Introduction
CVD continues to be a leading cause of morbidity and mortality among adults in Western countries. Cigarette smoking, high blood pressure, high serum total cholesterol and LDL-cholesterol, low serum HDL-cholesterol, diabetes mellitus and advanced age are considered the main risk factors for CVD(Reference Castelli1, Reference Wilson, D'Agostino and Levy2).
A large number of epidemiological studies have associated a diet rich in fruits and vegetables with a reduction in CVD risk factors(Reference Panagiotakos, Pitsavos and Arvaniti3). This is presumably due to the presence in plant foods and certain beverages of a variety of compounds including different kinds of antioxidants, such as vitamin C, vitamin E, polyphenols and carotenoids. Polyphenols in particular have been associated with a reduction of the risk of different diseases in several epidemiological studies(Reference Arts and Hollman4).
The French paradox(Reference Renaud and De Lorgeril5), i.e. the low prevalence of CVD in certain French regions with a high intake of saturated fats, has been put down to the consumption of red wine. This effect has been attributed mainly to the presence of polyphenols, a large group of compounds present in plant foods and beverages that have demonstrated a strong in vitro antioxidant capacity due to their ability to scavenge free radicals and to chelate metals(Reference Bravo6). Ethanol can also improve the bioavailability of polyphenols, as well as playing a specific cardioprotective role.
The possible benefits of grape and wine consumption in relation to CVD have prompted researchers to conduct many in vivo trials to study the effects of grape products (grape juice, grape seed, grape skin, polyphenol-rich extracts) on different CVD risk factors. Most reviews have focused only on wine(Reference Leighton and Urquiaga7) or have dealt with some specific aspects of grape and wine flavonoids' mechanisms of action, such as their effects on endothelial dysfunction(Reference Pérez-Vizcaíno, Duarte and Andriantsitohaina8). However, no paper has systematically discussed the in vivo trials performed during recent years in relation to grape products and CVD risk.
The aim of the present paper is to review the trials published during the last 13 years (thirty-four in animals and forty-one in human subjects) examining the effects of supplementation with grape products on CVD risk factors.
Grape products used in clinical trials
Clinical trials have evaluated the in vivo effects of grapes, wine, grape skin, grape seeds, grape pomace and grape polyphenol extracts. Table 1 summarises the main compositional characteristics of these products.
Table 1 Main compositional characteristics of grapes and polyphenol-rich derived products(Reference Girar, Mazza and Mazza9, Reference Falchi, Bertelli and Lo Scalzo14, Reference Bravo and Saura-Calixto28, Reference Monagas, Hernández-Ledesma and Gómez-Cordovés30, Reference Goñi, Martín and Saura-Calixto34)

Grapes (Vitis vinifera L.) contain high concentrations of polyphenols, especially flavonoids. The amount and composition of biologically active compounds present in grapes and grape products vary greatly according to the species, variety, maturity, seasonal conditions, production area and yield of the fruit(Reference Girar, Mazza and Mazza9). The main grape polyphenols are anthocyanins in red grapes and flavan-3-ols in the case of white grapes; red grapes contain more total polyphenols than white grapes. Grape seeds and skins are also an important dietary source of flavonoids, and seeds contain significant amounts of proanthocyanidins or condensed tannins. The composition of the seeds is much more diverse than that of the skins(Reference Girar, Mazza and Mazza9). Some in vivo studies have dealt with the effects of the intake of the original grapes after freeze-drying(Reference Cui, Cordis and Tosaki10, Reference Zern, West and Fernández11). Other studies have investigated the effects of the intake of grape seeds or grape peels separately(Reference Ruf, Berger and Renaud12–Reference Falchi, Bertelli and Lo Scalzo14).
The most common commercial product derived from grapes is wine, a moderately alcoholic drink made by fermentation of juice extracted from fresh, ripe grapes. The processing of grapes to yield wine transforms the polyphenols present in grapes, and as a result the main polyphenols in wine are flavan-3-ols, flavan-3,4-diols, anthocyanins and anthocyanidins, flavonols, flavones, condensed tannins and a characteristic biologically active compound, resveratrol – a stilbene whose concentration can range from 1·5 to 3 mg/l(Reference Girar, Mazza and Mazza9, Reference Shahidi and Naczk15). Consequently, given the polyphenol contents of the raw material, red wine exhibits a much higher phenolic content than white wine. Several in vivo studies have examined the effects of the intake of red(Reference Badía, Sacanella and Fernández-Solá16–Reference Naissides, Mamo and James19) and white(Reference Williams, Sutherland and Whelan20, Reference Pignatelli, Ghiselli and Buchetti21) wine.
