Management Implications
Seeds and fragments of invasive plant species can be introduced to new continents, countries, and regions of a country as contaminants in soil. Importing soil or moving soil among sites within a country can occur for construction and building purposes. Therefore, moving soil should be done under specific conditions, with the soil inspected to verify it is free of invasive species. Stationary soil steaming as a nonchemical control method has the potential to disinfect soil contaminated with invasive plant propagules. Steam has a high energy density and a high heat-transfer capability. Wet steam immediately increases the surface temperature of plant material with a destructive effect. The outcome varies depending on temperature and duration of exposure. Higher temperatures are more efficient but may also have side effects, including change of soil physical and chemical characteristics, which should be considered, depending on the intended soil use. We found that invasive plant propagative materials were controlled by soil steaming and that steam is a potential method for avoiding dispersal of invasive species. However, steaming measures can be adapted based on target species. The method is safe and does not pose environmental effects caused by chemical soil disinfection methods. It has a rapid effect and no residual impacts; therefore, soil can be used as soon as it has cooled to the ambient temperature.
Introduction
Invasive species may modify soils they are occupying in ways that increase their own fitness relative to native species. Positive feedback happens if increased invasive fitness furthers the degree or extent of soil modification, which in turn further favors these invasives over natives (Jordan et al. Reference Jordan, Larson and Huerd2007). Noxious weeds develop characteristics such as rapid growth rates, high seed production, and extended growing periods, all of which provide them with an advantage over native plants in occupying the soil (Sheley et al. Reference Sheley, Manoukian and Marks1996). Long-distance dispersal of plant propagules determines large-scale phenomena of greatest conservation concern, such as the spread of invasive plants (Trakhtenbrot et al. Reference Trakhtenbrot, Nathan, Perry and Richardson2005). The most effective method for managing noxious weeds is to prevent their invasion using a combination of methods aimed at limiting encroachment (Sheley et al. Reference Sheley, Manoukian and Marks1996). Limiting weed seed dispersal, containing neighboring weed infestations, minimizing soil disturbances, detecting and eradicating weed introductions early, and establishing competitive grasses following proper grass management have been proposed by Sheley et al. (Reference Sheley, Manoukian and Marks1996) to prevent noxious weeds from spreading. Over the last centuries, significant human-mediated long-distance dispersal of plants is evident in the large number of plant species that have naturalized in regions outside their native ranges (Hodkinson and Thompson Reference Hodkinson and Thompson1997; Mack and Lonsdale Reference Mack and Lonsdale2001). Importing soil or moving soil within a country, for example, soil that must be transported in connection with construction and building of railways and roads, can spread numerous diseases and pests, including invasive plant propagative materials. Therefore, the process of moving soil should be done only when the soil has been inspected and verified to be free of serious diseases and pests.
Recently, increasing attention has been paid to soil steaming as a soil disinfection method, particularly because the use of methyl bromide has been prohibited (Samtani et al. Reference Samtani, Ajwa, Weber, Browne, Klose, Hunzie and Fennimore2011) and because soil steaming has been proven to be a promising tactic for soil disinfection, including the control of agricultural weed seeds (Melander and Jørgensen Reference Melander and Jørgensen2005; Nishimura et al. Reference Nishimura, Asai, Shibuya, Kurokawa and Nakamura2015; Peruzzi et al. Reference Peruzzi, Raffaelli, Frasconi, Fontanelli and Bàrberi2012; Raffaelli et al. Reference Raffaelli, Martelloni, Frasconi, Fontanelli, Carlesi and Peruzzi2016; Van Loenen et al. Reference Van Loenen, Turbett, Mullins, Fielden, Wilson, Leifert and Seel2003). Various soil-steaming methods such as sheet steaming and steaming using fixed tube pipes have been developed and used for supplying steam in open fields and horticultural greenhouse settings (Gay et al. Reference Gay, Piccarolo, Ricauda Amimonino and Tortia2010; Raffaelli et al. Reference Raffaelli, Martelloni, Frasconi, Fontanelli, Carlesi and Peruzzi2016). A steam treatment breaks the natural thermal equilibrium of soil, forcing a multiphase high-temperature flow through its pores and quickly enhancing soil temperature (Gay et al. Reference Gay, Piccarolo, Ricauda Amimonino and Tortia2010). The thermal behavior of the soil depends on the specific heat transport mechanisms that are involved or forced by machines and tools used for disinfestation and on the condition, physical structure, and moisture content of the soil (Gay et al. Reference Gay, Piccarolo, Ricauda Amimonino and Tortia2010). Soil steaming is usually more expensive than other nonchemical methods, but cheaper than chemical soil fumigation (Peruzzi et al. Reference Peruzzi, Raffaelli, Ginanni and Mainardi2002, Reference Peruzzi, Raffaelli, Ginanni, Lulli, Fontanelli and Frasconi2008). It has a rapid effect and no residual effects (Materazzi et al. Reference Materazzi, Iandolo, Triolo and Vannacci1987; Peruzzi et al. Reference Peruzzi, Raffaelli, Frasconi, Fontanelli and Bàrberi2012); therefore, the soil can be used as soon as it has cooled to the ambient temperature (Luvisi et al. Reference Luvisi, Materazzi and Triolo2006).
