CHD are multifactorial disorders in which diet is known to play a major role. Dietary fatty acid composition may have an effect on the development of thrombosis and atherosclerosis(Reference Knapp1, Reference Kwon, Snook and Wardlow2), although no conclusive agreement regarding this matter has yet been reached. In general terms, SFA may promote the formation of arterial thrombi, linoleic acid is antithrombotic, and oleic acid and other MUFA probably have little effect on arterial thrombosis(Reference Knapp1). Thus, Thijssen et al. (Reference Thijssen, Hornstra and Mensink3) reported that different authors found no effect of dietary SFA on platelet function, thromboxane (TX) generation or the excretion of TXB2 and prostacyclin I2 (PGI2, determined as 6-keto-PGF1α). Sinclair(Reference Sinclair4) postulated that a deficiency of arachidonic acid might lead to atherosclerosis; moreover, from a slightly different viewpoint, a diet high in SFA and low in essential fatty acids contributes to CVD(Reference Lee and Hwang5). Hornstra(Reference Hornstra6, Reference Hornstra7) and Harris et al. (Reference Harris, Miller and Tighe8) indicate that a diet high in fish oils or n-3 fatty acids decreases platelet synthesis of the series-2 eicosanoids (e.g. TXA2). Moreover, both endothelial production of PGI2 and platelet synthesis of TXA2 decrease after consumption of n-3 fatty acids. However, in men, the effects of diets with a decreased linoleic/linolenic acid ratio differ from that of diets containing fish long-chain n-3 fatty acids because it further decreases TXB2 but increases the PGI2 productions(Reference Chan, McDonald and Gerrard9).
Previous studies by our research group suggest that culinary oil modifies platelet aggregation and thrombogenesis in postmenopausal women(Reference Sánchez-Muniz, Oubiña and Benedí10–Reference Sánchez-Muniz, Oubiña and Ródenas12). Moreover, hypercholesterolaemic subjects display increased platelet aggregation rate and thrombogenesis with respect to their normocholesterolaemic counterparts(Reference Sánchez-Muniz, Oubiña and Benedí10–Reference Miller13).
n-3 Fatty acids, such as α-linolenic, eicosapentaenoic and DHA, are considered important components of a balanced diet and are thought to be effective in preventing and treating CVD(Reference Gebauer, Psota and Harris14). The nutrient and phytochemical composition of walnuts differentiates them from other nuts. Walnuts are rich in polyunsaturated α-linoleic, α-linolenic and γ-linolenic fatty acids, display a balanced n-6/n-3 ratio(Reference Nus, Ruperto and Sánchez-Muniz15) and contain the antioxidant tocopherol(Reference Nus, Ruperto and Sánchez-Muniz15, Reference Tapsell, Gillen and Patch16) and other beneficial compounds, including proteins of high biological value (e.g. arginine), fibre, vitamins, tannins, folates and polyphenols(Reference Nus, Ruperto and Sánchez-Muniz15, Reference Tapsell, Gillen and Patch16), all of which may provide additional antiatherogenic properties(Reference Anderson, Teuber and Gobeille17, Reference Almario, Vonghavaravat and Wong18). Frequent intake of walnuts correlates inversely with myocardial infarction or mortality due to vascular ischaemic disease, regardless of other risk factors, such as age, overweight, hypertension, smoking and lack of exercise(Reference Iwamoto, Imaizumi and Sato19–Reference Sabate22). Due to their potential health benefits, increased walnut consumption has been recommended(Reference Feldman20, 24). However, at present, walnut consumption remains relatively low in Spain (2·65 g/d in 2004) and other Mediterranean countries(Reference Aranceta Bartrina, Pérez Rodrigo, Ruiz Vadillo, Salas-Salvadó, Ros Róala and Sabaté Casellas25). Consequently, several strategies, the most important of which has been the inclusion of walnuts in functional foods, have been adopted to increase their intake(Reference Serrano, Cofrades and Ruiz-Capillas26).
