jialal 36 ), reduction of lipopolysaccharide-stimulated levels of the proinflammatory cytokine TNF-α( Reference Mol, de Rijke and Demacker 37 ), and decrease of proinflammatory IL-1β by inhibition of 5-lipoxygenase, an enzyme involved in synthesis of inflammatory prostaglandins( Reference Devaraj and Jialal 38 ). It is noteworthy that there is an interaction between inflammation and oxidative stress, implying that one condition triggers the other( Reference Garcia-Bailo, El-Sohemy and Haddad 39 ). Oxidative stress is increased through the production of reactive oxygen and nitrogen species by immune cells. And these species do promote inflammation through the activation of NF-κB and the subsequent transcription of proinflammatory cytokines( Reference Garcia-Bailo, El-Sohemy and Haddad 39 ). Therefore, an anti-inflammatory effect of vitamin E may be reasonable via both direct anti-inflammatory and also antioxidative pathways.
Supplementation of vitamin E alone might not be as favourable as compared to its combined supplementation with other antioxidants, because antioxidative action of vitamin E leads to the formation of oxidized vitamin E, which has pro-oxidative potential if not regenerated. Vitamin C as a hydrophilic antioxidant is involved in the regeneration of oxidized vitamin E( Reference Packer, Slater and Willson 40 ). This could explain why vitamin E used particularly in combination with the other antioxidants, especially vitamin C, mostly supplemented in the present study, was most strongly associated with lower hs-CRP levels. Accordingly, Garcia-Bailo et al ( Reference Garcia-Bailo, El-Sohemy and Haddad 39 ) suggested that the combined administration of vitamin C and vitamin E might be more effective in reducing oxidative stress and inflammation than utilising either micronutrient on its own. Furthermore, it was proposed that there is a ‘vitamin E regeneration system’ based on the complex interaction of several antioxidants, which has to be considered when investigating vitamin E effectiveness( Reference Nwose, Jelinek and Richards 41 ). Results from the present investigation support this hypothesis.
α-Tocopherol is the only form of vitamin E used in supplements( Reference Wolf 42 ), and a moderate-to-strong correlation was observed in the present study between average daily intake amounts of vitamin E from supplements and α-tocopherol levels in serum. Results from linear regression analyses for serum levels of α-tocopherol as exposure were comparable to those found for intake amounts of vitamin E from supplements, i.e., higher α-tocopherol levels tended to be associated with lower levels of hs-CRP in regular vitamin E supplement users, especially when they ingested other antioxidants as well. On the contrary, the association tended to be positive if other antioxidants were not ingested. Results regarding serum α-tocopherol levels were, however, not significant, possibly because the number of subjects in each quartile was low since measurements were available only in a subsample of the study population. In previous studies both positive and negative associations between α-tocopherol levels and CRP concentrations were found, whereas the concomitant use of (antioxidant) supplements was not considered in those studies( Reference van Herpen-Broekmans, Klopping-Ketelaars and Bots 43 , Reference Cooney, Franke and Wilkens 44 ). Results from the present study suggest that the simultaneous use of other antioxidant supplements could be an effect modifier that needs to be taken into account.
In line with the present investigation, supplementation of vitamin E alone was not associated with reduced CRP levels in two intervention studies, where 800 and 400 IU levels of vitamin E/d were used respectively( Reference Block, Jensen and Dalvi 45 , Reference Kaul, Devaraj and Grundy 46 ), with the exception of another trial that used an extremely high amount of 1200 IU vitamin E/d( Reference Devaraj and Jialal 47 ) (1 IU equating to 0·67 mg RRR-α-tocopherol). However, combined supplementation of 800 IU vitamin E together with twenty-three other ingredients (among them vitamin C, β-carotene, Se, and Zn) reduced CRP levels in a randomized, double-blind, placebo-controlled trial( Reference Church, Earnest and Wood 48 ).