The Norwegian Nature Diversity Act, which was enacted on July 1, 2009 (Norwegian Ministry of Climate and Environment 2009), focuses on relocated soils as an important source for spread of invasive alien plant species. Enterprises responsible for projects that include relocation of soil are also responsible for alien species that are spread by their relocation activity. As an example, an important part of the regulation on wild oat (Avena fatua L.) is to prohibit spread by moving soil from one location to another (Norwegian Ministry of Agriculture and Food 2015).
Excavated construction and contaminated soils are often disposed of at landfills, and the recycling rate for high-quality purposes is low. The need for increased resource efficiency and decreased climate impacts is crucial for global sustainable development. Soil reuse can reduce the environmental burden associated with obtaining new soil and decrease CO2 emissions by transportation to disposal sites (Magnusson et al. Reference Magnusson, Lundberg, Svedberg and Knutsson2015). In this study, we discuss the first results of an experiment set up to test a soil-steaming method using a prototype device to disinfect soil masses infested with propagative plant materials of invasive plant species as a potential method for on-site soil disinfection. Because soil steaming has previously been shown to be effective against weeds in greenhouse and field conditions, we hypothesized that stationary soil steaming will sufficiently kill invasive plant propagules and thereby allow reuse of soil that would otherwise be disposed of due to contamination. We aimed to find the appropriate soil temperature and exposure duration to kill invasive plant propagules in contaminated soils by using the prototype device.
Materials and Methods
To target propagative plant materials of selected invasive plant species [bigleaf lupine (Lupinus polyphyllus Lindl.); ornamental jewelweed (Impatiens glandulifera Royle); A. fatua, one population from Poland, and one from Norway; Canada goldenrod (Solidago canadensis L.); and Bohemian knotweed (Reynoutria x bohemica Chrtek & Chrtková)] incorporated in soil masses using steam, three pot experiments were conducted at the Norwegian Institute of Bioeconomy Research station, Ås, Norway.
Plant Material Collection and Preparation
Mature seeds of A. fatua were collected from Ås (59.663°N, 10.790°E) and mature seeds of L. polyphyllus and I. glandulifera were collected from Kolbotn (59.811°N, 10.797°E) in September and October 2019. Seeds were collected from a large number of individual plants. The pooled seeds were stored under dry conditions at room temperature in paper bags for 6 to 9 wk and then transferred into small glass containers with lids until they were used in the experiments. To break dormancy, L. polyphyllus and I. glandulifera seeds were chilled at 4 C for 4 wk. Avena fatua seeds were tested both without pretreatment and moistened for 12 h before steaming to test the effect of steam on seeds with higher moisture content. Rhizome fragments of S. canadensis and R. x bohemica were collected the day before the experiments in July 2020 from Ås. The tests of R. x bohemica included two rhizome lengths, 5 and 10 cm.
Experimental Treatments and Data Collection
Three experiments were carried out. In the first experiment, we tested four different soil temperatures with an exposure duration of 90 s. In the second and third experiments, we tested different exposure durations at 99 C.