There are some alcohol-free products derived from wine. The most common of these is grape juice, which has been used in several in vivo studies(Reference Osman, Maalej and Shanmunagayagam22–Reference Castilla, Dávalos and Teruel24). Grape juice differs little in composition from grapes except that it lacks dietary fibre and oil. Its characteristic polyphenols include ellagic acid, an acid hydrolytic product of ellagitannins(Reference Girar, Mazza and Mazza9). Also, for some intervention studies dealcoholised red wine has been prepared(Reference Ruf, Berger and Renaud12, Reference De Rijke, Demacker and Assen25) in order to consider the possible effect of ethanol on the bioavailability of polyphenols and to differentiate the positive effects on CVD due to polyphenols and those due to ethanol. To the authors' knowledge, no in vivo study has considered solely the possible positive effects of dealcoholised white wine.
Because grape juice has a high energy value due to its high sugar content, other products derived from grapes have been studied. The most common of these are products derived from grape pomace, a by-product of wine-making consisting of pressed skins, seeds and stems. Traditionally, grape pomace was used as a source of different products, such as ethanol, tartrates, citric acid, grape seed oil, grape seed tannins, hydrocolloids and anthocyanins(Reference Girar, Mazza and Mazza9); however, with growing interest in the beneficial effects of grapes over the last few years, grape pomace-derived products have come to be used in intervention studies(Reference Bobek, Ozdin and Hromadova26, Reference Martin-Carrón, Goñi and Larrauri27) because of its high concentrations of phenolic compounds and dietary fibre, another beneficial component(Reference Bravo and Saura-Calixto28). Specific preparations of grape products rich in both dietary fibre and polyphenols have also been used to check the possible combined effect of these compounds(Reference Pérez-Jiménez, Serrano and Tabernero29).
Finally, several commercial products have been developed during the last few years, mainly in the form of pills. A recent study(Reference Monagas, Hernández-Ledesma and Gómez-Cordovés30) compared the antioxidant capacity of up to thirteen commercial products derived from grape skins, grape pomace and grape leaves; most of these products are supplied in solid form, although some of them came in the form of liquid concentrates and syrups. Anthocyanidin-3-glucosides presented the largest concentration, although the profiles of the ingredients derived from skin and those derived from leaves were different. However, in this and in other studies(Reference Monagas, Hernández-Ledesma and Garrido31) it has been noted that the polyphenol content of these supplements differs significantly, which means that the biological activities derived from them will be different. Some of them have been employed in in vivo studies(Reference Carbonneau, Leger and Monnier32–Reference Vinson, Proch and Bose33).
In short, different compounds may contribute to the observed effects depending on the tested grape product: polyphenols and ethanol in wine, polyphenols and sugar in grape juice, polyphenols and dietary fibre in grape seeds and grape skins, or just polyphenols in extracts(Reference Girar, Mazza and Mazza9, Reference Shahidi and Naczk15, Reference Bravo and Saura-Calixto28, Reference Monagas, Hernández-Ledesma and Gómez-Cordovés30, Reference Goñi, Martín and Saura-Calixto34).
Trials reviewed
Table 2 summarises the selected in vivo studies performed on animals. Thirty-four articles have been considered. They include studies on supplementation with freeze-dried grapes, grape pomace, grape peel, grape seed, polyphenols from grapes, polyphenols from grape seed, extracts from white grape seed, extracts from red grape seed, a grape product rich in both dietary fibre and polyphenols, grape juice, red and white wine, dealcoholised red wine, red wine powder and red wine extracts. The studies were performed on rats, apoE-deficient mice, rabbits (normal and Watanabe heritable hyperlipidaemic), hamsters, gerbils, ovariectomised guinea-pigs, chickens, monkeys and dogs. The number of animals in each study was between twenty and 180, with an average of fifty-three subjects. The duration of the studies was between 1 week and 6 months, with an average of 2 months.
