1 Introduction
22q11.2 deletion syndrome (22q11DS) is a common microdeletion syndrome with a prevalence of at least 1 to 4,000 live births, and is characterized by high rates of medical and psychiatric comorbidities as well as cognitive deficits Reference McDonald-McGinn, Sullivan, Marino, Philip, Swillen and Vorstman[1]. The 22q11DS is currently being considered the most commonly known genetic syndrome associated with schizophrenia, with psychotic illness occurring in about one-third of adults with 22q11DS Reference Schneider, Debbane, Bassett, Chow, Fung and van den Bree[2].
Previous studies have shown that some cognitive deficits including lower baseline full-scale IQ, decline in verbal IQ, and deficits in visual memory and executive functioning, predict the later onset of psychotic disorders [Reference Gothelf, Feinstein, Thompson, Gu, Penniman and Van Stone3–Reference Antshel, Shprintzen, Fremont, Higgins, Faraone and Kates5]. Thus, it seems that cognitive deficits are endophenotypes involved in the pathways leading to psychosis in 22q11DS.
While positive symptoms like delusions, hallucinations and disorganized speech have traditionally been the focus of schizophrenia research, negative symptoms are another core feature of the disorder, constituting risk factors for the evolution of schizophrenia [Reference Foussias and Remington6, Reference Lyne, Joober, Schmitz, Lepage and Malla7]. A large multisite research on clinical high-risk cohort, North American Prodromal Longitudinal Study (NAPLS), showed that early and persistent negative symptoms confer risk for later development of psychotic disorders in non-22q11DS individuals Reference Piskulic, Addington, Cadenhead, Cannon, Cornblatt and Heinssen[8]. Negative symptoms are often accompanied by cognitive deficits Reference Basso, Nasrallah, Olson and Bornstein[9] and social impairments Reference Lincoln, Mehl, Kesting and Rief[10].
Following the above-mentioned findings in non-22q11DS schizophrenia, greater focus is being given to the identification of negative, in addition to positive subthreshold symptoms in 22q11DS [Reference Tang, Yi, Moore, Calkins, Kohler and Whinna11–Reference Schneider, Van der Linden, Menghetti, Glaser, Debbané and Eliez13]. Also, similarly to idiopathic schizophrenia, in previous 22q11DS studies, negative symptoms were associated with neurocognitive deficits Reference Schneider, Van der Linden, Menghetti, Glaser, Debbané and Eliez[13] and executive dysfunction [Reference Schneider, Eliez, Birr, Menghetti, Debbané and Van der Linden14, Reference Maeder, Schneider, Bostelmann, Debbané, Glaser and Menghetti15]. Recent a large study showed a high prevalence of positive and negative SPS in 22q11DS, 33% and 28%, respectively, with highest rates manifested during adolescence and young adulthood Reference Weisman, Guri, Gur, McDonald-McGinn, Calkins and Tang[16].
To date, only a few studies investigated the association between cognitive deficits and subthreshold psychotic syndrome (SPS) in 22q11DS individuals. One study, found that both positive and negative SPS are more common in individuals with lower IQ scores Reference Weisman, Guri, Gur, McDonald-McGinn, Calkins and Tang[16]. Another study reported that lower verbal skills increase the likelihood of prodromal or overt psychotic symptoms Reference Kates, Russo, Wood, Antshel, Faraone and Fremont[17].
In a previous study, we compared, cross-sectionally, the rates of SPS among individuals with 22q11DS, typically developing (TD) controls and individuals with Williams syndrome (WS) Reference Mekori-Domachevsky, Guri, Yi, Weisman, Calkins and Tang[12]. We found that both 22q11DS and WS had similarly higher rates of positive subthreshold symptoms compared to TD. In addition, the 22q11DS had higher rates of negative SPS than WS and TD, despite having higher mean IQ scores than the WS group.
There are only a few published longitudinal studies that looked at the evolution of SPS in 22q11DS individuals [Reference Antshel, Fremont, Ramanathan and Kates18, Reference Schneider, Armando, Pontillo, Vicari, Debbané and Schultze-Lutter19]. In Schneider et al. Reference Schneider, Armando, Pontillo, Vicari, Debbané and Schultze-Lutter[19], 89 individuals with 22q11DS were evaluated twice in a 32 months interval. Transition rate to full-blown psychosis was 27% in those with ultra-high risk (UHR) condition.
