Some pregnant women develop gestational diabetes mellitus (GDM) when their pancreatic function is not able to overcome the insulin resistance produced by the pregnant state. The prevalence of GDM ranges from 1 to 25 % depending on patient demographics and screening practices( Reference Moyer 1 ). GDM is increasing worldwide probably because of the increases in mean maternal age and weight( Reference Dabelea, Snell-Bergeon and Hartsfield 2 , Reference Kim, Saraiva and Curtis 3 ). It grows in parallel with the prevalence of obesity and type 2 diabetes in the general population. In both chronic conditions, dietary habits and lifestyles play a crucial role as major determinants of risk. During the past years, international associations have attempted to differentiate between pre-existing diabetes that is first diagnosed during pregnancy and the transient pregnancy-related disorder in glycaemic metabolism, which is only secondary to pregnancy-related insulin resistance( 4 , Reference Hod, Kapur and Sacks 5 ). These organisations acknowledge the actual epidemic of underdiagnosed type 2 diabetes and glycaemic metabolic disorders in women of reproductive age when they get pregnant.
Well-documented evidence has demonstrated the impact of dietary habits in the risk of type 2 diabetes and obesity. The current state of the art in nutritional epidemiology is to rely on the assessment of overall dietary patterns instead of merely assessing isolated nutrients or foods. A seemingly important risk factor for GDM is a Western-style dietary pattern. On the contrary, following a high-quality dietary pattern such as the traditional Mediterranean diet can be useful for the prevention of GDM( Reference Guasch-Ferré, Becerra-Tomás and Ruiz-Canela 6 , Reference Schulze, Fung and Manson 7 ). However, few studies have been performed on the role of overall dietary patterns and the risk of GDM. There is limited evidence (none from randomised trials) that an overall dietary pattern rich in fruit, vegetables, whole grains and fish but low in red and processed meat, refined grains and high-fat dairy products (similar to the Mediterranean diet) may reduce the risk of developing GDM and that a dietary pattern with a high consumption of processed meats, cholesterol and fat (similar to the Western-style dietary pattern) may increase the risk of GDM( Reference Schoenaker, Mishra and Callaway 8 ).
Dietary patterns can be assessed through two major different approaches: the a priori approach calculates a score according to the compliance with the current dietary guidelines and the hypothesis being studied. Although the same a priori scores can be used in different populations, they may not be suitable for some of them. The a posteriori (post hoc) approach consists in data-driven empirical combinations of foods and nutrients using multiple statistical methods (e.g. factor analysis) to explain a considerable part of the total variability in dietary habits( Reference Martínez-González, García-López and Bes-Rastrollo 9 , Reference Kant, Leitzmann and Park 10 ). The limitation of this approach is that it might involve some degree of subjectivity of the dietary patterns identified and that it does not necessarily reflect the typical dietary patterns (e.g. Mediterranean dietary pattern (MDP)). Besides, the statistical method used in a posteriori approach has some inherent methodological limitations (e.g. the establishment of predefined food groups). On the other hand, this approach has the advantage of being a dietary pattern obtained from the study population, increasing the internal validity.
To our knowledge, the available evidence for the association between dietary patterns and GDM risk has been mainly obtained using a priori scores( Reference He, Yuan and Chen 11 – Reference Zhang, Tobias and Chavarro 16 ) and there is still lack of evidence on the associations between the adherences to empirically derived dietary patterns and GDM incidence. Furthermore, to the best of our knowledge, there is not an a priori score available to assess the Western dietary pattern (WDP).
Thus, the aim of this study was to investigate the association between the adherence to empirically derived dietary patterns and GDM risk in a cohort of university graduates in Spain (the Seguimiento Universidad de Navarra (SUN) Project), using the a posteriori approach and to assess the association of healthy lifestyles with the prevention of GDM defining an overall healthy score including both diet and lifestyle.
Methods
Study population
The SUN project is a multipurpose, prospective and dynamic Spanish cohort of university graduates designed to study associations between several socio-demographic, nutrition and lifestyle characteristics and the incidence of multiple chronic diseases. Beginning in 1999, it is constantly open. In brief, a mailed questionnaire regarding dietary habits, lifestyles and health conditions was used to invite Spanish graduates to participate in the study. After the baseline evaluation, which was considered to imply informed consent, participants receive a follow-up questionnaire every 2 years through mailed or Web-based questionnaires. The study protocol was supported by the Institutional Review Board of the University of Navarra. The design and methods used in the SUN project have been formerly described in detail elsewhere( Reference Martinez-Gonzalez, Sanchez-Villegas and De Irala 17 , Reference Segui-Gomez, de la Fuente and Vazquez 18 ).