Table 2 Published trials in animals on effects of grapes, wine and derived products on CVD risk factors in animals*

l-NAME, N-nitro-l-arginine-methyl ester; RWPC, red wine polyphenols concentrate.
* When not indicated expressly, grapes are red grapes and wine is red wine.
Table 3 summarises the selected in vivo studies performed on human subjects. Forty-one articles have been considered. They include studies on supplementation with freeze-dried grapes, grape peel, grape seed, polyphenols from grapes, polyphenols from grape seed, grape juice, concentrated grape juice, red and white wine, dealcoholised red wine and red wine polyphenol extracts. The studies include healthy subjects, subjects with coronary artery disease, type 2 diabetic patients, pre- and postmenopausal women, hypercholesterolaemic subjects, hypertensive subjects, men with stable IHD and haemodialysed subjects. The number of participants in each study was between eight and sixty-nine, with an average of twenty-four subjects. The duration of the studies was between 1 and 16 weeks, with an average of 25 d, plus eight studies which were based on an acute intake.
Table 3 Published trials on effects of grapes, wine and derived products on CVD risk factors in human subjects*

* When not indicated expressly, grapes are red grapes.
Effects on blood pressure: endothelial function
The US National High Blood Pressure Education program estimates that a reduction of 5 mmHg in systolic blood pressure (SBP) translates into a 14 % reduction in deaths by stroke, a 9 % reduction in deaths by heart disease and a 7 % reduction in overall mortality(35). This has generated an interest in the search for new bioactive dietary compounds that can reduce blood pressure, among them polyphenols.
However, when considering the effects of grape products on blood pressure, the ethanol content of red wine, one of the main grape products, should not be disregarded. It is well known that there is a linear relationship between alcohol intake and blood pressure(Reference Puddey, Beilin and Vandogen36). Although several epidemiological studies have suggested that beer and spirits consumption may be associated with higher blood pressure (particularly SBP) than wine consumption(Reference Klatsky, Friedman and Armstrong37, Reference Bulpitt, Shipley and Semmence38), an in vivo trial(Reference Zilkens, Burke and Hodgson39) showed that daily consumption of 40 g alcohol as either red wine or beer for 4 weeks resulted in similar increases in SBP and heart rate. Therefore, studies on the effects of grape products on blood pressure should be focused more on grape products lacking ethanol.
All the reviewed studies on animals dealing with the effects of grape polyphenols on blood pressure showed a hypotensive effect of them. Grape skin extract, red wine polyphenols and red wine extract significantly reduced blood pressure (in several cases, both SBP and diastolic blood pressure) in normotensive and hypertensive rats, where hypertension was induced by N-nitro-l-arginine-methyl ester (l-NAME) or by deoxycorticosterone acetate(Reference Diebolt, Bucher and Andriantsitohaina40–Reference Ranaivo, Diebolt and Andriantsitohaina44).
In the case of human subjects, a significant reduction in SBP and diastolic blood pressure was observed in hypertensive or coronary artery disease subjects after the intake of grape juice(Reference Park, Kim and Kang45). Also, the intake of a grape product rich in both dietary fibre and polyphenols by normotensive subjects for 16 weeks led to a non-significant decrease in SBP and diastolic blood pressure(Reference Monagas, Hernández-Ledesma and Gómez-Cordovés30). This is an interesting result, since assays on the effects of supplementation on blood pressure are usually performed on hypertensive subjects, and these might be expected to present a more marked effect if administered this product, which is rich in both polyphenols and dietary fibre. However, another study did not find any effect of dealcoholised red wine on blood pressure(Reference Zilkens, Burke and Hodgson39). Only one of the reviewed trials found that grape polyphenols had adverse effects on blood pressure; there, increased blood pressure was observed in hypertensive subjects when polyphenols were combined with vitamin C(Reference Ward, Hodgson and Croft46).
As to mechanisms that would explain the generally observed hypotensive effect of polyphenols, it has been suggested that they promote the release by the vascular endothelium of NO, a compound with vasorelaxing and anti-aggregating effects and that in the long term induces the expression of protective genes for the cardiovascular system(Reference Diebolt, Bucher and Andriantsitohaina40, Reference Chou, Keevil and Aeschlimann47). It has also been proposed that wine may regulate the endothelial NO synthase gene in endothelial cells, through transcriptional and post-transcriptional factors(Reference Wallerath, Poleo and Li48).