In Experiment 1, for which the aim was to find the minimum temperature effective against propagative plant materials of selected invasive plant species, we used four target soil temperatures of 60, 70, 80, and 99 C with an exposure duration of 90 s. Experiment 2 included three target exposure durations of 30, 90, and 180 s at 99 C. For all treatments, four replicates of 50 seeds of each species/each population of a species were placed in PP-fleece bags (9 by 7 cm) and received the proper pretreatment. Bags including seeds and rhizome fragments were covered by the soil at a depth of 7 cm in 60 by 40 by 20 cm plastic containers with holes (baskets to allow steam to pass freely). Soil was obtained from a local soil retailer. Soil type was loamy (10% to 25% clay, 25% to 50% silt, <3% organic matter) with a moisture content of 39.6%. Soils inside the baskets were steamed. The bags including steamed seeds as well as rhizome fragments were taken out from the baskets immediately after the steaming treatment and stored outdoors in shade at ambient air temperature until being transported to the greenhouse. Each opened bag and rhizome fragment was placed on the soil surface in a 12-cm-diameter pot (1 L) and covered by a thin layer of soil. Potting soil (80 vol% sphagnum peat, 10 vol% composted bark, 10 vol% sand) limed and fertilized with NPK (950:40:220 mg L−1) with a pH of 5.5 to 6.5 (Tjerbo torvfabrikk AS, Rakkestad, Norway) was used. The pots were placed in a greenhouse (21/16 C and 14/10 h for day/night; relative humidity: 68%) in a completely randomized design and watered from the bottom with tap water when needed throughout the experimental period. Potential seed germination and rhizome sprouting were followed for 28 d. For L. polyphyllus only, seed germination was followed for 35 d. Numbers of germinated seeds were counted every 7 d. Non-steamed seeds and rhizome fragments were used as controls. A total of 144 treated pots ([3 species (L. polyphyllus, I. glandulifera, S. canadensis) + 1 species (A. fatua) * 2 populations * 2 pretreatments + 1 species (R. x bohemica) * 2 rhizome lengths] * 4 temperatures * 4 replications) in Experiment 1 and 108 treated pots ([3 species (L. polyphyllus, I. glandulifera, S. canadensis) + 1 species (A. fatua) * 2 populations * 2 pretreatments + 1 species (R. x bohemica) * 2 rhizome lengths] * 3 exposure durations * 4 replications) in Experiment 2 as well as 36 control pots ([3 species (L. polyphyllus, I. glandulifera, S. canadensis) + 1 species (A. fatua) * 2 populations * 2 pretreatments + 1 species (R. x bohemica) * 2 rhizome lengths] *4 replications) were evaluated.
In Experiment 3, we tested the effect of different steaming durations of 90, 180, and 540 s at 99 C on propagative plant material germination/sprouting. For L. polyphyllus, I. glandulifera, and A. fatua, 30 seeds for each replicate were placed in a bag and received the proper pretreatment. Lupinus polyphyllus and I. glandulifera seeds were chilled for 2 wk, but A. fatua was only tested with 12-h moistening pretreatment in this experiment. Bags including seeds and rhizome fragments were exposed to steam similarly to Experiments 1 and 2. Steamed propagative plant materials were placed on the soil surface in 56 by 27 by 5 cm boxes, which were divided in three (number of replicates in Experiment 3) and covered by a thin layer of soil. Seed germination and rhizome sprouting were followed in the same way as in Experiments 1 and 2. Non-steamed propagative plant materials were used as controls (three replicates for L. polyphyllus, I. glandulifera, and A. fatua (Norway) and five replicates for S. canadensis and R. x bohemica). A total of 18 treated boxes ([4 species (L. polyphyllus, I. glandulifera, A. fatua (Norway), S. canadensis) + 1 species (R. x bohemica) * 2 rhizome lengths] * 3 exposure durations) and 6 control boxes [4 species (L. polyphyllus, I. glandulifera, A. fatua (Norway), S. canadensis) + 1 species (R. x bohemica) * 2 rhizome lengths] were evaluated.
