The ability to process and respond to rewards is essential for adaptive functioning as it allows for adjustment of behavior following positive outcomes and promotes the pursuit of new rewarding experiences (Casey, Duhoux, & Malter Cohen, Reference Casey, Duhoux and Malter Cohen2010; Forbes & Casement, Reference Forbes and Casement2019). Theoretical frameworks implicate dysregulation in reward responsiveness as an important mechanism of psychopathology. For example, the Reinforcement Sensitivity Theory (RST; Gray and McNaughton, Reference Gray and McNaughton2003) posits that individual differences in reward and punishment processing predict behavior, cognition, and psychopathology. RST has provided a theoretical basis for classification models related to general psychopathology (Bijttebier, Beck, Claes, & Vandereycken, Reference Bijttebier, Beck, Claes and Vandereycken2009) and more specifically for internalizing disorders (Nusslock, Abramson, Harmon-Jones, Alloy, & Hogan, Reference Nusslock, Abramson, Harmon-Jones, Alloy and Hogan2007). Research has indicated that sensitivity to reward discriminates between anxiety and depression, negatively predicting depression and positively predicting anxiety (Katz, Matanky, Aviram, & Yovel, Reference Katz, Matanky, Aviram and Yovel2020). Furthermore, RST has been used to suggest how individual differences may influence the etiology (Kimbrel, Reference Kimbrel2008) and severity (e.g. Brown, Chorpita, and Barlow, Reference Brown, Chorpita and Barlow1998) of internalizing psychopathology.
Many internalizing disorders are characterized by deficits in reward sensitivity (e.g. Craske, Meuret, Ritz, Treanor, and Dour, Reference Craske, Meuret, Ritz, Treanor and Dour2016; Treadway and Zald, Reference Treadway and Zald2013). An extensive body of research suggests that dysfunctional reward circuitry plays a central role in the development of depression (Russo & Nestler, Reference Russo and Nestler2013; Treadway & Zald, Reference Treadway and Zald2011) and anxiety disorders (Harrewijn, Schmidt, Westenberg, Tang, & van der Molen, Reference Harrewijn, Schmidt, Westenberg, Tang and van der Molen2017; Silk, Davis, McMakin, Dahl, & Forbes, Reference Silk, Davis, McMakin, Dahl and Forbes2012). Functional magnetic imaging (fMRI) studies have shown that blunted striatal activation to rewards is associated with concurrent depressive disorders and symptoms in youth and adults (Forbes et al., Reference Forbes, Christopher May, Siegle, Ladouceur, Ryan, Carter and Dahl2006; Pizzagalli et al., Reference Pizzagalli, Holmes, Dillon, Goetz, Birk, Bogdan and Fava2009), whereas social anxiety and behavioral inhibition are associated with enhanced striatal activation to rewards in youth (Bar-Haim et al., Reference Bar-Haim, Fox, Benson, Guyer, Williams, Nelson and Ernst2009; Guyer et al., Reference Guyer, Nelson, Perez-Edgar, Hardin, Roberson-Nay, Monk and Ernst2006). However, recent work has discovered inconsistencies across fMRI studies of internalizing disorders (Müller et al., Reference Müller, Cieslik, Serbanescu, Laird, Fox and Eickhoff2017).
Event-related potentials (ERPs) are another way to measure neural reward responsiveness. The reward positivity (RewP) is an ERP component that indexes reinforcement learning and reward system activation (Proudfit, Reference Proudfit2015). The RewP demonstrates good psychometric properties, including good internal consistency and test–retest reliability (Levinson, Speed, Infantolino, & Hajcak, Reference Levinson, Speed, Infantolino and Hajcak2017) and concurrent validity with other indicators of reward sensitivity, such as self-report reward sensitivity, reward learning behavior (Bress & Hajcak, Reference Bress and Hajcak2013), observed and self-reported positive emotionality (Kujawa et al., Reference Kujawa, Proudfit, Kessel, Dyson, Olino and Klein2015), and fMRI-based activation in the medial prefrontal cortex and striatum (Carlson, Foti, Mujica-Parodi, Harmon-Jones, & Hajcak, Reference Carlson, Foti, Mujica-Parodi, Harmon-Jones and Hajcak2011; Foti, Carlson, Sauder, & Proudfit, Reference Foti, Carlson, Sauder and Proudfit2014).