Our research group has studied the effect of adding walnut paste, as a functional ingredient, to restructured meat and has found that consumption of this restructured meat significantly increases the concentration of certain endogenous antioxidants(Reference Canales, Benedí and Nus27, Reference Nus, Frances and Librelotto28). Moreover, a related study(Reference Olmedilla-Alonso, Granado-Lorencio and Herrero-Barbudo29) found that volunteers who consumed walnut-enriched meat displayed significantly lower LDL-cholesterol levels than those who ate low-fat meat (LM). Thus, it can be hypothesised that the intake of restructured beef steaks and sausages containing walnuts may also modify aggregation, TXA2, PGI2 and the thrombogenic ratio (TXA2/PGI2) in individuals at increased CHD risk. The present study aims to compare the effects of meat enriched in walnut paste (WM) with those of LM on platelet aggregation, TXB2, 6-keto-protaglandin F1α (6-keto-PGF1α) and the TXB2/6-keto-PGF1α ratio in volunteers consuming both meats. Moreover, because hypercholesterolaemia and obesity have been related to hyperaggregability and hypercoagulability(Reference Basili, Pacini and Guagnano30–Reference Vignini, Nanetti and Moroni32), this study also analyses the effect of basal serum cholesterol levels and BMI on platelet aggregation and eicosanoid production in these volunteers.
Subjects and methods
Subjects
Twenty-five volunteers selected among the 144 candidates for the study were recruited through announcements in the media and hospitals. The volunteers had to fulfil the following eligibility criteria: (1) age, men ≥ 45 years; women ≥ 50 years and postmenopausal and (2) BMI ≥ 25 to < 35 kg/m2. The volunteers with a BMI ≥ 30 kg/m2 were defined as obese. Moreover, at least one of the following criteria was also required: serum total cholesterol (TC) ≥ 5·69 mmol/l; smoking habit ( ≥ 10 cigarettes per day) and/or hypertension (systolic pressure ≥ 140 mmHg and/or diastolic pressure ≥ 90 mmHg). Exclusion criteria included familiar hypercholesterolaemia and/or type 1 diabetes, treatment with any lipid-lowering, antihypertensive or anti-inflammatory drugs and hormone replacement therapy.
Although many subjects were conscious of displaying three or more risk factors for CHD, some of them were not finally selected because they did not show a regular dietary habit of high meat consumption ( ≥ 5 times/week). In addition, twenty-two volunteers were excluded for different reasons such as the habitual use of drug therapy. Three participants did not complete all the blood extractions and were excluded from the study. The characteristics of volunteers at the start of the dietary intervention are presented in Table 1.
Table 1 Basal characteristics of participants at study entry
(Mean values with their standard errors of twenty-two volunteers)
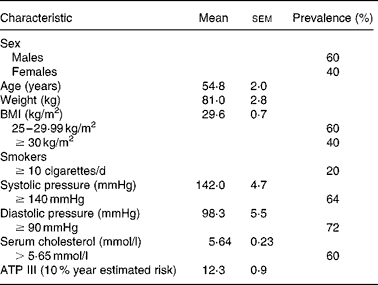
ATP, Adult Treatment panel.
Procedures followed were in accordance with the standards of the Ethics Committee of the University Hospital of Puerta de Hierro (Madrid, Spain) and the Helsinki Declaration, as indicated in the guidelines of the Scientific Technologic Project AGL 2001-2398-C03. Volunteers provided informed consent previous to the start of the study.
Study design
Volunteers were randomly assigned to follow a non-blinded, crossover, placebo-controlled study, consisting of two 5-week experimental periods. The two periods were separated by a 4- to 6-week washout interval during which the subjects returned to their usual diet. During the WM period, the volunteers consumed four 150 g restructured WM steaks and a 150 g ration of WM sausages per week, all containing 20 % WM. During the LM period, the volunteers consumed four 150 g restructured LM steaks and a 150 g ration of LM sausages each week. Table 2 shows the composition of the two types of meat. Further information is available in Serrano et al. (Reference Serrano, Cofrades and Ruiz-Capillas26). It was firmly recommended that all other meats and meat derivatives be excluded from the diet during the experimental periods.