In the current study, we wished to evaluate longitudinally the psychiatric and neurocognitive functioning of individuals with 22q11DS and to identify the neurocognitive deficits that predict the emergence of positive and negative SPS. We used a relatively short evaluation interval of 12-20 months to be able to detect immediate changes and response to treatment. In addition to TD controls, we also included the WS group in the current longitudinal follow-up study, since our previous studies suggested the importance of including a control group with another neurogenetic syndrome in the attempt to identify the specific phenotypical features of 22q11DS, that are beyond the nonspecific effect of having intellectual disability [Reference Mekori-Domachevsky, Guri, Yi, Weisman, Calkins and Tang12, Reference Zarchi, Diamond, Weinberger, Abbott, Carmel and Frisch20].
Specifically, we formulated the following main aims and hypotheses:
1. The 22q11DS group will show higher conversion rates to either psychotic disorders or more severe SPS over time, compared to WS; 2. General cognitive deficits as well as specific deficits in the executive and social cognition domains would be associated with the presence of negative SPS in 22q11DS, but not in WS; 3. In individuals with 22q11DS, both baseline global neurocognitive performance (GNP) scores and scores of neurocognitive subdomains of the Penn Computerized Neurocognitive Battery (CNB)will predict the presence and severity of negative but not positive SPS at follow-up. Similarly, GNP scores at baseline will predict the emergence and persistence of negative SPS. 4. Finally, we wished to investigate the potential effect of psychiatric medications on 22q11DS trajectories. We assumed that 22q11DS individuals treated with psychiatric medications will demonstrate more robust improvement in SPS and neurocognitive functioning relative to 22q11DS individuals not treated with psychiatric medications.
2 Methods and measures
2.1 Participants
In a previous publication Reference Mekori-Domachevsky, Guri, Yi, Weisman, Calkins and Tang[12] we described the recruitment procedure and baseline evaluation of 102 individuals- 22q11DS (n = 50), WS (n = 20) and TD (n = 32) controls at the Behavioral Neurogenetics Center, Tel Aviv. Of the 102 individuals, 93 (88.2%) returned for follow-up- 22q11DS (n = 44), WS (n = 19) and TD (n = 30) and were included in the final longitudinal analyses. The mean interval between the baseline and follow-up visits was 15.2 months (SD = 2.1, range 12-20 months) (Table 1). The study was approved by the Institutional Review Board of Sheba Medical Center. After providing a complete description of the nature of the study, informed consent was obtained from all participants and from the parents of minors. Additional details on the participants’ characteristics and their recruitment are listed in Table 1 and Appendix A in Supplementary material.
2.2 Measures
2.2.1 Psychiatric evaluation
All individuals with 22q11DS and WS and their main caregivers were interviewed by a trained psychologist using the Hebrew version with the Structured Interview for Prodromal Symptoms (SIPS) version 4 Reference Miller, McGlashan, Rosen, Cadenhead, Ventura and McFarlane[21]. The Scale of Prodromal Symptoms (SOPS) is composed of 19 items, each representing a different possible SPS, divided into four groups: positive, negative, disorganized and general symptoms. Each item on the SIPS was rated on a seven-point scale (0 - absent, 1- questionably present, 2 - mild, 3 - moderate, 4 - moderately severe, 5 - severe but not psychotic, 6 - severe and psychotic/extreme) Reference McGlashan, Walsh and Woods[22]. A participant was considered both at baseline and at follow-up assessments to have “positive SPS” when at least one positive symptom was rated at a level of ≥3, and with “negative SPS” when at least two negative or disorganized symptoms were rated at a level of ≥3 Reference Tang, Yi, Moore, Calkins, Kohler and Whinna[11].
Table 1 Demographics, intelligence and adaptive functioning of the study groups at baseline.