The RewP has been associated with multiple internalizing disorders. For example, a blunted RewP has been associated with depression in children (Belden et al., Reference Belden, Irvin, Hajcak, Kappenman, Kelly, Karlow and Barch2016), adolescents (Bress, Smith, Foti, Klein, & Hajcak, Reference Bress, Smith, Foti, Klein and Hajcak2012; Bress, Meyer, & Hajcak, Reference Bress, Meyer and Hajcak2015a; Burani et al., Reference Burani, Mulligan, Klawohn, Luking, Nelson and Hajcak2019), and adults (Brush, Ehmann, Hajcak, Selby, & Alderman, Reference Brush, Ehmann, Hajcak, Selby and Alderman2018; Burkhouse, Gorka, Afshar, & Phan, Reference Burkhouse, Gorka, Afshar and Phan2017; Foti et al., Reference Foti, Carlson, Sauder and Proudfit2014; Foti & Hajcak, Reference Foti and Hajcak2009; Liu et al., Reference Liu, Wang, Shang, Shen, Li, Cheung and Chan2014; Whitton et al., Reference Whitton, Kakani, Foti, Van't Veer, Haile, Crowley and Pizzagalli2016). A blunted RewP has also been shown to prospectively predict the onset of depression in adolescents (Bress et al., Reference Bress, Smith, Foti, Klein and Hajcak2012, Reference Bress, Foti, Kotov, Klein and Hajcak2013; Bress, Meyer, & Proudfit, Reference Bress, Meyer and Proudfit2015b; Nelson, Perlman, Klein, Kotov, & Hajcak, Reference Nelson, Perlman, Klein, Kotov and Hajcak2016) and adults (Bress & Hajcak, Reference Bress and Hajcak2013; Foti & Hajcak, Reference Foti and Hajcak2009; Proudfit, Reference Proudfit2015; Weinberg, Liu, Hajcak, & Shankman, Reference Weinberg, Liu, Hajcak and Shankman2015). Similarly, a blunted RewP has been associated with generalized anxiety symptoms in children (Kessel, Kujawa, Hajcak Proudfit, & Klein, Reference Kessel, Kujawa, Hajcak Proudfit and Klein2015) and trait anxiety in college students (Gu, Huang, & Luo, Reference Gu, Huang and Luo2010). In contrast, one study found that a larger RewP is associated with greater social anxiety symptoms in children (Kessel et al., Reference Kessel, Kujawa, Hajcak Proudfit and Klein2015). Findings from another study suggest that the RewP demonstrates the opposite relationship with depression and social anxiety symptoms, such that a more blunted RewP is associated with greater depression symptoms, whereas a more enhanced RewP is associated with greater social anxiety symptoms (Nelson & Jarcho, Reference Nelson and Jarcho2021). Additional research has shown that hypomania is related to an enhanced RewP (Glazer, Kelley, Pornpattananangkul, & Nusslock, Reference Glazer, Kelley, Pornpattananangkul and Nusslock2019).
Extant studies on the RewP and psychopathology have largely focused on individual disorders. However, depression and anxiety are highly comorbid (Kessler, Chiu, Demler, Merikangas, & Walters, Reference Kessler, Chiu, Demler, Merikangas and Walters2005b; Watson, Reference Watson2005a), and it is possible that the unique relationship between the RewP and particular disorders and symptoms might be due to higher-order subfactors of psychopathology. Factor analytic studies of the latent structure of psychopathology have organized internalizing disorders into two empirical classes: distress disorders (major depressive disorder [MDD], dysthymia, GAD, and posttraumatic stress disorder [PTSD]), and fear disorders (panic disorder, agoraphobia, social phobia, and specific phobia) (Eaton et al., Reference Eaton, Krueger, Markon, Keyes, Skodol, Wall and Grant2013; Vollebergh et al., Reference Vollebergh, Iedema, Bijl, de Graaf, Smit and Ormel2001). Researchers have hypothesized that higher-order subfactors could help reveal fundamental biological mechanisms shared across disorders (Watson, Reference Watson2005a) and this has been recently demonstrated using other neurobiological measures of emotional reactivity (Beatty, Ferry, Eaton, Klein, & Nelson, Reference Beatty, Ferry, Eaton, Klein and Nelson2023; Nelson, Perlman, Hajcak, Klein, & Kotov, Reference Nelson, Perlman, Hajcak, Klein and Kotov2015). In addition, one investigation found that the RewP was negatively associated with the distress subfactor, but was unrelated to the fear subfactor (Burkhouse et al., Reference Burkhouse, Gorka, Afshar and Phan2017). However, no study has examined the relationship between the RewP and higher-order psychopathology subfactors in youth.
The relationship between the RewP and internalizing psychopathology has largely been examined using monetary reward. Social reward might be a more salient incentive in the context of internalizing disorders, given that deficits in social functioning play a critical role in the etiology and maintenance of depression and social anxiety (Badcock & Allen, Reference Badcock and Allen2003). Research has differentiated between ‘domain-general’ and ‘domain-specific’ neural reward systems. A domain-general neural reward system suggests that a common neural network underlies response to all types of rewards. For example, the same brain regions and neural pathways might activate when a person gets a bonus (monetary reward) or receives a compliment (social reward). A domain-specific neural reward system suggests that different types of rewards are processed by distinct neural reward pathways. Functional MRI research has supported both theories, with some studies indicating shared neural reward pathways in response to non-social and social rewards (Daniel & Pollmann, Reference Daniel and Pollmann2014; Izuma, Saito, & Sadato, Reference Izuma, Saito and Sadato2008; Lin, Adolphs, & Rangel, Reference Lin, Adolphs and Rangel2012), suggesting a common, domain-general neural reward system. However, other studies have found that the anticipation of social and monetary rewards is associated with activation in different neural regions, indicating dissociable, domain-specific neural networks for monetary and social reward (Chan et al., Reference Chan, Li, Li, Zeng, Xie, Yan and Jin2016; Rademacher et al., Reference Rademacher, Krach, Kohls, Irmak, Gründer and Spreckelmeyer2010). Similarly, ERP research has found moderate correlations between the RewP elicited by monetary and social reward (Ait Oumeziane, Jones, & Foti, Reference Ait Oumeziane, Jones and Foti2019; Banica, Schell, Racine, & Weinberg, Reference Banica, Schell, Racine and Weinberg2022; Ethridge et al., Reference Ethridge, Kujawa, Dirks, Arfer, Kessel, Klein and Weinberg2017; Nelson & Jarcho, Reference Nelson and Jarcho2021; Pegg, Arfer, & Kujawa, Reference Pegg, Arfer and Kujawa2021), suggesting that both overlapping and specific neural responses to reward likely exist (Sescousse, Caldú, Segura, & Dreher, Reference Sescousse, Caldú, Segura and Dreher2013). Despite these findings, most studies have assessed the RewP to a single reward type in relation to psychopathology.