Table 2 Energy content, proximate composition and some compound contents of meat enriched in walnut paste and low-fat meat*
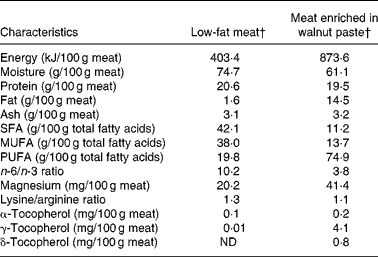
ND, not detected.
* Adapted from Serrano et al. (Reference Serrano, Cofrades and Ruiz-Capillas26).
† Restructured meat and sausages.
Dietary control and compliance
The subjects received frozen LM and WM products once a week. Special emphasis was given to compliance and management of intake with regard to frequency, dates and numbers of steaks consumed. The volunteers completed a dietary record throughout the study in order to allow us to check compliance with the intervention and to confirm substitution of the meat products in their ordinary meals, while maintaining a mixed diet throughout the study periods. Dietary energy and nutrient intakes were calculated using food composition tables(Reference Moreiras, Carbajal and Cabrera33). In order to avoid any possible dietary misunderstanding, volunteers recorded the amount and type of food consumed on a daily basis. Compliance was also assessed by measuring plasma γ-tocopherol concentrations after each experimental period(Reference Olmedilla Alonso, Granado Lorencio and Herrero Barbudo34). Most participants (80 %) enjoyed WM, although they considered this meat type to have a particular taste; however, a large percentage of volunteers (40 %) disliked the low palatability of LM.
Anthropometric and blood pressure measurements
Trained staff measured weight, height, BMI and systolic and diastolic blood pressures of the participants at the start and at weeks 3 and 5 of both dietary periods.
Sample collection
Overnight fasting blood samples were collected from all participants at baseline and at 3 and 5 weeks. During each visit to the hospital, the blood samples and dietary records of each participant were collected. In addition, blood pressure and body weight were recorded. Serum was separated by at 1500 g at 4°C, for 30 min within 1 h of sampling. TC was determined in serum samples using a Technicon RA-500 autoanalyser (Tarrytown, NY, USA) and standard enzymatic procedures (Boehringer Mannheim, Mannheim, Germany). Commercially available quality controls (Precinorm reference 225053 and Precilip reference 781827; Boehringer Mannheim) were included in all assays.
Blood samples were mixed with 3·8 % trisodium citrate (9:1 (v/v), blood/citrate). The anticoagulated blood was centrifuged at 200 g for 10 min to prepare platelet-rich plasma. Platelet counts on platelet-rich plasma samples were done in a haemocytometer, diluting platelet-rich plasma with saline solution. The number of platelets in platelet-rich plasma samples was adjusted with saline solution to 300 000/mm3. Platelet aggregation was determined using ADP (Chromopag ADP, IZASA, Barclona, Spain) as the aggregating agent with an electronic aggregometer (model 500, Chronolog Corporation, IZASA-Coulter, Havertown, PA, USA), as reported by Cardinal & Flower(Reference Cardinal and Flower35). Data were expressed as the maximum aggregation rate at 5 min (cm/5 min) and the time required for reaching the maximum aggregation rate (min).
TXB2, a stable metabolite of TXA2, and 6-keto-PGF1α, a stable metabolite of PGI2, were extracted from citrated plasma samples using silica microcolumns (Chromabond® C18) coupled to a vacuum system (Manifold Vacuum Gauge Controller; J.T. Baker, Phillipsburg, NJ, USA)(Reference Hishinuma, Yu and Takabatake36). After extraction at pH 3, an aliquot of the dry residue was reconstituted with the assay buffer for TXB2 determination (TXB2/2,3-Dinor-TXB2(125I) RIA kit Izotop, Budapest, Hungary) and another for the PGI2 analysis (6-keto-PGF1α/2,3-dinor-6-keto-PGF1α (125I) RIA kit Izotop). The radioactivity of all tubes was measured using a Packard Mod Cobra auto-gamma counting system (Packard Instrument Company, Inc., Packard-Becker B.V.; BK Groningen, The Netherlands).