For the present analysis, we used the latest available database from the 1 December 2015 (13 777 women). We included 13 233 women who had responded to the first questionnaire before the 1 March 2013 to make sure that they would have been in the cohort for a sufficient time as to be able to complete the first follow-up questionnaire (2 years to have received the first follow-up questionnaire and nine additional months to account for late responses). Up to that date, 3555 pregnant women were identified. Women were excluded from the analyses if they had been previously diagnosed of diabetes (n 30) or reported extremely low (below percentile 1) or high (above percentile 99) values for total energy intake before pregnancy (n 70). The total energy intake was calculated using the semiquantitative FFQ with the last available information for nutrients included in food composition tables for Spain (see the ‘Dietary exposure assessment’ section). We excluded low and high values in order to assure the correct fulfilment of the FFQ by the participants. The final available population included 3455 pregnant women.
Dietary exposure assessment
Dietary intake was assessed using a semiquantitative FFQ with 136 food items previously validated and described in detail( Reference Martinez-Gonzalez, de la Fuente-Arrillaga and Nunez-Cordoba 19 , Reference Martin-Moreno, Boyle and Gorgojo 20 ). The validity( Reference Fernandez-Ballart, Pinol and Zazpe 21 ) and reproducibility( Reference De la Fuente-Arrillaga, Ruiz and Bes-Rastrollo 22 ) of this questionnaire have recently been re-evaluated. Each item was composed by a typical portion size and consumption frequencies were ranked in nine categories from ‘never or almost never’ to ‘≥6 times/d’ (the used FFQ are available at http://www.unav.edu/departamento/preventiva/infoinvsun). Then, daily food consumption was calculated by multiplying the portion size of each food item by its consumption frequency. Specific nutrient intake was evaluated by a trained dietitian updating nutrient data bank with the last available information included in food composition tables for Spain( Reference Moreiras, Carbajal and Cabrera 23 , Reference MataixVerdu 24 ). Nutrient scores were computed through a computer software designed for this objective.
Assessment of other variables
First/baseline questionnaire also gathered information on several characteristics: socio-demographic covariates (e.g. age, sex, marital status, academic degree and employment), anthropometric measurements (e.g. weight, height), lifestyle and health-related habits (e.g. physical activity, alcohol consumption, smoking status), family history of major diseases (e.g. diabetes) and clinical covariates (e.g. obstetric history, prevalence of chronic diseases, medication use). Self-reported anthropometric measurements have demonstrated a high validity in a subsample of this cohort( Reference Bes-Rastrollo, Perez Valdivieso and Sanchez-Villegas 25 ). Physical activity was expressed in metabolic equivalent tasks (MET) per week, calculated from the time spent on each of the activities multiplied by a multiple of the RMR (MET) score according to published guidelines( Reference Ainsworth, Haskell and Whitt 26 ). Furthermore, an adequate correlation between information from questionnaires and objective measurements have been shown in a subsample of this cohort (Spearman’s coefficient of 0·51 (P=0·002))( Reference Martinez-Gonzalez, Lopez-Fontana and Varo 27 ).
Gestational diabetes mellitus assessment
The outcome of interest was GDM incidence. Pregnant women who reported a new diagnosis of GDM in the biennial questionnaire were considered possible incident cases. An additional specific questionnaire was sent to these participants, requesting their medical reports. Afterwards, a panel of medical doctors (blinded to baseline characteristics of women) confirmed each case. For these analyses, we only worked with confirmed cases (n 173).
Assessment of dietary patterns
The 136 food items included in the FFQ were classified in twenty-six predefined food groups. The grouping criteria were based on the similarity of nutrient profiles or the usual culinary use for the different foods. In order to identify major dietary patterns of participants, we applied a principal component analysis to these groups to identify the fewest number of factors that could explain the highest proportion of the variance from the original groups( Reference Utts 28 ). To determine the number of factors to retain, we used the Scree test, eigenvalues >1 and the interpretability of the obtained factors. According to current recommendations in Nutritional epidemiology( Reference Willet 29 ), food groups with absolute factor loading ≥0·30 were considered relevant components of the dietary patterns. Food groups with absolute loadings <0·30 were excluded from the final model (legumes, shortbread and butter, vegetable margarine, biscuits, juices, jam, honey and alcohol).