These hypotheses have only been partially proven. In an ex-vivo experiment with aortic rings from rats incubated with red grape polyphenols, there was a two-fold increase in the NO-Fe(diethyldithiocarbamate)2 electron paramagnetic resonance, which indicates the generation of NO; this NO production was completely prevented by the NO synthase inhibitor l-NAME(Reference Andriambeloson, Kleschyov and Muller49). Similarly, after supplementing rats with red wine and observing an anti-thrombotic effect(Reference Wollny, Aiello and Di Tommaso50), the effects produced by red wine were prevented by l-NAME, indicating the involvement of NO in this process. Moreover, the effects of l-NAME could be reverted by l-arginine, the precursor of NO synthesis in vascular endothelium but not by its stereoisomer, d-arginine. In human subjects, although an acute intake of polyphenols did not significantly increase NO production(Reference Matsuo, Nakamura and Takahashi51), the supplementation to healthy volunteers with grape juice for 14 d led to a significant increase in platelet-derived NO production (from 3·5 (sem 1·2) to 6·0 (sem 1·5) pmol/108 platelets(Reference Freedman, Parker and Li52)).
Another parameter that has been studied to determine the effect of grape polyphenols on endothelial function has been the determination of effects on vasodilatation and, particularly, on flow-mediated dilatation of the brachial artery, which is considered to be an early marker of alterations in endothelial function. In studies in animals, red wine polyphenols induced endothelium-dependent relaxation in rat aorta(Reference Andriambeloson, Kleschyov and Muller49), red wine and dealcoholised red wine induced vasodilatation in isolated vessels from rats(Reference Boban, Modun and Musc53) and grape skin extract had a vasodilator effect on the mesenteric cardiovascular bed of rats(Reference Soares de Moura, Costa Viana and Souza42). In human subjects, an improvement in endothelium-dependent vasodilatation has been reported after the acute intake of white wine or red wine with a meal by subjects with IHD(Reference Hashimoto, Kim and Eto54); also it has been observed that an acute intake of red grape polyphenol extract, red wine or dealcoholised red wine cause an increase in flow-mediated dilatation, peaking at 60 min(Reference Hashimoto, Kim and Eto54, Reference Lekakis, Rallidis and Andreadou55). Nevertheless, in another study(Reference Boban, Modun and Musc53) red wine, but not polyphenols from red wine, produced an enhancement of endothelial response, despite a similar catechin concentration in both products. Intake of grape juice or grape seed extract for 2–3 weeks also caused a significant increase in flow-mediated dilatation, compared with the control group(Reference Stein, Keevil and Wiebe56, Reference Clifton57). Interestingly, in one of these studies(Reference Clifton57), the addition of quercetin to the grape seed extract nullified this effect, indicating maybe that the provided amount of antioxidants was excessive, thus becoming pro-oxidants.
Also, grape polyphenols may have a reducing effect in cardiac fibrosis, a process occurring in cases of hypertension which is produced by an excessive accumulation of collagen and is associated with an increase in alterations of cardiac and vascular functions(Reference Bernatova, Pechanova and Babal41).
Effects on lipid profile
Several studies have focused on the possible positive effects of grape products on different lipid profile parameters, particularly total cholesterol, and likewise on LDL- and HDL-cholesterol, TAG and apolipoproteins. The results of these trials are summarised in Table 4.
Table 4 Summary of observed effects of grapes, wine and derived products on lipid profile in the reviewed studies
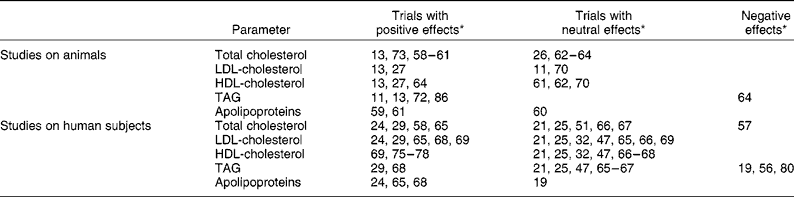
* Numbers correspond to references in the Reference section.