Statistics
Data are presented as means with their standard errors. Food and nutrient consumption was analysed by paired Student's t test. The percentage change from the baseline TXB2 and PGI2 concentration was determined a priori to be the primary outcome variables. The present study was designed to have a power of 80 % to detect a 20 % difference between the two meat-diet consumptions in the TXB2 and PGI2 response. A pooled sd of 30 % for the change from baseline TXB2 and PGI2, based on previous studies(Reference Oubiña, Sanchez-Muniz and Ródenas11, Reference Sánchez-Muniz, Oubiña and Ródenas12, Reference Mensink, van Houwelingen and Kromhout37), was assumed for this calculation. The statistical power would be approximately 70 %, when compared with subgroups with nine to eleven participants classified according to BMI (higher or lower than 30 kg/m2) or cholesterol levels (higher or lower than 2200 mg/l).
Aggregation and eicosanoid changes were analysed by a two-factor (time and treatment) repeated-measures ANOVA. When there was a significant time × treatment interaction, the change over time within each group was assessed using a one-factor ANOVA, while a paired Student's t test was used to compare differences at the same week between the two treatments. The inter-subject effects of TC and BMI were also tested. The linear adjustment between time of treatment and differences between the two periods was analysed by Pearson's product-moment correlations. Data were significant at P < 0·05. The SPSS 15.0 statistical package was employed.
Results
Most of the important differences between WM and LM were in their fatty acid profiles. The WM diet displayed higher PUFA values and a lower n-6/n-3 ratio than the LM diet (Table 2).
Food intake did not significantly differ throughout both the periods. SFA energy contribution was lower during the WM period, while PUFA n-6 and PUFA n-3 energy contributions and the n-6/n-3 ratio were significantly higher (Table 3). Data on BMI, platelet aggregation, eicosanoid production and the thrombogenesis ratio at basal conditions and after weeks 3 and 5 of each period are presented in Table 4. BMI was not affected throughout the study. Non-significant time × treatment interaction (P>0·1) was found for maximum aggregation rate and the time for maximum aggregation. Maximum aggregation rate was significantly higher at week 5 (P < 0·05) of the WM period than at the same week of the LM period. A significant time × treatment interaction was found for TXB2 (P = 0·048) and the TXB2/6-keto-PGF1α ratio (P = 0·025). TXB2 values were significantly lower at week 5 (P < 0·05) of the WM period than at the same week of the LM period. 6-keto-PGF1α increased (P = 0·037) throughout the WM period but no significant differences (P>0·1) were found between the two diets. The thrombogenic ratio decreased with the WM diet (P = 0·048) and was significantly lower (P < 0·05) at week 5 of the WM period than at the same week of the LM period (Table 4).
Table 3 Daily energy intake of macronutrients and fatty acid energy contribution during intervention and control periods
(Mean values with their standard errors of twenty-two volunteers)

%EN, percentage energy.
Table 4 Effect of low-fat meat and meat enriched in walnut paste consumption on BMI, platelet aggregation and thrombogenesis markers after the 3- and 5-week study
(Mean values with their standard errors of twenty-two volunteers)

TX, thromboxane.
a,b Mean values within a row for the same type of meat with unlike superscript letters were significantly different (P < 0·05).
* Mean value was significantly different from that for week 5 of the low-fat meat period (P < 0·05).
A significant correlation was found between the week of treatment and differences in TXB2 production between the two dietary periods, Y = 12·28 − 26·67X(r 0·296; P = 0·019), where Y is the difference expected between TXB2 WM and TXB2 LM values, and X is the week of treatment.
Differences in the thrombogenic ratio between the two meat periods were significantly correlated with treatment week, Y = 0·290 ;− 0·61X(r 0·318; P = 0·011), where Y is the difference expected in the thrombotic ratio between the WM and LM periods, and X the week of treatment.