In accordance with the factor loadings of the food groups, we denominated the first and the second factors a WDP and a MDP, respectively. Then, a score was calculated for each participant summing the standardised consumption of each food group weighted by the coefficient of each factor score and the resulting quantitative score was grouped into quartiles.
Definition of the low-risk score for gestational diabetes mellitus
Taking into account the most important risk factors for gestational diabetes( Reference Solomon, Willett and Carey 30 , Reference Zhang and Ning 31 ), having a BMI<25 kg/m2, age≤28 years and a low adherence to an unhealthy dietary pattern (lowest quartile for the score on the adherence to WDP) were considered low-risk factors. We created a binary variable for each of the three factors, obtaining 1 if the participants met the criteria for the low-risk factor and 0 if they did not meet the criteria.
Statistical analysis
We used logistic regression models to assess the association between quartiles of adherence to both the Western and the MDP and the risk of GDM, using the lowest quartile as the reference category. We estimated age-adjusted and multivariable-adjusted OR and 95 % CI. Potential confounders included in the multivariable model were age (continuous), baseline BMI (continuous), family history of diabetes (yes/no), smoking status (never/former/current), physical activity (continuous), previous number of pregnancies (0/1/2/≥3) and any previous multiple pregnancy (yes/no).
Subsequently, we defined the three new variables (BMI<25 kg/m2, age≤28 years and lowest quartile for the score on the adherence to WDP) with two categories (yes or no) each to include them in the low-risk score and we calculated their OR and their 95 % CI, adjusting each for the same potential confounders described before (except variables BMI<25 kg/m2 and age≤28 years that were not adjusted for BMI and age, respectively). Then, we defined a low-risk score for GDM variable summing these three new variables (ranging from 0 to 3 points) and calculated the adjusted OR and 95 % CI for the three upper scores (1, 2, 3) taking as the reference category those women with 0 points.
We used STATA version 12.0 (StataCorp LP) for all the analyses. P values were two-sided and statistical significance was set at the conventional cut-off point of P<0·05.
Results
Characteristics of the 3455 analysed pregnant women according to their adherence to the low-risk Score for GDM risk (0–3) are shown in Table 1. More than the half of the participants were adhered to two low-risk factors at least. Women who obtained a higher score (2 or 3) were on average younger, nulliparous, had a lower frequency of family history of diabetes, had lower BMI, were more physically active, consumed less fast food and snacks and they also had on average lower total energy intake.
Table 1 Characteristics of 3455 pregnant women in the Seguimiento Universidad de Navarra cohort according to low-risk ScoreFootnote * for gestational diabetes mellitus (GDM) risk (Mean values and standard deviations; absolute numbers and percentages)
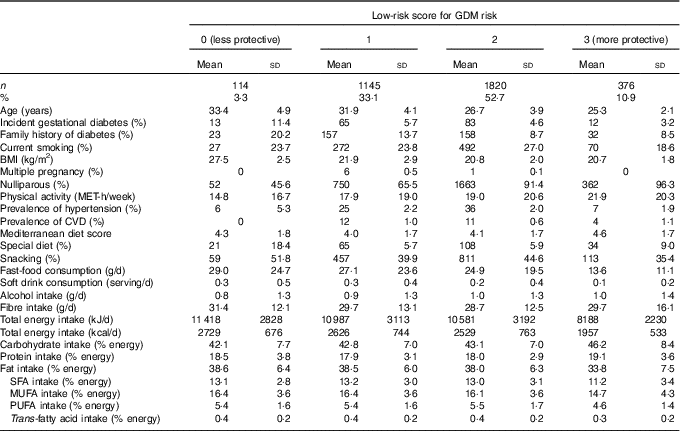
MET, metabolic equivalent tasks.
* Low-risk score: BMI<25 kg/m2, age≤28 years and lowest quartile for the score on the adherence to Western dietary pattern.