In the few studies that have compared the neural response to monetary and social rewards in relation to internalizing psychopathology, there are a number of critical confounds and limitations. For example, several studies have examined the neural response to social feedback but failed to include a non-social comparison condition (Freeman et al., Reference Freeman, Ethridge, Banica, Sandre, Dirks, Kujawa and Weinberg2022; Kujawa, Arfer, Klein, & Proudfit, Reference Kujawa, Arfer, Klein and Proudfit2014a; van der Veen, van der Molen, van der Molen, & Franken, Reference van der Veen, van der Molen, van der Molen and Franken2016) or compared experimental paradigms that are not matched on basic task properties (Banica et al., Reference Banica, Schell, Racine and Weinberg2022; Ethridge et al., Reference Ethridge, Kujawa, Dirks, Arfer, Kessel, Klein and Weinberg2017). With these confounds, it is not possible to make more refined domain-specific interpretations about how neural responses to different reward types are related to psychopathology. One study utilized monetary and social tasks that were matched on trial structure, timing and feedback stimuli, and found that depression and social anxiety demonstrated negative and positive, respectively, associations with the domain-general RewP, while social anxiety was also positively associated with domain-specific RewP to social dislike feedback (Nelson & Jarcho, Reference Nelson and Jarcho2021). These results suggest that it is important to minimize task confounds when exploring the association between domain-general v. domain-specific neural activation and psychopathology.
The RewP has also been shown to index familial risk for psychopathology. For example, a blunted RewP has been observed in children and adolescents with a family history of depression (Foti, Kotov, Klein, & Hajcak, Reference Foti, Kotov, Klein and Hajcak2011; Freeman et al., Reference Freeman, Ethridge, Banica, Sandre, Dirks, Kujawa and Weinberg2022; Kujawa, Proudfit, & Klein, Reference Kujawa, Proudfit and Klein2014b; Kujawa & Burkhouse, Reference Kujawa and Burkhouse2017). These investigations have focused on discrete categorical disorders, despite research suggesting that higher-order subfactors may better reveal fundamental neurobiological mechanisms shared across multiple psychiatric disorders (Watson, Reference Watson2005b). To date, no study has examined familial psychopathology subfactors in relation to the RewP.
The present study examined the association between adolescent (throughout this paper, we use the term ‘adolescents’ to refer to the entire sample, encompassing individuals aged 13 to 22 years; while recognizing that some participants fall into the young adult category, we have chosen this term for consistency and clarity in addressing the developmental context of our study) and parental psychopathology subfactors (distress, fear/obsessions, and positive mood) and the adolescent RewP to monetary and social reward. Adolescents also completed monetary and social feedback tasks that were matched in trial structure, timing, and feedback stimuli in a counterbalanced order, and the RewP was measured in response to monetary and social feedback. We hypothesized that the adolescent distress subfactor would be negatively associated with the RewP, whereas the fear/obsessions and positive mood subfactors would be positively associated with the adolescent RewP. We hypothesized that the associations would be present for the domain-general RewP. We hypothesized a similar relationship for parental distress, fear/obsessions, and positive mood subfactors and the adolescent RewP. Finally, we tested whether parental psychopathology was associated with the adolescent RewP independent of adolescent psychopathology.
Method
Participants
The sample was obtained from a longitudinal investigation of the development of reward sensitivity and depression across adolescence. Participants were initially recruited from the community using online and flier postings and a commercial mailing list of families with an 8 to 14-year-old girl within a 30-mile radius of Stony Brook University. The current paper is limited to the T3 sample (ages 13 to 22) since this was the only assessment in which participants completed the social reward task. The T3 sample included 193 females (M = 17.33 years-old, s.d. = 1.97) who identified as White (87.0%), Black (6.2%), more than one race (5.7%), Native Hawaiian/other Pacific Islander (0.5%), and Hispanic (10.4%). The parent who accompanied the participant to the lab session was primarily the mother (86.0%). Inclusion criteria were fluency in English and ability to read and understand questionnaires. Exclusion criteria were the presence of a significant developmental or medical disability; participants were allowed to meet criteria for psychiatric disorders. Participants were financially compensated $5 for the monetary reward task and $20/hour for completing the study visit. Informed consent was obtained from all individual participants included in the study, and the research protocol was approved by the Institutional Review Board at Stony Brook University.