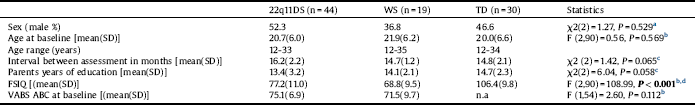
22q11DS: 22q11.2 deletion syndrome; ABC: Adaptive behavioral composite; FSIQ: Full-scale IQ; n.a: Not available; Paternal years of education: Average parents number of years in a formal educational setting; TD: Typically developing; VABS: Vineland Adaptive Behavior Scale; WS: Williams syndrome.
a Pearson chi square test.
b Analysis of variance.
c Kruskal–Wallis test.
d On post-hoc tests WS<22q11DS <TD.
Data on UHR non-22q11DS individuals have shown that those with persistence and worsening of subthreshold psychotic symptoms are at high risk to convert to psychosis Reference Dominguez, Wichers, Lieb, Wittchen and van Os[23]. Based on these findings and because of our limitation of a small sample size, we divided our 22q11DS sample into two groups; and applied these criteria to both the positive and negative subthreshold symptoms.
One group was ‘persistent/emergent' (n = 29 for negative SPS, n = 17 for positive SPS) meeting subthreshold psychosis symptoms at both time points (persistent), or not meeting subthreshold symptoms criteria at baseline but subsequently meeting subthreshold psychosis symptoms criteria or converted to psychosis at follow-up (emergent) Reference Tang, Moore, Calkins, Yi, McDonald-McGinn and Zackai[24]. The other group was ‘low risk ' (n = 15 for negative SPS, n = 27 for positive SPS), not meeting subthreshold psychosis symptoms criteria at either time point or did so at baseline but not at follow-up.
All 22q11DS and WS individuals underwent semi-structured psychiatric evaluation using the DSM-5 version of the schedule for affective disorders and schizophrenia for school-age children (K-SADS), or the Structured Clinical Interview for Axis I (SCID) as previously described Reference Gothelf, Schneider, Green, Debbane, Frisch and Glaser[25].
2.2.2 Cognitive and adaptive functioning evaluations
All participants completed IQ assessment using the age-appropriate version of the Wechsler test as previously described Reference Gothelf, Schneider, Green, Debbane, Frisch and Glaser[25] and completed the CNB. Adaptive functioning of the participant was assessed by Vineland Adaptive Behavior Scale version 2 (VABS). For more details on the CNB and VABS, see Appendix A in Supplementary material.
2.2.3 Assessment of psychiatric medications' history
We inquired about the status of psychiatric medications of all 22q11DS and WS individuals both at baseline and at follow-up, and also collected the data from their electronic medical records. The psychiatric medications screened included antipsychotics, antidepressants, anxiolytics, mood stabilizers, and stimulants.
Each individual was rated as ‘positive' for psychiatric medication treatment, if either at baseline or at follow-up, he or she were on any of the above-mentioned psychiatric medications.
2.3 Data analysis
Data were analyzed using SPSS software system for Windows Version 20.0. and SAS System for Windows. Categorical variables were compared by Pearson χ2 tests for proportions' analysis. Continuous variables were tested for normality using the Shapiro-Wilk test. Neurocognitive data were compared using analysis of variance (ANOVA), and the intervals between assessments in months and parents years of education were compared with the nonparametric Kruskal–Wallis test.
For between-groups comparisons, change in frequency of positive and negative subthreshold symptoms from baseline to follow-up, two logistic regressions, one for positive and one for negative SPS, were conducted with group (22q11DS vs. WS) by SPS status (present or absent) at baseline as predictors and SPS status at follow-up as the outcome variable.
We calculated the positive and negative SOPS scores by summing up the five positive items and the six negative items of the SOPS, respectively. Since the SOPS scores were skewed, we compared SOPS positive and negative total scores among groups at baseline and at follow-up using the nonparametric Kruskal–Wallis test for comparing three groups, and the Mann–Whitney U test for comparing two groups. The Wilcoxon signed-rank test was used to detect changes between baseline and follow-up SOPS scores.
For the adjusted probability of the presence of SPS in 22q11DS and WS after controlling for the CNB neurocognitive domain scores, a one-way analysis of covariance (ANCOVA) was performed, using the SAS's PROC GLIMMIX procedure with a binomial distribution.
The summary of the cross-sectional and longitudinal comparison of CNB domain scores among groups is presented in Supplementary Table 1. The ANOVA simple effects test estimation of combined baseline and follow-up CNB scores.