Steaming Method
The steaming prototype device (Soil Steam International AS, Sandefjord, Norway) used for the experiments has a steaming container with the following specifications: 190 by 144 by 88 cm dimensions, 130 kg weight, and 120 by 80 cm effective steaming area (Figure 1A). The steam generator has a production capacity of 250 kg h−1 with an effective calorific rating of 167,000 kcal h−1 and a working pressure of 0.5 bar. The fuel consumption (diesel) is 19.2 L h−1. When the steam enters the non–air proof chamber, it is distributed over the soil. If the soil sample reaches the saturation point, for example, if the soil temperature reaches 98 to 100 C in the whole mass, the steam consumption is reduced significantly, causing an imbalance between steam supply and demand. In such a case, excessive steam exits from the top of the chamber, causing no significant pressure change.

Figure 1. (A) Steaming rig and (B) container of prototype device used in the experiments. Photos: Belachew Asalf Tadesse.
All bags and rhizome fragments in the same replicate of each combination of target temperature and exposure duration were placed at the bottom of one basket (60 by 40 by 20 cm) and covered by a 7-cm soil layer. Each basket was placed in the steaming container, and 10 thermocouples were placed in the soil (Figure 1B). When the container lid was closed, steam released from the top with a constant temperature of ca. 150 C and vacuumed from the bottom of the container. Soil temperature was monitored by means of PT1000 sensors connected to a cRIO-9073 data logger (National Instruments, Austin, TX 78759-3504, USA). Steaming and vacuum were shut off when at least 5 of the 10 thermocouples had reached the target soil temperature. Exposure duration was considered to be the time at which the target soil temperature was measured by 5 of the 10 thermocouples in the rig. The basket was removed from the steaming container when the post-steaming exposure duration of either 30, 90, 180, or 540 s was completed. The samples were then removed from the basket and the warm soil immediately after steaming.
Processing of Soil Temperature Data
The individual temperature measurements from each of the 10 thermocouples were used to calculate the average soil temperature during the steaming for each combination of target soil temperature and exposure duration in each replicate. The maximum values of each of the mean soil temperature curves during the steaming process were extracted. Comparison of the target and actual maximum mean soil temperature showed that it was generally difficult to reach the exact target temperatures. Actual maximum mean soil temperature values were allocated to four temperature intervals of 59.3 to 68.5, 73.9 to 75.6, 76.9 to 83.0, and 94.1 to 99.2 for target temperatures of 60, 70, 80, and 99 C, respectively, where the average temperature for each interval is presented in this paper as the actual maximum mean soil temperature (64, 75, 79, and 98 C, respectively). Examples of mean soil temperature curves are shown in Figure 2.

Figure 2. Examples of soil temperature curves in Experiment 1 in which target temperatures were 60, 70, or 80 C followed by an exposure duration of 90 s. Each temperature curve is the average of 10 measurements. The gray horizontal bar shows the period with steam entering the steaming container with the samples (Figure 1A). The black horizontal bar indicates the exposure duration, i.e., the period after steaming stopped until the basket with the seeds (Figure 1B) was removed from the container.
Statistical Analysis
Considering the cumulative distribution function of the standard log-logistic distribution, seed germination was modeled with the function F(t) in Equation 1 using the extension package drc for the software environment R (R Development Core Team 2011; Ritz and Streibig Reference Ritz and Streibig2005):
where F(t) denotes the fraction of seed germinating between the onset of the experiment (at time 0) and time t. The upper limit parameter d denotes the proportion of seeds that germinated during the experiment out of the total number of seeds present at the beginning of the experiment (50). Parameter b is proportional to the slope of F at time t equal to parameter t 50, when 50% of the seeds that germinated during the experiment had germinated. The estimation and the model-checking procedures were based on treating the data as event times recording the time it took for germination (the event of interest) to occur (Ritz et al. Reference Ritz, Pipper and Streibig2013).
The rhizome sprouting response to different temperatures and exposure durations was modelled with the function y in Equation 2, using the extension package drc for the software environment R (R Development Core Team 2011; Ritz and Streibig Reference Ritz and Streibig2005):
where y is the response and depends on the dose x. Parameter b denotes the relative slope around the point of inflection, which is ED50, the dose required to reduce the response halfway between the upper (1) and lower (0) limit. If the curve is decreasing from the upper limit, b is positive, and if it is increasing, b is negative. We fit a two-parameter log-logistic model for binomial data where the response was rhizome fragments sprouting (1 = upper limit) or not (0 = lower limit) (Ritz et al. Reference Ritz, Baty, Streibig and Gerhard2015).