Total cholesterol, LDL-cholesterol and HDL-cholesterol
Nine of the reviewed trials in animals reported a positive effect of grapes and derived products on plasma total cholesterol. This was observed after supplementation with grapes, grape pomace, grape juice, red grape skin, white grape seed, red and white grape extracts rich in procyanidins, red wine, red wine polyphenols, dealcoholised red wine or white wine enriched with red wine polyphenols, to rats fed with a cholesterol-rich diet, to hamsters (fed with a normal or with an atherogenic diet), to ovariectomised guinea-pigs or to rabbits(Reference Zern, West and Fernández11, Reference Martín-Carrón, Saura-Calixto and Goñi13, Reference Shanmuganayagam, Warner and Krueger23, Reference Martin-Carrón, Goñi and Larrauri27, Reference Vinson, Teufel and Wu58–Reference Auger, Rouanet and Vanderlinde61).
Four similar studies in animals reported no effect on plasma total cholesterol(Reference Bobek, Ozdin and Hromadova26, Reference Cestaro, Simonetti and Cervato62–Reference Araya, Rodrigo and Orellana64). So, it cannot be said overall that grapes and derived products present positive effects on plasma cholesterol in animal studies. However, one of these studies(Reference Bobek, Ozdin and Hromadova26) did find a reduction in cardiac cholesterol and in the half-times of serum [14C]cholesterol decay curves, while another reported a decrease in aorta total cholesterol(Reference Yamakoshi, Kataoka and Koga63).
As regards studies in human subjects, although a significant reduction in total cholesterol has been observed, for example of 12 % after the intake of a grape seed extract(Reference Vinson, Teufel and Wu58) or of 6–11 % after the intake of concentrated grape juice(Reference Castilla, Dávalos and Teruel24, Reference Castilla, Echarri and Davalos65), many other studies found no change in this parameter after the intake of grapes or derived products(Reference Pignatelli, Ghiselli and Buchetti21, Reference De Rijke, Demacker and Assen25, Reference Chou, Keevil and Aeschlimann47, Reference Freedman, Parker and Li52, Reference Cordain, Melby and Hamamoto66, Reference Cacetta, Burke and Mori67).
In the case of LDL-cholesterol, significant reductions have been observed in studies in both animals(Reference Martín-Carrón, Saura-Calixto and Goñi13, Reference Martin-Carrón, Goñi and Larrauri27) and in human subjects(Reference Castilla, Dávalos and Teruel24, Reference Pérez-Jiménez, Serrano and Tabernero29, Reference Vinson, Proch and Bose33, Reference Castilla, Echarri and Davalos65, Reference Zern, Wood and Greene68, Reference Naissides, Mamo and James69), although other studies have reported no effect either in animals(Reference Zern, West and Fernández11, Reference Hayek, Fuhrman and Vaya70) or in human subjects(Reference Pignatelli, Ghiselli and Buchetti21, Reference Castilla, Dávalos and Teruel24, Reference Chou, Keevil and Aeschlimann47, Reference Cordain, Melby and Hamamoto66, Reference Cacetta, Burke and Mori67).
The differences in the results of these assays may have to do with the design of the study, the polyphenol content of the tested product or its storage conditions. For example, it has been noticed that the higher the initial concentration of plasma cholesterol, the greater the reduction(Reference Pérez-Jiménez, Serrano and Tabernero29, Reference Vinson, Proch and Bose33, Reference Naissides, Mamo and James69). This would explain the lack of effects in some of the cited studies in human subjects, which targeted normocholesterolaemic subjects. The importance of a proper design can be seen from the fact that in the studies where some change has been observed in lipid profile, this change has affected several parameters(Reference Bobek, Ozdin and Hromadova26, Reference Araya, Rodrigo and Orellana64).