Two logistic regressions were conducted to assess whether baseline GNP predicts the presence of negative and positive SPS at follow-up. We also calculated positive and negative SOPS scores by summing up the five positive items and the six negative. We conducted two linear regressions to determine whether baseline GNP predicts positive and negative total SOPS scores at follow-up. Additional multivariate logistic and linear regressions were performed to assess if the association between GNP scores at baseline and the presence and severity of negative SPS are driven by any of the five CNB subdomains. Furthermore, two logistic regressions were conducted to clarify whether or not baseline GNP scores predict the persistence/emergence of negative and positive subthreshold symptoms.
To analyze the potential effect of psychiatric medication treatments on individuals with 22q11D, ANOVA-RMs was applied using medication as the between-subjects factor, and GNP score as well as the mean total positive or negative SOPS scores at baseline and follow-up as the within-subject factors.
3 Results
3.1 Conversion to psychotic disorders and longitudinal change in rates of subthreshold psychotic symptoms
The baseline comparison among 22q11DS, WS and TD was previously described Reference Mekori-Domachevsky, Guri, Yi, Weisman, Calkins and Tang[12]. Two individuals with 22q11DS (4.5%), and none from the WS and TD groups, converted to psychotic disorders between baseline and follow-up evaluations. Case studies information is presented in Appendix B in Supplementary material.
There was a significant effect of group (22q11DS vs. WS) by positive SPS status at baseline (present or absent) on positive SPS status at follow-up (P = 0.009) with 22q11DS decreasing from 43% at baseline to 16% at follow-up while WS increasing from 32% to 53%. There was no significant effect of group by negative SPS status at baseline on negative SPS status at follow-up (Table 2).
We then analyzed the SOPS scores as continuous variables, summing the total positive and negative symptom scores. In line with the categorical analyses, we found similar but nonsignificant improvement in mean positive SOPS scores (Z = 1.43, P = 0.053) from baseline to follow-up in 22q11DS, and a nonsignificant change in positive SOPS scores (Z = 0.45, P = 0.565) from baseline to follow-up in WS, There was no significant change in mean negative SOPS scores from baseline to follow-up (Table 2).
A summary of the cross-sectional and longitudinal comparisons of CNB domain scores among groups is presented in Appendix B and Supplement Table 1 in Supplementary material.
3.2 Association between neurocognitive functions and subthreshold psychotic symptoms in individuals with 22q11DS and WS
One-way ANCOVA was conducted to compare the probability of the presence of negative prodromal symptoms between 22q11DS and WS after controlling for baseline neurocognitive domain scores. There was a significant group effect on the presence of baseline negative SPS after controlling for GNP, executive function and social cognition scores (Table 3). The adjusted probabilities for these domains were significantly higher for 22q11DS than for WS individuals, indicating a stronger association between these neurocognitive domains and negative symptoms in 22q11DS compared to WS.
Table 2 Longitudinal within and between group comparisons of rates of subthreshold psychotic syndromes, severity of subthreshold psychotic symptom scores.

22q11DS: 22q11.2 deletion syndrome; SOPS: Scale of prodromal symptoms; SPS: Subthreshold psychotic symptoms; T1: Baseline evaluation; T2: Follow-up evaluation; TD: Typically developing; WS: Williams syndrome.
a Pearson chi square test.
b Logistic regression with group (22q11DS vs. WS) by SPS status (present or absent) at baseline as predictors and SPS status at follow-up as the outcome variable.
c Ideational richness was excluded from the negative symptoms between groups baseline and longitudinal analyses since it is known to be highly affected by the cognitive level.
d Kruskal-Wallis test for three-group comparisons followed by Mann-Whitney for two-group post hoc comparisons.
e Wilcoxon signed-rank test to compare change from baseline to follow-up scores in 22q11DS and in WS.
3.3 Effect of neurocognitive deficits at baseline on subthreshold psychotic symptoms at follow-up in individuals with 22q11DS
Logistic regression revealed that baseline GNP scores were significantly associated with the presence of negative SPS at follow-up (B = −2.36, SE = 0.93, P = 0.011), accounting for 24% of the variance in negative SPS. GNP did not predict the presence of positive SPS at follow-up (B = −0.63, SE = 0.86, P = 0.467). The linear regression analyses of SOPS scores were in line with the SOPS categorical analyses. Baseline GNP scores predicted total negative SOPS scores [F(1, 43) = 4.34, P = 0.043], accounting for 9.4% of the variance, but not total positive SOPS scores [F(1, 43) = 2.36, P = 0.132].