The assessment of the individual fits was done by inspecting the graphical analysis of the residuals. Post hoc comparisons of parameters were based on pairwise t-tests adjusted for multiple testing using the single-step approach (Tukey’s range test) implemented in the extension package drc multcomp (Hothorn et al. Reference Hothorn, Bretz and Westfall2008).
Results and Discussion
Some alien plant species, especially garden plants, show strong vegetative (e.g., rhizomes) and sexual (seeds) reproductive capacities, which together represent dispersal and resistance organs contributing to species invasiveness and affecting the native community (Gioria et al. Reference Gioria, Pyšek and Moravcová2012). Seeds are the most resistant plant organs, and preventing their germination from the seedbank impedes a species’ recruitment of individuals and thus its persistence in a community (Regan et al. Reference Regan, McCarthy, Baxter, Panetta and Possingham2006; Richardson and Kluge Reference Richardson and Kluge2008), yet this factor is often underestimated (De Wilde et al. Reference De Wilde, Buisson, Yavercovski, Willm, Bieder and Mesléard2017).
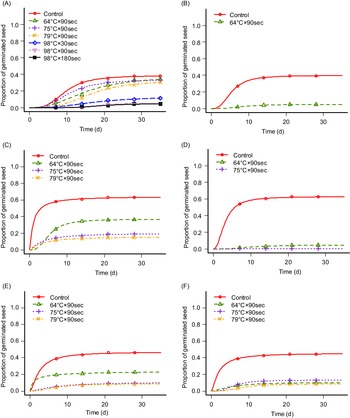
Heat has been proposed as contributing to weed control by destroying weed seeds and seedlings. However, the response of different species to heating varies (Thompson et al. Reference Thompson, Jones and Blair1997). Heat can be used separately in multiple forms such as steam, fire, dry heat, forced hot air, electric fields, or electromagnetic energies, or in combination. Soil steaming for 30 min at 82 C has been suggested to kill most weed seeds in the soil or at 100 C for resistant weed seeds (Shurtleff Reference Shurtleff1983). This was achieved in our experiment for the tested species, though the exposure duration was considerably shorter (Table 1; Figure 3). However, seed germination was inhibited at lower temperatures for I. glandulifera (Table 1; Figure 3B) in accordance with Oliver et al. (Reference Oliver, Berge, Solhaug and Fløistad2020). We have analyzed the results from Experiments 1 and 2 for each test group together and made germination curves showing where seed germination occurred (Table 1; Figure 3). There was no germination for any test group at 98 C, except for L. polyphyllus (Figure 3A). However, in Experiment 3, in which we tested exposure durations of 90 (1.5 min), 180 (3 min), and 540 s (9 min) at 98 C, germination was only observed for L. polyphyllus on exposure duration of 90 s (7.7%). In Experiment 3, the germination rates in the controls were 35.0%, 51.6%, and 74.4% for L. polyphyllus, I. glandulifera, and A. fatua, respectively. Though L. polyphyllus seeds germinated following exposure at 98 C, the germination rate was low. Seed germination was 13.3%, 5.0%, and 4.7% after exposures of 30, 90, and 180 s, respectively, not significantly different from one another but significantly different from untreated seeds with their germination rate of 38.5% (Table 1). The same results were achieved for S. canadensis and R. x bohemica, for which rhizome fragment sprouting was inhibited by increasing temperature (P ≤ 0.01 and P ≤ 0.001, respectively) (Figure 4). Flinn and Pringle (Reference Flinn and Pringle1983) and Granstrom and Schimmel (Reference Granstrom and Schimmel1993) reported the death of several species’ rhizomes at a temperature of ca. 60 C with exposure durations of 5 and 10 min.