Measures
Inventory of depression and anxiety symptoms – expanded version (IDAS-II)
The IDAS-II (Watson et al., Reference Watson, O'Hara, Naragon-Gainey, Koffel, Chmielewski, Kotov and Ruggero2012) is a 99-item factor-analytically derived self-report inventory of empirically distinct dimensions of depression and anxiety symptoms. Each item assesses symptoms over the past two weeks on a five-point Likert scale ranging from 1 (Not at all) to 5 (Extremely). Individual items are scored to create a total of 18 subscales. Higher-order distress, fear/obsessions, and positive mood subfactors were calculated using the factor weights from Watson et al. (Reference Watson, O'Hara, Naragon-Gainey, Koffel, Chmielewski, Kotov and Ruggero2012). The IDAS-II subscales demonstrated good to excellent internal consistency in adolescents and parents (α ranged from 0.71–0.89 and 0.66–0.89, respectively). The parent suicidality subscale showed poor internal consistency (α = 0.23).
Procedure
At the beginning of the laboratory visit, adolescents were told that they would be completing a social evaluation study with peers across the United States. Participants were also told that they would be completing a monetary guessing task. An electroencephalography (EEG) cap was applied to their head and then participants completed the monetary and social reward tasks in a counterbalanced order. At the end of the task participants were informally asked about their experience with the task, and nearly all participants reported high task engagement and believing the veracity of the feedback.
Experimental tasks
Both experimental tasks were administered using Presentation software (Neurobehavioral Systems, Inc., Albany, CA, USA), and were presented in a counterbalanced order.
Monetary Task. Participants completed the doors monetary task (Proudfit, Reference Proudfit2015)). For each trial, participants were presented with an image of two identical doors and asked to select the door containing a monetary prize. Participants were told that if they chose the correct door, they would win $0.50, while if they chose the incorrect door they would lose $0.25. ‘Correct’ selection of the monetary win was indicated by a green arrow pointing upward (↑). ‘Incorrect’ selection of the monetary win was indicated by a red arrow pointing downward (↓). The task consisted of three blocks of 20 trials (60 total), and there was an equal number of win and loss outcomes (30 each).
Social Task. The social task stimuli consisted of 120 images of age-matched peers (60 males and 60 females) presenting positive facial expressions. Images were compiled from multiple sources (internet databases of non-copyrighted images and photographs). All images were cropped to a standardized size (560 × 857 pixels, 72 pixels per inch) and edited such that the individuals pictured were presented from the shoulders up and on a solid gray background. Each trial consisted of a pair of faces matched by gender (i.e. two male-presenting faces or two female-presenting faces). Gender and age determination of photographs were visually assessed by the research team. Participants were asked to provide a digital photo of themselves that they were told would be sent to other age-matched peers across the country. Participants were told that those peers would receive a notification on their smartphone asking them to view the image of the participant and give a rating of whether they would ‘like’ or ‘dislike’ the participant, based on that photo. Participants were told that later in the lab session, after enough time had elapsed for the purported peers to have rated their photo, they would be asked to guess which peers ‘liked’ them.
The social task (Distefano et al., Reference Distefano, Jackson, Levinson, Infantolino, Jarcho and Nelson2018) was identical to the monetary task, except pictures of gender-matched peers were presented instead of doors. Participants were presented with pairs of gender-matched peers of a similar age to the participant. There were an equal number of trials with male and female peers (30 each, 60 total). Prior to the task, participants were told that the peers were other participants who viewed and rated the digital photograph they submitted to the study. Participants were asked to select the person that they thought said they would ‘like’ the participant. Correct selection of the person that said they would like the participant was indicated by a green arrow pointing upward (↑). Incorrect selection of the person that said they would like the participant was indicated by a red arrow pointing downward (↓), indicating that person would dislike the participant. Participants experienced 16 trials of correct feedback from female-presenting pairs, 14 trials of correct feedback from male-presenting pairs, 14 trials of incorrect feedback from female-presenting pairs, and 16 trials of incorrect feedback from male-presenting pairs.
EEG recording and data reduction
Continuous EEG was recorded using an elastic cap with 34 sintered Ag/AgCl electrodes placed according to the 10/20 system. Electrooculogram (EOG) was recorded using four additional facial electrodes: two placed approximately 1 cm outside of the right and left eyes and two placed approximately 1 cm above and below the right eye. Data were recorded using the ActiveTwo system. The EEG was digitized with a sampling rate of 1024 Hz using a low-pass fifth order sinc filter with a half-power cutoff of 204.8 Hz. A common mode sense active electrode producing a monopolar (nondifferential) channel was used as a recording reference.
EEG data were analyzed using BrainVision Analyzer 2 (Brain Products, Gilching, Germany). Data were referenced offline to the average of left and right mastoids, band-pass filtered (0.1 to 30 Hz), and corrected for eye movement artifacts (Gratton, Coles, & Donchin, Reference Gratton, Coles and Donchin1983). A semiautomatic procedure was employed to detect and reject artifacts. The criteria applied were a voltage step of more than 50 μV between sample points, a voltage difference of 300 μV within a trial, and a maximum voltage difference of less than 0.5 μV within 100-ms intervals. These intervals were rejected from individual channels in each trial. Visual inspection of the data was then conducted to detect and reject remaining artifacts. Feedback-locked epochs were extracted with a duration of 1000 ms, including a 200 ms pre-stimulus and 800 ms poststimulus interval. The 200 ms pre-stimulus interval was used as the baseline.