To determine whether the association between baseline GNP scores and the presence of negative SPS is driven by any of its components, we repeated the regressions entering the five subdomains composing the GNP at baseline as potential predictors of follow-up negative SPS or total negative SOPS scores. None of the five subdomains were significantly associated with the presence of negative SPS (B = 0.49, SE = 0.34, P = 0.143) or with total negative SOPS scores [F(5, 36) = 0.31, P = 0.903].
Table 3 Adjusted probability of the presence of baseline negative subthreshold psychotic symptoms of 22q11.2 deletion syndrome and Williams syndrome.
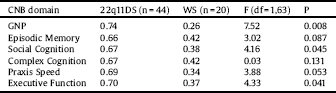
22q11DS: 22q11.2 deletion syndrome; CNB: Computerized neurocognitive battery; GNP: Global neurocognitive performance; WS: Williams syndrome.
In addition, GNP at baseline significantly predicted the persistence/emergence of negative SPS (n = 29) at follow-up (B = −2.76, SE = 1.03, P = 0.007), accounting for 29.7% of the variance of negative SPS. In line with the other analyses, GNP scores at baseline did not predict the persistence/emergence of positive SPS (n = 17), (B = −0.48, SE = 0.66, P = 0.471).
3.4 Effect of pharmacotherapy on change in severity of subthreshold symptoms and cognitive deficits in individuals with 22q11DS
Twenty of the 44 individuals with 22q11DS (45%) were on psychiatric medications (some were on more than one medication). The psychotropic medication status of individuals with 22q11DS or WS is presented in Appendix B in Supplementary material. Table 4 shows the results of the comparison between 22q11DS individuals who were and were not treated with psychiatric medications at baseline and at follow-up. The groups did not differ in age, sex distribution, FSIQ or baseline GNP scores.
To learn about the difference in change in SOPS or GNP scores between 22q11DS treated with psychiatric medications and those not treated with psychiatric medications we conducted two ANOVA-RMs, with baseline and follow-up positive, negative SOPS mean total scores or GNP as within-subject factors and psychiatric medication treatment (yes or no) as a between-subjects factor. There was a significant effect of psychiatric medication status by time on GNP efficiency score [F (1,42) = 5.86, P = 0.020]. GNP of individuals with 22q11DS treated with psychiatric medication improved (from baseline GNP −0.99 ± 0.53 to follow-up GNP −0.77 ± 0.42) whereas GNP scores of 22q11DS no treated with psychiatric medication worsened (from baseline −0.89 ±0.40 to follow-up −1.05 ± 0.72). We found no significant effect on cognitive change for any of the specific group of psychiatric treatments (antipsychotics, stimulants, and antidepressants; see Appendix B in Supplementary material). There was no significant time effect of psychiatric medication status (yes or no) on mean positive SOPS scores [F(1,42) = 3.28, P = 0.077] or mean negative SOPS scores [F(1,42) = 2.59, P = 0.115].
Table 4 Comparison between individuals with 22q11.2 deletion syndrome treated vs. not treated with psychiatric medications.
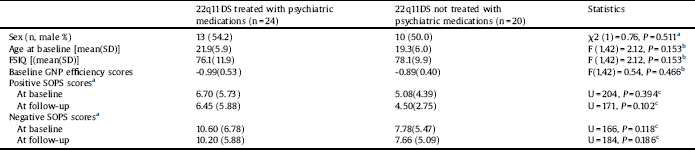
22q11DS: 22q11.2 deletion syndrome; FSIQ: Full-scale IQ; GNP: Global neurocognitive performance; SOPS: Scale of prodromal symptoms.
a Pearson chi square test.
b Analysis of variance.
c Mann–Whitney U test.