Table 1. Germination response of Lupinus polyphyllus, Impatiens glandulifera, and Avena fatua (two populations from Poland and Norway) to steaming treatment at soil temperatures of 64, 75, and 79 C applied for 90 s.a

a d×100 denotes the percentage of seeds germinated during the experiment (with standard errors in brackets). T 50 denotes the time (days) when 50% of the seeds that germinated during the experiment had germinated (with standard errors in brackets). The parameters were calculated based on Equation 1. No germination is shown by a dash (—). Numbers for each parameter marked with different letters are significantly different for each species/population of a species.

Figure 3. Germination curves of (A) Lupinus polyphyllus, (B) Impatiens glandulifera, and (C–F) Avena fatua ([C] population from Poland with no seed pretreatment, [D] population from Poland with 12-h seed pre-moistening treatment, [E] population from Norway with no seed pretreatment, [F] population from Norway with 12 h seed pre-moistening treatment) after exposure to steaming at soil temperatures of 64, 75, 79, and 98 C. Germination curves are shown only where germination occurred with the respective temperatures. Points are the means of exact counts, and lines are estimated germination curves based on counts (Equation 1).

Figure 4. Dose–response curves of the mortality of rhizome fragments of (A) Reynoutria x bohemica (solid red and dotted green curves correspond to 5- and 10-cm rhizome lengths, respectively) and (B) Solidago canadensis in response to exposure to steaming at soil temperatures of 64, 75, 79, and 98 C applied for 90 s. Points are the means of exact counts, and lines are estimated dose–response curves based on counts (Equation 2).
Steam has a high energy density and a high heat-transfer capability. Wet steam immediately increases the temperature of plant surface tissues with destructive effects. The thermal control technology is based on the plant thermoenergy exchange at high temperatures and uses the thermomethod for disturbing or removing the vital functions of the parts of a plant (Sirvydas et al. Reference Sirvydas, Lazauskas, Vasinauskiene and Kerpauskas2002). To define the lower limit of effectiveness, we considered lower temperatures of 60, 70, and 80 C. Increasing temperature resulted in decreasing seed germination ability of two tested populations of A. fatua (Table 1; Figure 3C–F) as well as sprouting of rhizome fragments of S. canadensis (Figure 4). Seed germination of I. glandulifera was inhibited by temperatures above 64 C (Table1; Figure 3B), and sprouting of rhizome fragments of R. x bohemica was inhibited by temperatures above 79 C (Figure 4). However, increased temperature did not significantly change the seed germination rate of L. polyphyllus (Table 1; Figure 3).
Though temperatures equal to 82 C for 30 min were suggested by Shurtleff (Reference Shurtleff1983) to be needed for inhibition of germination in most weed species, low temperature–short duration steam treatment of agricultural soils (temperatures of 50 to 60 C for 3 min with an 8-min resting period) has been suggested by Van Loenen et al. (Reference Van Loenen, Turbett, Mullins, Wilson, Fielden, Seel and Leifert2002, Reference Van Loenen, Turbett, Mullins, Fielden, Wilson, Leifert and Seel2003) for 100% kill. Such differences in results can be attributed to the different steaming methods, experimental conditions, and weed species. The temperature and exposure time are the important interactive factors that influence the efficiency of control (Nishimura et al. Reference Nishimura, Asai, Shibuya, Kurokawa and Nakamura2015). While some research has shown that the primary factor in reducing heat exposure times is the maximum temperature required for killing (Hoyle and McEloroy Reference Hoyle and McEloroy2012; Vidotto et al. Reference Vidotto, De Palo and Ferrero2013), an inverse relationship has been reported between optimal temperature and exposure time (Dahlquist et al. Reference Dahlquist, Prather and Stapleton2007; Melander and Jørgensen Reference Melander and Jørgensen2005). Steam treatments at 50 to 60 C for 11 min have been reported to destroy most weed seeds and reduce problems of phytotoxicity and reinfestation (Dawson et al. Reference Dawson, Johnson, Adams and Last1965; Raffaelli et al. Reference Raffaelli, Martelloni, Frasconi, Fontanelli, Carlesi and Peruzzi2016), which may persist after steaming at higher temperatures (Van Loenen et al. Reference Van Loenen, Turbett, Mullins, Fielden, Wilson, Leifert and Seel2003).