Feedback-locked ERPs were averaged separately for gain and loss feedback on the monetary task, and like and dislike feedback on the social feedback task. The ERP response to monetary and social feedback was scored by using the mean activity in a 100-ms window around the peak of the win/like and loss/dislike difference waveform within a 250 to 350-ms time frame at a pooling of electrodes FC1, FC2, Cz, and FCz. See online Supplemental Materials for reliability of the RewP.
Data analysis
A total of 193 adolescents and their parents attended the T3 lab visit. Of these families, four adolescents and 12 parents did not complete the IDAS-II, three adolescents declined to complete the social task, eight adolescents were missing more than 50% trials (e.g. due to poor signal/artifacts), and two adolescents experienced equipment failure, resulting in a final sample of 163 adolescents and their parents.
To compare the neural response to monetary and social feedback, we conducted a Task (monetary v. social) × Outcome (favorable [gain/like] v. unfavorable [loss/dislike]) repeated measures analysis of variance. The association between the ERP response to monetary and social feedback was examined using Pearson's correlation coefficients.
The relationship between adolescent psychopathology subfactors and adolescent neural response to monetary and social reward was examined via mixed-model analysis of covariance (ANCOVA), with Task (monetary v. social) as the within-subject factor and adolescent age, distress, fear/obsessions, and positive mood subfactors included as covariates. Adolescent subfactor main effects were followed-up with partial correlations using subfactor residuals (e.g. distress independent of fear/obsessions and positive mood) and the mean RewP (averaged across monetary and social tasks) controlling for adolescent age. Reward Type X Adolescent Subfactor interactions were followed-up with partial correlations using subfactor residuals and monetary RewP (i.e. monetary RewP difference score independent of social RewP difference score) and social RewP residuals, controlling for adolescent age. Identical analyses were conducted for parental subfactors and adolescent neural response to rewards; analyses also controlled for parent sex (father = 0, mother = 1). All ANCOVA analyses were conducted in IBM SPSS Statistics, Version 26.0 (Armonk, NY, USA).
Results
Monetary and social reward tasks
Figure 1 displays the grand average waveforms and scalp distributions for the ERP response to monetary and social feedback. Results indicated a main effect of task, F(1, 162) = 238.09, p < 0.001, ηp2 = 0.60, such that the neural response during monetary trials was greater compared to social trials. Results also indicated a main effect of outcome, F(1, 162) = 160.55, p < 0.001, ηp2 = 0.50, such that the neural response to favorable feedback (i.e. monetary gain, social like) was greater compared to unfavorable feedback (i.e. monetary loss, social dislike). There was a positive moderate correlation between the monetary and social RewP, r(163) = 0.38, p < 0.001. There was no Task × Outcome interaction, F(1, 162) = 3.04, p = 0.08, ηp2 = 0.02.
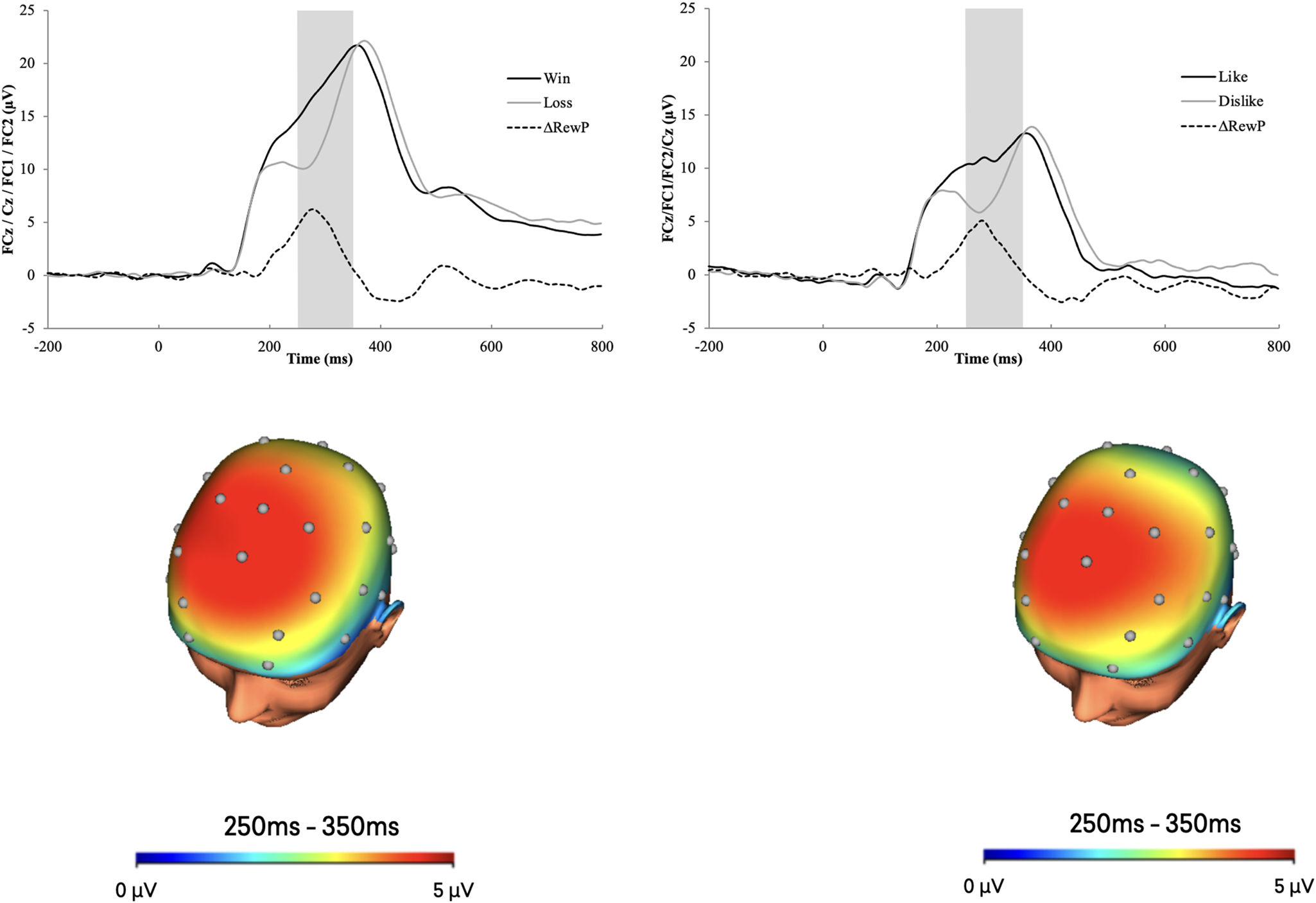
Figure 1. ERP waveforms (top) and scalp distributions (bottom) for the monetary (left) and social (right) tasks.
Note. The shaded region of the waveforms shows the segment from 250 to 350 ms where the mean activity was scored at a pooling of electrodes FC1, FC2, Cz, and FCz. The monetary RewP is represented by the gain-loss difference, and the social RewP is represented by the like-dislike difference. ms = millisecond. The scalp distributions use different scales.