The two major dietary patterns (WDP and MDP) identified in the factor analysis procedure explained together 16·6 % of the total variance in the consumption of the predefined 26 food groups. Absolute factor loadings ≥0·30 for each dietary pattern are presented in Table 2. The WDP was characterised by a high consumption of red meat, high-fat processed meats, potatoes, commercial bakery products, whole dairy products, fast foods, sauces, pre-cooked foods, eggs, soft drinks and sweets and chocolates. In contrast, the MDP was characterised by a high consumption of vegetables, fruits, fish, whole grain bread, low-fat dairy products, nuts, olive oil and poultry.
Table 2 Factor loadings for the two major dietary patterns in the Seguimiento Universidad de Navarra Project from Principal Component Analysis (PCA)Footnote *
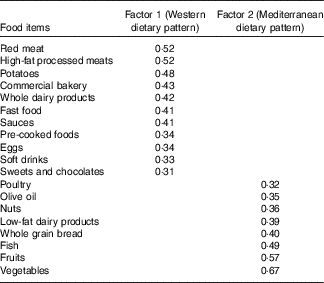
* The first factor (Western dietary pattern) explains 9·0 % of the total variance, and the second factor (Mediterranean dietary pattern) explains 7·6 % of the total variance.
During a mean follow-up of 10·3 (sd 3·3) years, we identified 173 incident cases of GDM.
OR and 95 % CI for GDM risk according to quartiles of adherence to both dietary patterns are shown in Table 3. On one hand, a direct borderline significant association was found in the multivariable model between the highest quartile of adherence to WDP and GDM incidence using as the reference the lowest quartile of adherence to WDP (adjusted OR 1·56; 95 % CI 1·00, 2·43; P=0·05). On the other hand, no association was found between quartiles of adherence to the MDP and GDM incidence (adjusted OR 1·08; 95 % CI 0·68, 1·70 for the highest quartile of adherence compared with the lowest quartile) after adjusting for age, baseline BMI and other potential confounders.
Table 3 Gestational diabetes mellitus (GDM) risk according to quartiles of adherence to the Western dietary pattern and Mediterranean dietary pattern in the Seguimiento Universidad de Navarra project (Odds ratios and 95 % confidence intervals)
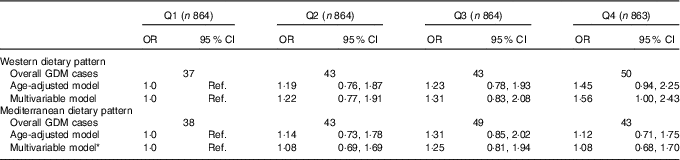
Ref., reference category.
* Adjusted for age, baseline BMI, family history of diabetes, smoking status, physical activity, number of pregnancies before and multiple pregnancies.
When we categorised ‘low risk’ lifestyle factors, a healthy body weight (BMI<25 kg/m2), young age (≤28 years) and low adherence to an unhealthy dietary pattern (lowest quartile for the score on the adherence to WDP), we observed that all of them were significantly (albeit low adherence to an unhealthy dietary pattern was only in the threshold of significance) and independently associated with a lower risk of GDM (Table 4). Then, we gave one point to women for each low-risk lifestyle factor, and we observed that an increasing number of low-risk factors was monotonically inversely associated with GDM risk (Table 4). Women who adhered to all three low-risk factors had a 76 % lower risk of GDM (OR 0·24; 95 % CI 0·10, 0·55) compared with women who did not adhere to any factor before pregnancy.
Table 4 Combined score of gestational diabetes mellitus (GDM) risk according to low-risk factors (Numbers and percentages of cases; odds ratios and 95 % confidence intervals)
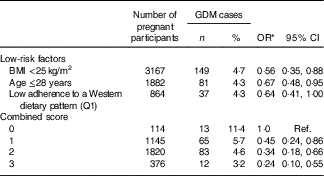
Q, quartile; Ref., reference category.
* Adjusted for family history of diabetes, smoking status, physical activity, number of pregnancies before and multiple pregnancies, and additionally for the other two low-risk factors in the analysis of independent risk factors.
Discussion
In this large cohort of Spanish university graduates pregnant women, two major dietary patterns were identified by using a principal component analysis: the WDP and the MDP. Together, they explained 16·6 % of the total variability in dietary intake. Although this variability may be considered low, it is similar to those found in other studies( Reference Bédard, Garcia-Aymerich and Sanchez 32 ).