No differences were observed throughout the treatments for maximum aggregation rate or time for maximum aggregation due to BMI or basal cholesterol levels. The effect of BMI and treatment on TXB2 levels and the thrombogenic ratio is shown in Fig. 1. TXB2 decreased most in obese individuals (P = 0·050). The thrombogenic ratio of volunteers with the highest basal TC values who consumed WM tended to decrease more than those who consumed LM after week 5 (P = 0·066; Fig. 2).

Fig. 1 Comparison of the effects of 5-week meat enriched in walnut paste (WM) v. low-fat meat (LM) consumption on changes in (a) thromboxane (TXB2) values and (b) the thrombogenic ratio (TXB2/6-keto-PGF1α) in overweight (BMI < 29·99 kg/m2, n 13) and obese (BMI ≥ 30 kg/m2, n 9) volunteers. Repeated measures followed by Bonferroni post hoc study (BMI effect: (a) P = 0·050 and (b) P = 0·181; meat effect: (a) P = 0·023 and (b) P = 0·023; BMI × meat interaction: (a) P = 0·149 and (b) P = 0·121). Change (after 5 weeks minus basal) of bars indicated by a line and an asterisk was significantly different. Basal (![]() ) and after 5 weeks (
) and after 5 weeks (![]() ).
).

Fig. 2 Comparison of the effects of 5-week consumption of meat enriched in walnut paste (WM) v. low-fat meat (LM) on changes in (a) thromboxane (TXB2) values and (b) the thrombogenic ratios (TXB2/6-keto-PGF1α) in low basal total cholesterol (TC) volunteers ( < 2200 mg/l or < 5·64 mmol/l, n 9) and high basal TC volunteers ( ≥ 2200 mg/l, n 13). Repeated measures followed by Bonferroni post hoc study (cholesterol effect: (a) P = 0·200 and (b) P = 0·066; meat effect: (a) P = 0·079 and (b) P = 0·035; cholesterol × meat interaction: (a) P = 0·185 and (b) P = 0·073. Change (after 5 weeks minus basal) of bars indicated by a line and an asterisk was significantly different. Basal (![]() ) and after 5 weeks (
) and after 5 weeks (![]() ).
).
Discussion
To the best of our knowledge, this is the first study of the effect of WM on platelet aggregation and eicosanoid production. Moreover, no studies on the benefits of LM on these parameters have been carried out either.
BMI was not affected by WM diet consumption. These results are interesting, taking into account both the high-fat and energy contents of walnut(Reference Nus, Ruperto and Sánchez-Muniz15), and therefore their potential effect on body weight. Although no scientific papers on the effects of meat and/or walnut consumption on platelet aggregation, eicosanoid production and the thrombogenic ratio are available for a direct comparison of data, the results of studies on the effect of antioxidants, vitamins and PUFA on aggregation and thrombogenesis suggest that differences in some dietary compounds such as SFA, PUFA (total, n-6 and n-3) and the n-6/n-3 and linoleic acid/linolenic acid ratios between the two periods explain, at least partially, the variations between maximum aggregation values observed at week 5. The lower linoleic to linolenic acid ratio of the WM diet, together with other walnut compounds, must affect the platelet reaction capacity. In a previous study, platelet aggregation increased after ADP stimulation when oleic acid was substituted by palmitic acid(Reference Sánchez-Muniz, Oubiña and Ródenas12). According to Chan et al. (Reference Chan, McDonald and Gerrard9), oleic, linoleic and linolenic acids in platelet phospholipids reflect the fatty acid composition of the diet, although little linolenic acid is incorporated into phospholipids. Nonetheless, both 18 : 3 and the linoleic/linolenic ratio influenced the levels of longer-chain n-3 fatty acids, especially EPA, in platelet phospholipids.