Adolescent subfactors
Table 1 displays bivariate correlations between adolescent and parental subfactors and adolescent monetary and social RewP. See online Supplementary Materials for additional information on severity of psychopathology. Analyses of adolescent subfactors and adolescent neural response to social and monetary reward indicated main effects of adolescent distress, fear/obsessions, and positive mood (Table 2). As shown in Fig. 2, the adolescent fear/obsessions subfactor was positively associated with adolescent neural response to domain-general reward (i.e. across monetary and social reward), r(160) = 0.19, p = 0.02. In contrast, the adolescent distress and positive mood subfactors were negatively associated with adolescent neural response to domain-general reward, r(160) = −0.16, p = 0.04; r(160) = −0.22, p = 0.004, respectively.
Table 1. Correlations between parental and adolescent psychopathology subfactors and adolescent neural response to monetary and social reward

Note. ** p < 0.01, * p < 0.05.
Table 2. ANCOVA results for the adolescent and parent psychopathology subfactors and the adolescent RewP

Note. Separate analyses were conducted for adolescent and parental subfactors and adolescent neural response to rewards.

Figure 2. Scatterplots displaying the association between parental (left) and adolescent (right) distress (top), fear/obsessions (middle) and positive mood (bottom) subfactors and the domain-general RewP (average of monetary and social; i.e. mean of gain-loss difference score and like-dislike difference score), at a pooling of electrodes FC1, FC2, Cz, and FCz.
Parental subfactors
Analyses of parental subfactors and adolescent neural response to social and monetary reward indicated main effects of parental distress and fear/obsessions (Table 2). As shown in Fig. 2, the parental fear/obsessions subfactor was positively associated with adolescent neural response to domain-general reward, r(159) = 0.18, p = 0.03. In contrast, the parental distress subfactor was negatively associated with adolescent neural response to domain-general reward, r(159) = −0.22, p = 0.004. Results also indicated a Reward Type X Parental Positive Mood interaction (Table 2). As shown in Fig. 3, follow-up analyses indicated that the parental positive mood subfactor was negatively associated with adolescent neural response to monetary reward, r(159) = −0.24, p = 0.002, but was not associated with adolescent neural response to social reward, r(159) = 0.11, p = 0.17. Results did not differ based on parent sex.

Figure 3. Scatterplots displaying the association between parental positive mood symptoms the domain-specific monetary (left; i.e. gain-loss difference score) and social (right; i.e. like-dislike difference score) RewP, at a pooling of electrodes FC1, FC2, Cz, and FCz.
Independence of adolescent and parental subfactors
When adolescent and parental subfactors were included as simultaneous independent variables, results again indicated main effects of the adolescent distress, F(1, 154) = 4.56 p = 0.034, η p2 = 0.03, adolescent fear/obsessions, F(1, 154) = 5.50, p = 0.020, η p2 = 0.03, adolescent positive mood, F(1, 154) = 9.81, p = 0.002, η p2 = 0.06, parental distress, F(1, 154) = 9.76, p = 0.002, η p2 = 0.06, parental fear/obsessions subfactors, F(1, 154) = 5.67, p = 0.018, η p2 = 0.04, and Reward Type X a Parental Positive Mood interaction F(1, 154) = 7.14, p = 0.008, η p2 = 0.04.