A highest adherence to the WDP, characterised mainly by a high consumption of red meat, high-fat processed meats, potatoes, commercial bakery, whole dairy products, fast food, sauces, pre-cooked foods, eggs, soft drinks and sweets and chocolates, was associated with a 56 % higher relative risk of GDM. In contrast, greater adherence to the MDP did not present any association with GDM.
The finding of an outstanding Western-style dietary pattern in this Mediterranean cohort is surprising. These results show an alarming departure in Spain from the traditional Mediterranean diet, especially among young people, who seem to adopt Western-style diets more easily. Nevertheless, the MDP was the second factor identified. These findings are in accordance with previous studies performed in Mediterranean countries, including Spain( Reference Sánchez-Villegas, Delgado-Rodríguez and Martínez-González 33 – Reference León-Muñoz, Guallar-Castillón and Graciani 35 ). In our cohort of university graduates, it is likely that this departure from the traditional MDP could be explained because highly educated people are more likely to be involved in work duties that frequently preclude that they may prepare their own foods and that their work environment may influence their dietary pattern( Reference Sánchez-Villegas, Delgado-Rodríguez and Martínez-González 33 – Reference León-Muñoz, Guallar-Castillón and Graciani 35 ).
The principal component analysis method might involve some degree of subjectivity, and the two major dietary patterns identified do not necessarily reflect the typical WDP and MDP. In this sense, for example, consumption of vegetables was higher in the highest quartile of adherence to WDP than in the lowest quartile. Even with the inherent methodologic limitations of this statistical method (e.g. the establishment of predefined food groups), we found an association between high adherence to WDP and higher risk of GDM.
Furthermore, our findings are in line with previous studies that have investigated the association of dietary patterns with GDM risk. However, to the best of our knowledge, the majority of these studies between dietary patterns and GDM risk have been obtained using a priori scores( Reference He, Yuan and Chen 11 – Reference Zhang, Tobias and Chavarro 16 ). Ours is probably the first study that found an association between adherence to empirically derived dietary patterns and GDM incidence.
The majority of the available evidence between diet and GDM risk has come from the results of the Nurses’ Health Study II cohort, in the USA. They reported that lowering the intake, before getting pregnant, of foods with high haeme iron (such as red meat), sugar-sweetened cola, potatoes, fatty foods (such as high-fat processed meats and fast food) and sweets can reduce the incidence of GDM, especially among the high-risk population( Reference Bao, Tobias and Hu 36 – Reference Chen, Hu and Yeung 39 ). Some of these results have been consistently reported in studies assessing other populations( Reference Qiu, Frederick and Zhang 40 , Reference Qiu, Zhang and Gelaye 41 ) apart from the Nurses’ Health Study II cohort. Besides, a healthier dietary pattern, similar to MDP (i.e. the alternate Mediterranean (aMED)), was reported to be associated with a reduced risk of GDM( Reference Tobias, Zhang and Chavarro 14 ). However, the magnitude of the relative risk reduction for GDM in that study was stronger for the Dietary Approaches to Stop Hypertension, and alternate Healthy Eating Index dietary patterns than for the aMED. These plant-based dietary patterns decrease the risk of GDM mainly because of the lower energy intake of the diet, the amounts of fibre, mineral and vitamin intakes and their anti-inflammatory and antioxidant properties.
We observed that a low-risk lifestyle before pregnancy (i.e. maintaining a healthy body weight, getting pregnant before 28 years and avoiding a Western-style dietary pattern) was inversely and strongly associated with GDM risk. Women at low risk for all three lifestyle risk factors had a 76 % lower risk of GDM than those women who did not adhere to any factor.