4 Discussion
In this longitudinal study, we investigated the trajectories of SPS and neurocognitive functioning in individuals with 22q11DS in comparison to TD as well as WS controls. There was no overall increase in the severity of subthreshold symptoms from baseline to follow-up, but a decrease in positive SPS in the 22q11DS group was detected. Yet, we found that two individuals with 22q11DS and SPS converted to psychosis, whereas none of the TD and WS controls converted to psychosis. The association between neurocognitive deficits and negative SPS was significantly more robust in 22q11DS compared to WS. Baseline GNP scores predicted the presence, persistence and emergence of negative SPS at follow-up in individuals with 22q11DS. Finally, we found that 22q11DS individuals maintained on psychiatric medications displayed significant improvement in overall cognitive functioning compared to unmedicated 22q11DS individuals.
4.1 Conversion to psychosis and subthreshold psychotic symptoms longitudinal trajectory in 22q11DS
In our sample, 4.5% of 22q11DS individuals developed psychotic disorders at the time interval between baseline to follow-up, i.e., 3.8% per year. In previous longitudinal 22q11DS studies of different cohorts, looking at age range similar to our cohort, the mean reported conversion rates to psychosis were 2.4%- 6.7% per year [Reference Schneider, Armando, Pontillo, Vicari, Debbané and Schultze-Lutter19, Reference Gothelf, Schneider, Green, Debbane, Frisch and Glaser25–Reference Tang, Moore, Calkins, Yi, McDonald-McGinn and Zackai27].
Of note, out of a sample of 44, the two 22q11DS individuals who converted to psychotic disorders in our cohort had both positive and negative subthreshold symptoms at baseline, and the conversion rates in our cohort among those with positive SPS at baseline was 8.8% per year. In two recent studies, the conversion rates in 22q11DS individuals with positive SPS were 10.1% Reference Schneider, Armando, Pontillo, Vicari, Debbané and Schultze-Lutter[19] and 15.3% Reference Tang, Moore, Calkins, Yi, McDonald-McGinn and Zackai[27]. The conversion rates per year of individuals with 22q11DS with positive SPS, are in the lower range of the rates reported in clinical UHR youth studies, in which conversion rates varied and ranged from 6.1% to 38.6% per year [Reference Addington, Liu, Buchy, Cadenhead, Cannon and Cornblatt28, Reference Ruhrmann, Schultze-Lutter and Klosterkotter29].
It has been demonstrated that the administration of psychotropic medications, e.g., antipsychotics and anti-depressants/anti-anxiety medications, to UHR individuals, postpone the onset of psychotic disorders and lower the annual conversion rates to psychosis Reference Fusar-Poli, Bonoldi, Yung, Borgwardt, Kempton and Valmaggia[30]. Similarly, the somewhat lower rates of conversion to psychosis in our subthreshold cohort as well as Schneider et al's cohort Reference Schneider, Armando, Pontillo, Vicari, Debbané and Schultze-Lutter[19] could be related to the high rate of medication that reached 45% of 22q11DS individuals in our sample.
Although conversion to psychosis occurred in 8.8% of our 22q11DS individuals with SPS, in a substantial proportion of the patients with 22q11DS there was an improvement in SPS from baseline to follow-up, especially for positive SPS. Similar trajectory of SPS exists in non-22q11DS UHR population. For example, in the NAPLS study there was an overall mean improvement in SPS from first to second evaluation and then an escalation in SPS from second to third evaluation Reference Piskulic, Addington, Cadenhead, Cannon, Cornblatt and Heinssen[8], highlighting the need to further assess SPS in 22q11DS longitudinal cohorts.
4.2 The association between 22q11DS cognitive deficits and negative subthreshold psychotic symptoms
In the present study, we further demonstrate the clinical importance of negative SPS in 22q11DS by showing that there is a strong and specific association between negative SPS and cognition in individuals with 22q11DS. We found that general cognitive deficits predict the persistence and emergence of negative SPS but not positive SPS in 22q11DS individuals at the 15-month follow-up, and is more strongly associated with the presence of negative SPS in 22q11DS compared to individuals with WS. These findings are in line with previous studies on non-22q11DS UHR, TD and help-seeking individuals, showing that negative SPS are often associated with poor neurocognitive functioning and predict conversion to psychosis [Reference Fusar-Poli, Borgwardt, Bechdolf, Addington, Riecher-Rossler and Schultze-Lutter31–Reference Seidman, Giuliano, Meyer, Addington, Cadenhead and Cannon34].