The main mechanism responsible for this hypolipidaemic effect would be reduction in intestinal cholesterol absorption, leading to an enhanced excretion of faecal neutral steroids and bile acids. This was first observed by Tebib et al. (Reference Tebib, Besancon and Rouanet71) in rats supplemented with low- and high-molecular-weight tannins (a class of polyphenols) from grapes and was later observed in other assays, for example, in rats supplemented with a grape seed extract(Reference Nakamura and Tonogai72). Similarly, polyphenols would reduce the intestinal absorption of dietary fat, since it has been observed that both normal and dealcoholised red wine reduced the postprandial concentrations of chylomicrons(Reference Pal, Naissides and Mamo73). Also, it has been observed that polyphenols, in this case from tea, with high molecular weight (such as tannins present in grapes) form complexes with bile, which causes disruption of micelles, leading to the precipitation of cholesterol in the intestinal lumen, which is a behaviour similar to that of water-soluble dietary fibre(Reference Ikeda, Imasoto and Sasaki74).
It is also interesting to note that other observed facts besides changes in plasma total cholesterol may also have a positive effect on lipid metabolism. For example, after supplementing of ovariectomised guinea-pigs with freeze-dried grapes, a positive change was found in the concentration and composition of LDL particles that were not readily taken up by the aorta, resulting in less accumulation of cholesterol in this tissue(Reference Zern, West and Fernández11). The authors of this study also suggested that grapes may reduce the secretion of LDL particles by the kidney.
As regards HDL-cholesterol, although some studies in animals have reported positive effects after supplementation with alcohol-free products(Reference Martín-Carrón, Saura-Calixto and Goñi13, Reference Martin-Carrón, Goñi and Larrauri27, Reference Araya, Rodrigo and Orellana64), in studies in human subjects increased HDL-cholesterol has only been observed once after the intake of red grape juice concentrate by haemodialysed patients(Reference Castilla, Dávalos and Teruel24), but did not take place in the trials with alcohol-free grape products(Reference Naissides, Mamo and James69, Reference Fuhrman, Lavy and Aviram75–Reference Avellone, Di Garbo and Campisi78). Therefore, this effect is presumably mainly related to the already-known ability of ethanol to increase HDL-cholesterol(Reference Fuhrman, Lavy and Aviram75) instead of polyphenol content.
Triacylglycerols
It is known that ethanol can increase plasma TAG(Reference Ayaori, Ishikawa and Yoshida79). One would therefore expect that if grapes and derived products had a positive effect on plasma TAG, this would only be so in the case of alcohol-free products. This tendency was observed in studies in animals, when supplementation with freeze-dried grapes, white grape peel, white grape seed, grape seed polyphenols or dealcoholised red wine to rats, ovariectomised guinea-pigs or apoE-deficient mice reduced plasma TAG(Reference Zern, West and Fernández11, Reference Martín-Carrón, Saura-Calixto and Goñi13, Reference Nakamura and Tonogai72), while supplementation with wine had neutral or negative(Reference Araya, Rodrigo and Orellana64) effects on this parameter.
Nevertheless, this aspect was less clear in the case of human subjects. Although supplementation to menopausal women with freeze-dried grapes reduced plasma TAG by up to 15 %(Reference Zern, Wood and Greene68) and the intake of a grape product rich in both dietary fibre and polyphenols had a similar effect on hypercholesterolaemic subjects(Reference Pérez-Jiménez, Serrano and Tabernero29), the intake of other non-alcoholic grape-derived products had neutral(Reference Pignatelli, Ghiselli and Buchetti21, Reference Castilla, Dávalos and Teruel24, Reference Chou, Keevil and Aeschlimann47, Reference Castilla, Echarri and Davalos65) or negative(Reference Stein, Keevil and Wiebe56, Reference O'Byrne, Devaraj and Grundy80) effects. Interestingly, the two tested products that had a positive effect on this parameter contained dietary fibre, and that may have contributed significantly to this effect.
Apolipoproteins
The effects of grapes and derived products on apolipoproteins have not been systematically studied. However, the results of a few trials have shown that the hypolipidaemic effect of grape polyphenols may be related to their effects on the concentrations of certain apolipoproteins. For instance, red wine or red wine polyphenol supplementation to rats increased apoA1 and reduced apoB(Reference Auger, Caporiccio and Landrault59, Reference Auger, Rouanet and Vanderlinde61). Similarly, the same effects were produced by the intake of freeze-dried grapes or concentrated grape juice by menopausal women or by healthy and haemodialysed subjects respectively(Reference Castilla, Dávalos and Teruel24, Reference Castilla, Echarri and Davalos65, Reference Zern, Wood and Greene68).