As in the present analysis, other observational studies examining associations between nutrients and hs-CRP also reported for antioxidants stronger inverse associations among women as compared to those in men( Reference Scheurig, Thorand and Fischer 3 ), and similar associations for glucosamine and chondroitin( Reference Kantor, Lampe and Vaughan 32 ). Furthermore, in two intervention studies, no effect of vitamin E in combination with vitamin C and with vitamin C and β-carotene was observed in men with high cholesterol or with elevated homocysteine levels( Reference Bruunsgaard, Poulsen and Pedersen 49 , Reference O'Doherty, Gilchrist and Young 50 ). However, Floegel et al. ( Reference Floegel, Chung and von Ruesten 4 ) found in the US National Health and Nutrition Examination Survey no significant interaction on the basis of sex, and reported combined results for an inverse association of antioxidant intake from diet plus supplements with CRP concentrations. Further randomized controlled trials especially in women are warranted to investigate the association between supplementation of antioxidants and CRP levels.
Vitamin E intake amounts of 78 mg/d are well above the German reference values for daily vitamin E intake, which is set at 13 mg for men and 14 mg for women in the age group of 51 to 64 years( 51 ). The actual European Tolerable Upper Intake Level for vitamin E was set at 300 mg/d in 2003( 52 ). During the subsequent years, meta-analyses of randomized trials reported an increased risk of mortality among users of high-dosage vitamin E supplements( Reference Miller, Pastor-Barriuso and Dalal 53 , Reference Bjelakovic, Nikolova and Gluud 54 ). According to a review article published in the year 2005, several large or long-term clinical trials support the safety of vitamins E and C in combination( Reference Hathcock, Azzi and Blumberg 55 ). However, the safety of antioxidative supplements is still debated( Reference Bjelakovic, Nikolova and Gluud 56 ), and should further be investigated.
The major limitation of the present analysis is the cross-sectional design; therefore, the observed association might not be causal. Furthermore, because of the inherent nature of observational studies, the said effect could be due to residual confounding, i.e., the subgroup of people with intake of several antioxidants could represent a healthier group particularly interested in health. However, analyses were adjusted for common lifestyle factors and use of other supplements. Moreover, a multitude of further important covariables was available for multivariable adjustment. Intake of dietary supplements over the previous 7 d may not truly reflect usual irregular intake behaviour. However, interview questions elicited details of participants' ‘regular intake’ and ‘intake as needed’, respectively, and only ‘regular intake’ of supplements was considered for analyses. The causal role of CRP levels and CVD progression is controversial( Reference Zacho, Tybjaerg-Hansen and Jensen 57 ). Nevertheless, CRP is still widely used as a clinical marker in contemporary studies( Reference Simental-Mendia, Rodriguez-Moran and Guerrero-Romero 33 , Reference Chen, Wan and Han 34 , Reference Wang, Jiao and Li 58 , Reference Ong, Allison and Cheung 59 ).
The major strength of the present study is the thorough assessment of supplements and medications used with detailed data on the composition of individual preparations, which allowed the conduct of dose–effect analyses. Moreover, serum levels of α-tocopherol were additionally measured which supported the main findings. Also a hs-CRP assay was used to detect lower CRP levels which have the potential to predict CVD events( Reference Pearson 1 ). Finally, the KORA F4 study represents a population-based survey; therefore, results are applicable to the general Southern German population.
Conclusion
Regular supplementation of vitamin E was associated with lower hs-CRP levels only in higher amounts and in combination with other antioxidants. Further intervention studies are needed to establish whether this association is causal. Furthermore, the safety of this kind of supplementation should be confirmed. If done so, the combined supplementation of vitamin E with other antioxidants could be a promising strategy for the prevention of inflammation-related diseases in the general population.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114515000902
Acknowledgements
We would like to thank the participants and the staff of the KORA F4 study for their efforts and contributions.
S. S., A. P., and B. T. designed the research; S. S., A. S., M. H., W. K., G. K., M. W., A. P., and B. T. conducted the research; S. S. and A. Z. performed the statistical analyses; S. S. wrote the paper and had primary responsibility for the final content; all authors read and approved the final manuscript.
The authors declare that they have no conflicts of interest.
The KORA research platform (KORA, Cooperative Research in the Region of Augsburg) was initiated and financed by the Helmholtz Zentrum München – German Research Center for Environmental Health, which is funded by the German Federal Ministry of Education and Research and by the State of Bavaria. Furthermore, KORA research was supported within the Munich Center of Health Sciences (MC Health), Ludwig-Maximilians-Universität, as part of an LMUinnovativ project. The funding agencies had no role in the design, analysis, findings, or the writing of this article.