One of the strongest risk factors for GDM is maternal age( 42 ). Although it may be seen as an apparently non-modifiable risk factor, women of reproductive age can freely choose the timeliness of their pregnancies and they may modify this factor. Therefore, the decisions of reproductive-age women regarding their age at pregnancy should be taken into account when considering potential interventions to reduce the risk of GDM. It is known that maternal BMI is another strong risk factor to develop GDM( 43 , Reference Bellamy, Casas and Hingorani 44 ) and Western-style dietary habits and lifestyles are important factors for weight gain( Reference Mozaffarian, Hao and Rimm 45 ). Moreover, it is known that a Western-style dietary pattern itself leads to insulin resistance because of higher availability of free fatty acids that increase TAG deposits in pancreatic islets, producing β-cell dysfunction( Reference Pereira, Kartashov and Ebbeling 46 ). In this context, knowledge acquired from studies on type 2 diabetes leads to think that subjects who develop type 2 diabetes tend to accumulate a higher amount of fat in their liver and pancreas than they can cope with, independently of their BMI( Reference Roy 47 ). Thus, hepatic fat accumulation is related to both the dietary pattern and the risk of diabetes, and a Western-style dietary pattern is likely to be an independent risk factor for GDM.
A study on the role of lifestyle factors before pregnancy in the development of GDM reported the importance of having a global ‘good score’ (following a healthy diet, maintaining a healthy body weight, being non-smoker and being physically active) in order to prevent GDM risk( Reference Zhang, Tobias and Chavarro 48 ). Some of these factors are consistent with our results.
Our study presents some potential limitations. Voluntary self-reported completion of the questionnaire may produce some degree of selection bias, which usually makes it more difficult to find associations. Nevertheless, variables such as self-reported weight and BMI have been validated in a subsample of the SUN project( Reference Bes-Rastrollo, Perez Valdivieso and Sanchez-Villegas 25 ). Furthermore, skepticism may arise from a dietary assessment conducted with a FFQ, which may be susceptible to information bias. However, the FFQ used has been repeatedly validated( Reference Martin-Moreno, Boyle and Gorgojo 20 – Reference De la Fuente-Arrillaga, Ruiz and Bes-Rastrollo 22 ) and probably the FFQ is the best available option to evaluate dietary habits of large samples that require to be monitored over long periods( Reference Willet 29 ).
Because of the fact that all participants were Spanish university graduates, we were not able to assess the effect of ethnicity. Most participants were Caucasians.
We did not assess diet during pregnancy because pregnant women are susceptible to change their dietary pattern as a consequence of their pregnancy. However, to the best of our knowledge, most dietary interventions performed during pregnancy do not significantly reduce the GDM risk and it seems that pre-pregnancy dietary habits have more relevance in GDM incidence( Reference Donazar-Ezcurra, López-del Burgo and Bes-Rastrollo 49 ).
Not all potential risk factors for GDM such as the presence of polycystic ovary syndrome or use of corticosteroid medication could be included in the analyses as this information was not collected in the questionnaires.
Because of the inherent characteristics of an observational study, we are not able to exclude possible residual confounding, although we adjusted our results for several potential confounders.
Despite these limitations, the study also has several strengths. It has a large sample size with a high retention rate and a prospective design with prolonged follow-up. The FFQ used has been repeatedly validated( Reference Martin-Moreno, Boyle and Gorgojo 20 – Reference De la Fuente-Arrillaga, Ruiz and Bes-Rastrollo 22 ). In addition, we were able to control for a wide array of potential dietary, lifestyle and demographic confounders.
In conclusion, a Western-style dietary pattern seems to be an independent risk factor for the development of GDM. These findings highlight the importance of acquiring a high-quality dietary pattern before pregnancy in order to prevent GDM besides global health benefits. Our results reinforce the importance of taking into account pre-gravid dietary recommendations and other two factors (low BMI and young age at pregnancy) and they should be considered for the prevention of GDM among women of reproductive age.
Acknowledgements
The authors thank the participants and the members of the SUN project.
This work was supported by the Spanish Government-Instituto Carlos III, the European Regional Development Fund (FEDER)(RD06/0045,CIBEROBN, grants PI10/02658, PI10/02293, PI13/00615, PI14/01668, PI14/01798,PI14/01764 and G03/140), the Navarra Regional Government (45/2011, 122/2014) and the University of Navarra.
C. L. B., M. A. M.-G., J. D. I. and M. B.-R. conceived, designed and conducted research. M. D.-E. and M. B.-R. analysed data and wrote the paper. C. L. B., M. A. M.-G., F. J. B.-G. and J. D. I. contributed to the discussion and reviewed/edited the manuscript. M. B.-R. had primary responsibility for final content. All authors read and approved the final manuscript.
None of the authors has any conflicts of interest to declare.