Discussion
The present study indicates that both adolescent and parental (i.e. familial risk) psychopathology subfactors are associated with the adolescent RewP. Adolescents with higher distress and positive mood had a more blunted domain-general RewP. Conversely, adolescents with higher fear/obsessions had a more enhanced domain-general RewP. Similarly, adolescents of parents with higher distress and positive mood had a more blunted domain-general and domain-specific monetary RewP, respectively. Adolescents of parents with higher fear/obsessions had a more enhanced domain-general RewP. Finally, the associations between parental and adolescent psychopathology subfactors and the adolescent RewP were independent of each other. Together, these findings indicate that both current adolescent psychopathology as well as risk for psychopathology are uniquely associated with the adolescent RewP.
The present study provides novel evidence that psychopathology subfactors demonstrate differential associations with the neural response to rewards. The results are in line with previous studies demonstrating that a blunted RewP is associated with distress disorders, such as depression (Belden et al., Reference Belden, Irvin, Hajcak, Kappenman, Kelly, Karlow and Barch2016; Bress et al., Reference Bress, Smith, Foti, Klein and Hajcak2012; Bress et al., Reference Bress, Meyer and Hajcak2015a; Burani et al., Reference Burani, Mulligan, Klawohn, Luking, Nelson and Hajcak2019), and distress symptoms/traits, such as generalized anxiety symptoms (Kessel et al., Reference Kessel, Kujawa, Hajcak Proudfit and Klein2015) in children and adolescents. The results also suggest that the common findings found across individual distress disorders may reflect the common variance amongst these categorical diagnoses. Anxiety disorders have been characterized by hyper-reactivity to emotional and motivational stimuli, which includes reward (Harrewijn et al., Reference Harrewijn, Schmidt, Westenberg, Tang and van der Molen2017; Silk et al., Reference Silk, Davis, McMakin, Dahl and Forbes2012). Consistent with existing literature showing an association between fear-based psychopathology (e.g. social anxiety) and an enhanced RewP in children (Kessel et al., Reference Kessel, Kujawa, Hajcak Proudfit and Klein2015) and adults (Nelson & Jarcho, Reference Nelson and Jarcho2021), the present study indicated a positive association between the adolescent fear/obsessions subfactor and the adolescent domain-general RewP. Together, the present study suggests there is a discriminate relationship with the distress and fear subfactors and the neural response to reward.
In contrast with our hypothesis, the adolescent positive mood subfactor was negatively associated with the adolescent domain-general RewP. These results are inconsistent with research showing a negative association between anhedonia and the RewP in undergraduates and emerging adults (Banica et al., Reference Banica, Schell, Racine and Weinberg2022; Mulligan, Eisenlohr-Moul, Eckel, & Hajcak, Reference Mulligan, Eisenlohr-Moul, Eckel and Hajcak2023). The positive mood subfactor is anchored by two scales: one assessing a healthy, adaptive form of positive affect (well-being) and another assessing a dysfunctional form of positive affect (euphoria; Watson and O'Hara, Reference Watson and O'Hara2017). Euphoria, which represents elevated mood, heightened energy and grandiosity/self-esteem, captures the elevated, expansive mood that is the hallmark of manic episodes (American Psychiatric Association, 2013; Gruber, Mauss, & Tamir, Reference Gruber, Mauss and Tamir2011). Previous studies have found that bipolar disorder is associated with deficits in reinforcement learning, suggesting that positive mood elevation (a defining feature of bipolar disorder) could be inversely associated with the neural correlates of reinforcement learning (Urošević, Halverson, Youngstrom, & Luciana, Reference Urošević, Halverson, Youngstrom and Luciana2018). However, it is important to note that the present sample did not include high rates of bipolar disorder (see online Supplemental Materials). The positive mood subfactor has received much less attention in the literature and future research is needed to better understand its association with the neural response to rewards.
The present study provides further evidence that psychopathology subfactors demonstrate domain-general, as opposed to domain-specific, associations with the neural response to rewards. Similar to a previous investigation (Nelson & Jarcho, Reference Nelson and Jarcho2021), the present study found that the association between psychopathology and the RewP did not differ as a function of the type of reward, even in a sample of adolescents for whom social feedback and information are particularly salient. It is important to note that these results do not rule out the possibility that different forms of psychopathology demonstrate distinct relationships with different aspects of reward processing (e.g. anticipatory reward processing), but they do highlight the importance of considering basic task and stimulus properties when attempting to make a domain-specific interpretation.
The present study adds to growing research indicating that the RewP also indexes risk for psychopathology. The present study extends this literature as results demonstrated a negative association between the parental distress subfactor and the adolescent domain-general RewP. While abnormal reward processing has been observed in the youth of depressed parents, there is limited knowledge on the influence of parental anxiety on reward processing in youth. One research study found a relationship between parental depression and offspring blunted RewP, only in the absence of parental anxiety (Kujawa et al., Reference Kujawa, Proudfit and Klein2014b). The present study indicates that parental fear/obsession psychopathology should also be examined in relation to offspring neural response to reward.