4.3 Longitudinal change in cognitive deficits and SOPS scores in 22q11DS individuals with and without psychiatric medications
To our knowledge, there are no publications on the longitudinal effect of psychiatric medications on the progression of SPS in 22q11DS. In the current study, we did not find any effect of psychiatric medications on the progression of SPS in individuals with 22q11DS. However, we found that 22q11DS individuals on psychiatric medications exhibited an improvement from baseline to follow-up in global cognitive functioning, the GNP measure. Our findings are in line with a recent paper Reference Kates, Olszewski, Gnirke, Kikinis, Nelson and Antshel[35] that showed that treatment with psychiatric medications, such as antipsychotics and mood stabilizers, may have a reparative effect on white matter in prodromal 22q11DS individuals, independent of the potential effect on psychosis.
Importantly, the two 22q11DS individuals in our study group that converted to psychotic disorders were treated with methylphenidate. Methylphenidate and other stimulants could potentially induce psychotic symptoms. In a previous study, none of the 15 children and adolescents treated with methylphenidate had developed psychosis in a 6 months follow-up Reference Green, Weinberger, Diamond, Berant, Hirschfeld and Frisch[36]. To our knowledge, there are no long-term studies on the safety of stimulants in 22q11DS. Such studies aimed to investigating whether or not stimulant treatment increases or decreases the risk of 22q11DS individuals to develop psychosis.
4.4 Limitations
Study limitations include the sample size that was too small to accurately assess rates of conversion to psychosis and to identify additional interactions that may exist between other potential risk factors for the progression of subthreshold symptoms and conversion to psychotic disorders, such as age, sex, comorbid psychiatric disorders, specific neurocognitive deficits and specific psychiatric treatments. Of note, we can not conclude that psychiatric medications affect the cognitive developmental trajectories of 22q11DS individuals because the participants were not randomized into the groups (e.g., self-selecting who was receiving medication). In addition, it is possible that the medicated group had more acute psychiatric symptoms, which could potentially attract psychiatric attention for treatment earlier or possibly those who received medication had greater mental health awareness, more resources, or greater levels of support, all of which would support the argument that they would do better psychiatrically.
4.5 Conclusions
In conclusion, the present study is among the first to evaluate longitudinally the developmental trajectory of SPS in 22q11DS compared to another neurogenetic syndrome, WS, and to assess the interactions among, neurocognition, psychiatric treatments, and SPS trajectories. Our study, as well as the other longitudinal study Reference Schneider, Armando, Pontillo, Vicari, Debbané and Schultze-Lutter[19] showed that the presence of SPS increases the annual conversion rates to ∼10%. Our study, consistent with accumulating evidence [Reference Schneider, Van der Linden, Menghetti, Glaser, Debbane and Eliez37, Reference Jalbrzikowski, Carter, Senturk, Chow, Hopkins and Green38], showed the important association between cognitive deficits and negative SPS in 22q11DS. The effects of pharmacotherapy should be included as potential covariates in 22q11DS longitudinal analyses also in cognitive studies.
Future large-scale ongoing studies that combine several large cohorts of 22q11DS will be able to capture the full scope of the complex interaction of genes, cognition and psychiatric trajectories on the evolution of psychosis in individuals with 22q11DS.
Role of the funding source
There was no involvement of the funding sources in study design, in the collection, analysis, and interpretation of data, nor in the writing of the report and the decision to submit the paper for publication. Prof. Doron Gothelf was supported by the Binational Science Foundation, grant number 2011378; the National Institute of Mental Health of the National Institutes of Health under Award Number U01MH101722. Dr. Omri Weisman is supported by a fellowship from the Sagol School of Neuroscience, Tel Aviv University.
Disclosure of interest
All authors declare that have no competing interest.
Acknowledgements
The authors are grateful to participants and their families. This work was performed in partial fulfillment of the requirements for a Ph.D. degree by Ronnie Weinberger at the Sackler Faculty of Medicine, Aviv University, Israel.
Appendix A Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.eurpsy.2017.10.010.







Comments
No Comments have been published for this article.