Propagules of perennial weeds and seeds with high primary dormancy are poorly affected and dry soil conditions greatly reduce the steaming technique’s effectiveness (Peruzzi et al. Reference Peruzzi, Raffaelli, Frasconi, Fontanelli and Bàrberi2012). Soil texture and humidity play an important role and significantly influence the efficiency of the treatment (Gay et al. Reference Gay, Piccarolo, Ricauda Amimonino and Tortia2010). Water is essential for improving thermal conductivity (Nishimura et al. Reference Nishimura, Asai, Shibuya, Kurokawa and Nakamura2015). The moisture produced from the steam promoted heat transmission to the core (Melander and Jørgensen Reference Melander and Jørgensen2005). Soil moisture at levels near field capacity in general yielded high heating efficiency values in relation to steaming disinfection methods (Gay et al. Reference Gay, Piccarolo, Ricauda Amimonino and Tortia2010). Seed moisture content (Egley Reference Egley1990; Melander and Kristensen Reference Melander and Kristensen2011; Nishimura et al. Reference Nishimura, Asai, Shibuya, Kurokawa and Nakamura2015; Thompson et al. Reference Thompson, Jones and Blair1997), seed structure, anatomy, and morphology (Horowitz and Taylorson Reference Horowitz and Taylorson1984; Jakobsen et al. Reference Jakobsen, Jensen, Bitarafan and Andreasen2019; Vidotto et al. Reference Vidotto, De Palo and Ferrero2013), and seed dormancy (Thompson et al. Reference Thompson, Jones and Blair1997) are known to affect the susceptibility to heating as well. However, the relative influence of any individual factor is difficult to detect (Dabbene et al. Reference Dabbene, Gay and Tortia2003), although maximum temperature and heat duration are considered foremost for germination reduction (Vidotto et al. Reference Vidotto, De Palo and Ferrero2013). In our experiments, seeds with higher moisture content had significantly lower germination compared with dry seeds receiving the same steaming treatment in the A. fatua Polish population (P = 0.012). Thompson et al. (Reference Thompson, Jones and Blair1997) showed the germination of imbibed A. fatua seeds stopped at temperatures of 75 C, which was achieved at higher temperatures in our experiment. Rhizome size did not influence rhizome mortality by steaming (P = 0.26), but a higher temperature was needed for 10-cm rhizome lengths to reach 50% rhizome mortality during the experiment compared with 5-cm rhizomes (Table 2).
Table 2. Parameters for the log-logistic dose–response curves of the mortality of Reynoutria x bohemica and Solidago canadensis rhizome fragments in response to exposure to steaming at soil temperatures of 64, 75, 79, and 98 C applied for 90 s.a

a ED50 denotes the dose (temperature) required to reduce the response halfway between the upper (1 = sprouted) and lower (0 = not sprouted) limits of the model during the experiment. The parameters were calculated based on Equation 2. Numbers for each parameter marked with different letters are significantly different for each pretreatment of a species.
Depending on the intended re-use of the soil, there may be undesired effects of steaming on microarthropods, microorganisms, and the natural soil microflora, in particular nitrifying bacteria, and these should be considered (Fenoglio et al. Reference Fenoglio, Gay, Malacarne and Cucco2006; Roux-Michollet et al. Reference Roux-Michollet, Czarnes, Adam, Berry, Commeaux, Guillaumaud, Le Roux and Clays-Josserand2008). In conclusion, preventing and controlling noxious weed encroachment depends on early eradication. Soil disinfection using steam in a soil relocation process can prevent introduction of propagative material to new regions. Our results showed a promising mortality level of invasive plant propagative materials by soil steaming, but the responses to temperature and duration differed depending on the species. Steam regulation should therefore be based on the differences in heat susceptibility of plant propagative material.
Acknowledgments
This work was done as a part of the project BIOIMMIGRANTS financed by the Research Council of Norway, grant no. 194051. We thank Henrik Antzée-Hyllseth, Andreas Beachell, Marit Helgheim, Vinh Hong Le, and Marta Bosque Fajardo (Norwegian Institute of Bioeconomy Research, Ås, Norway) for practical help, as well as Hans Kristian Westrum, Tobias Glemming, and Cornelis Arnoldussen (Soil Steam International AS, Sandefjord, Norway) for their collaboration. No conflict of interest has been declared.