The effect of treatment on time for maximum aggregation was more pronounced in individuals with serum cholesterol levels below 2200 mg/l (data not shown). Sanchez-Muniz et al. (Reference Sánchez-Muniz, Oubiña and Ródenas12) found significant differences in maximum aggregation between individuals with cholesterol levels above and below 2400 mg/l when a high-oleic acid diet was consumed, but reported that these differences were not significant with a palmolein diet. Sánchez-Muniz et al. (Reference Sánchez-Muniz, Oubiña and Benedí10) also reported that substitution of extra virgin olive oil by high-oleic sunflower oil significantly increased the aggregation rate. Platelets of hypercholesterolaemics tended to aggregate earlier than those of normocholesterolaemics during the high-oleic acid sunflower oil period, although not in the virgin olive oil period.
Platelet aggregation is modulated by the production of TXA2 and PGI2. An optimal balance of TXA2/PGI2 may be important in the prevention of thrombotic conditions. Evidence indicates that dietary fatty acids can alter this balance(Reference Kwon, Snook and Wardlow2, Reference Chan, McDonald and Gerrard9). Chan et al. (Reference Chan, McDonald and Gerrard9) found that TXB2 production in men significantly decreased when an oil mixture with a high-linoleic/linolenic ratio was substituted by an intermediate or low-linoleic/linolenic acid mixture. This effect was attributed to increased 20 : 5n-3 fatty acid levels in platelet phospholipids. The WM diet contains a lower concentration of SFA and linoleic/linolenic and n-6 PUFA/n-3 PUFA ratios that are 2·5 times lower than those of the LM diet, explaining, at least partially, the present results.
Our group found that the substitution of extra virgin olive oil by oleic acid-rich sunflower oil increased in vitro platelet aggregation and TXB2 production(Reference Oubiña, Sanchez-Muniz and Ródenas11). These findings suggested that variations in the concentration of minor oil constituents may account for some of the differences observed in TXB2 levels after both dietary periods(Reference Oubiña, Sanchez-Muniz and Ródenas11). Proanthocyanidins, naturally occurring plant metabolites commonly found in fruits, vegetables, nuts, seeds, flowers and bark(Reference Bagchi, Garg and Krohn38), form part of a specific group of polyphenolic compounds called flavonoids(Reference Bravo39). These compounds are reported to have anti-inflammatory and vasodilatory properties(Reference Bagchi, Garg and Krohn38, Reference Bagchi, Garg and Krohn40), to inhibit lipid peroxidation, platelet aggregation and capillary permeability and to affect, among others, the phospholipase A2, cyclo-oxygenase and lipoxygenase enzyme systems(Reference Bagchi, Garg and Krohn38, Reference Bagchi, Garg and Krohn40–Reference Robert, Godeau and Gavignet-Jeannin42).
One very interesting finding was the significant and negative adjustment between time of treatment and differences in TXB2 production between the two periods, suggesting, at least during the length of the study, that WM consumption partially inhibits, in a time-dependent manner, the TXB2 production.
Obesity has been related to hyperaggregability and thrombogenesis(Reference Basili, Pacini and Guagnano30–Reference Vignini, Nanetti and Moroni32, Reference Anfossi, Russo and Massucco43). Obese individuals show TXB2 excretion three times higher than slim people(Reference Basili, Pacini and Guagnano30). In agreement with these findings, we observed that the overweight individuals presented an average basal TXB2 concentration of approximately 240 pg/ml, while their obese counterparts approximately 700 pg/ml (data not shown). Although WM consumption did not significantly change the body weight or BMI in the same volunteers(Reference Canales, Benedí and Nus27), it decreased TXB2 production by 20·2 %. This decrease was more relevant (32·2 %) in obese volunteers (data not shown), suggesting that obese individuals are the real target for WM consumption.
No clear differences between normocholesterolaemic and hypercholesterolaemic individuals were found with regard to the effect of the WM diet on TXB2 production, as levels decreased by approximately 40 % in both cholesterol groups. Oubiña et al. (Reference Oubiña, Sanchez-Muniz and Ródenas11) reported that the dietary exchange of olive oil by high-oleic acid sunflower oil had a similar effect on TXB2 production in subjects whose cholesterol levels were above and below 6·21 mmol/l.