The present study also found that the parental positive mood subfactor was negatively associated with the adolescent domain-specific monetary RewP. Research indicates that adolescents experience more frequent high-intensity positive emotions as compared to adults (Larson, Csikszentmihalyi, and Graef, Reference Larson, Csikszentmihalyi and Graef1980; Larson and Richards, Reference Larson and Richards1994; Verma and Larson, Reference Verma and Larson1999). Parent and adolescent positive mood subfactors were weakly correlated. Considering age-related differences in emotional experiences, it is possible that the way parents and youth self-report positive mood differs as a function of age. It is currently unclear whether positive mood measured in adolescents and their parents reflect the same construct, which could explain why parental positive mood symptoms were associated with the adolescent domain-specific monetary RewP, whereas adolescent positive mood symptoms were associated with the domain-general RewP. The present study suggests that the type of reward might be relevant when considering risk for internalizing psychopathology symptoms: a blunted adolescent RewP to general v. specific (monetary) reward might represent a mechanism that distinguishes between internalizing subfactors (i.e. distress v. positive mood).
This study is one of the first to move beyond single disorders and examine higher-order subfactors of psychopathology in relation to neural response to monetary and social reward. Extant research on neurobiological risk factors has primarily focused on diagnostic groups, though known vulnerability factors tend to operate via multifinality (Cicchetti & Rogosch, Reference Cicchetti and Rogosch1996). This study facilitates more efficient identification of risk factors for psychopathology by examining neural response to monetary and social reward as a mechanism that distinguishes subfactors of internalizing psychopathology. Moreover, adolescence is a key period for the development of reward circuitry and is characterized by heightened neural reward sensitivity (Bjork et al., Reference Bjork, Knutson, Fong, Caggiano, Bennett and Hommer2004; Ernst et al., Reference Ernst, Nelson, Jazbec, McClure, Monk, Leibenluft and Pine2005; Forbes et al., Reference Forbes, Ryan, Phillips, Manuck, Worthman, Moyles and Dahl2010; Galvan, Hare, Voss, Glover, & Casey, Reference Galvan, Hare, Voss, Glover and Casey2007). Adolescent increased reward system activation coincides with developmentally normative increases in social exploration, risk-taking, and sensation seeking (Casey, Jones, & Hare, Reference Casey, Jones and Hare2008; Galvan, Reference Galvan2010) as well as increases in the emergence of psychopathology (Kessler et al., Reference Kessler, Berglund, Demler, Jin, Merikangas and Walters2005a; Lewinsohn, Clarke, Seeley, & Rohde, Reference Lewinsohn, Clarke, Seeley and Rohde1994). Thus, identifying individuals who fall on either extreme of the RewP spectrum (i.e. enhanced or blunted) may be useful in prioritizing youth in greatest need of preventative efforts, thus supporting initiatives for transdiagnostic staging models.
Finally, the present study had some limitations that should be taken into consideration. Prior research has reported age-related changes in reward sensitivity (Albert, Chein, & Steinberg, Reference Albert, Chein and Steinberg2013; Ethridge et al., Reference Ethridge, Kujawa, Dirks, Arfer, Kessel, Klein and Weinberg2017). Firstly, we did not collect specific measures of task engagement and believability of the task. While our experimental paradigms were designed to be as engaging and believable as possible, it is possible that variations in participants’ task engagement and believability could have contributed to individual differences in reward responsiveness. Moreover, while our monetary and social tasks were matched on basic task properties, they failed to disentangle the intrinsically rewarding experience of being correct from obtaining positive feedback. Future research is needed using experimental paradigms that account for this (e.g. Nelson and Jarcho, Reference Nelson and Jarcho2021). Prior research has reported age-related changes in reward sensitivity (Albert et al., Reference Albert, Chein and Steinberg2013; Ethridge et al., Reference Ethridge, Kujawa, Dirks, Arfer, Kessel, Klein and Weinberg2017). Many of the participants were around 18 years old, and it is possible that children and younger adolescents might show different associations between the internalizing symptoms and the RewP. In addition, the sample of adolescents was entirely female. Existing research suggests that there are gender differences in neural reward processing of monetary (Kujawa et al., Reference Kujawa, Proudfit and Klein2014b) and social (Guyer, Choate, Pine, & Nelson, Reference Guyer, Choate, Pine and Nelson2012; Spreckelmeyer et al., Reference Spreckelmeyer, Krach, Kohls, Rademacher, Irmak, Konrad and Gründer2009) stimuli. Future work should extend this work by examining familial history of psychopathology subfactors and the RewP across genders. Lastly, our sample was primarily Caucasian, which limits the generalizability of the findings. Future research should include more diverse samples to further elucidate the relationship between the RewP and internalizing psychopathology across different racial and ethnic groups.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291723003720.
Funding statement
Support for this research was provided through National Institute of Mental Health grant R01MH097767 awarded to B.D.N. and G.H.
Competing interests
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.