In agreement with Chan et al. (Reference Chan, McDonald and Gerrard9), the decrease in the dietary linoleic/linolenic ratio increased by 30 % 6-keto-PGF1α levels. Animals consuming a diet containing more than 4 % linoleic acid displayed lower or identical aortic PGI2 synthesis than those whose diet included less than 4 % linoleic acid(Reference Srivastava44). Low concentrations of linoleic acid decreased in vitro cyclo-oxygenase production by human platelets and thus the synthesis of both TXB2 and PGI2. At high concentrations, however, linoleic acid decreased cyclo-oxygenase and TX synthase activities, while PGI2 synthesis increased(Reference Srivastava44). Oubiña et al. (Reference Oubiña, Sanchez-Muniz and Ródenas11) reported that BMI and cholesterol levels did not significantly influence the effect of diet on prostacyclin production, and Sánchez-Muniz et al. (Reference Sánchez-Muniz, Oubiña and Ródenas12) also found that serum cholesterol levels had no significant effect on the modulation of eicosanoid production by the dietary exchange.
WM positively affected the thrombogenic ratio of volunteers. The differences between the thrombogenic ratios of both dietary periods increased over time. This finding suggests that WM has a strong effect on thrombogenesis. As previously commented, Chan et al. (Reference Chan, McDonald and Gerrard9) found that the thrombogenic ratio in men significantly improved when an oil mixture with a high-linoleic/linolenic ratio was substituted by an intermediate or low-linoleic/linolenic acid. Several walnut compounds may be involved. Thus, it has been suggested that tocopherol may decrease thrombogenesis(Reference Robert, Godeau and Gavignet-Jeannin42, Reference Szczeklik, Gryglewski and Domagala45). Furthermore, polyphenols, which are present in walnuts and some vegetables as condensed tannins, may decrease platelet aggregation and the enzymes that participate in eicosanoid production(Reference Murray, Pizzorno, Murray and Pizzorno41, Reference Robert, Godeau and Gavignet-Jeannin42, Reference Fine46).
The positive effect of WM on the thrombogenic ratio was relevant in the hypercholesterolaemic volunteers. By contrast, Oubiña et al. (Reference Oubiña, Sanchez-Muniz and Ródenas11) and Sánchez-Muniz et al. (Reference Sánchez-Muniz, Oubiña and Ródenas12) found that the serum cholesterol level did not modify the effect of diet on the thrombogenic ratio.
In conclusion, the effects on TXB2 and the thrombogenic ratio suggest that consumption of WM helps to decrease thrombogenic risk. Moreover, the results suggest that dietary treatment, more than BMI or basal TC, affected the thrombogenic ratio at week 5. Obese and hypercholesterolaemic individuals were more sensitive to the effect of WM. More studies are needed to better understand the physiological effect of changes induced by the consumption of meat enriched in WM, and to assess the role of some minor walnut compounds over a longer period of time.
Acknowledgements
Funds for this study were granted by the Spanish Ministerio de Educación y Ciencia, Project AGL 2001-2398-C03 and AGL 2005-07 204-C02-01/ALI and Consolider-Ingenio 2010, reference CSD2007-00 016. We are grateful for the valuable assistance in TX and prostacyclin determination given by the Department of Physiology of the Medical School of the Universidad Complutense of Madrid. Thanks are due to the Universidad Complutense of Madrid for the predoctoral fellowship of M. N. All authors have significantly contributed to the paper and agree with the present version of the manuscript. F. J. S.-M. has contributed to the study design, volunteers' selection, data discussion and writing of the paper. J. B., S. B. and A. C. have contributed to the data acquisition and analysis and writing of the paper, M. N. and J. L. have contributed to the volunteers' selection, data analysis and writing of the paper. The work has been performed according to the ethical bases of the Helsinki Declaration. Volunteers provided informed consent previous to the start of the study. The authors declare that there are no conflicts of interest.