Effects on platelet aggregation
Reducing platelet activity is a preventive strategy to reduce the development of atherosclerosis(Reference Shanmuganayagam, Warner and Krueger23). Several trials have studied the possible effects of grapes, wine and derived products on platelet aggregation.
In studies in animals, it has been observed that the intake of grape juice reduces platelet aggregation induced by collagen or by ADP as agonists, but not aggregation induced by phorbol-12-myristate-13-acetate(Reference Osman, Maalej and Shanmunagayagam22, Reference Shanmuganayagam, Warner and Krueger23).
In a trial in rats on the rebound effect on thrombin-induced platelet aggregation after alcohol withdrawal, it was observed that 18 h after deprivation of alcohol, 6 % ethanol increased the platelet response by 124 %, white wine by 46 %, and red wine reduced it by 59 %. This is an interesting aspect, since the increase in platelet aggregation after alcohol deprivation is sometimes associated with sudden death and stroke in humans(Reference Ruf, Berger and Renaud12).
The intake of grape juice by human subjects reduced platelet aggregation induced by collagen, ADP and phorbol-12-myristate-13-acetate; it also caused an increase in platelet-derived NO release and a decrease in platelet superoxide anion production(Reference Freedman, Parker and Li52, Reference Keevil, Osman and Reed81). The intake of a grape juice enriched in resveratrol also reduced thrombin-induced platelet aggregation, but this effect was absent after supplementation with a commercial grape juice(Reference Pace-Asciak, Rounova and Hahn82).
The intake of red wine and white wine by human subjects reduced thrombin-induced platelet aggregation and thromboxane B2 concentration, but only white wine reduced ADP-induced aggregation(Reference Pace-Asciak, Rounova and Hahn82).
The suppression of platelet-mediated thrombosis, then, is another mechanism that could potentially explain the beneficial effects of grape polyphenols in CVD. Specific studies should be performed with grape skins, grape seeds, grape pomace and polyphenols extracted from grapes.
Anti-atherosclerotic effect
Atherosclerosis is a key pathology in CVD, which, once started, cannot be prevented but may be retarded(Reference Frederiksen, Mortensen and Schrøder83). Therefore, a great deal of research has focused on the positive effect that grapes and derived products may have in preventing the progression of this pathology.
Effects on atheromatous plaque
Several studies have been performed to evaluate the effect of supplementation with grape polyphenols in the development of atherosclerotic lesions. Due to the nature of the measurements performed, they can only be developed in animals.
A significant reduction in the size of atherosclerotic lesions or in the accumulation of foam cells in aorta has been observed after supplementation with red wine, dealcoholised red wine, red wine polyphenols, white wine enriched in polyphenols, and white and red grape extracts to apoE-deficient mice and hamsters with an atherogenic diet and to rabbits and Watanabe heritable hyperlipidaemic rabbits(Reference Vinson, Teufel and Wu58, Reference Auger, Rouanet and Vanderlinde61, Reference Yamakoshi, Kataoka and Koga63, Reference Frederiksen, Mortensen and Schrøder83, Reference Waddington, Puddey and Croft84). Also, red wine or dealcoholised red wine supplementation to rats induced a marked prolongation of template bleeding time, a decrease in platelet adhesion to fibrillar collagen and a reduction in thrombus weight, while neither ethanol nor white wine affected these systems(Reference Wollny, Aiello and Di Tommaso50).
These studies found that there was an effect mainly in the early stages of atherosclerosis, during fatty streak formation. In contrast, another study that examined the mature phase of atherosclerosis reported no positive effects in terms of the speed of progression of the lesions, inhibition in the aortic root and brachicephalic trunk or changes in collagen content of atheromatous plaques after red wine powder supplementation to apoE-deficient mice(Reference Benteon, Skovenborg and Hansen85). Similarly, Stocker & O'Halloran(Reference Stocker and O'Halloran86) found a reduction in the aortic lesions in the aortic arch, but not in the aortic root after supplementing apoE-deficient mice with dealcoholised red wine. Since lesions in the aortic root are more developed than in the aortic arch, this may confirm that grape polyphenols are only able to inhibit atherosclerosis during its early stages, although this would be an important beneficial effect in any case.
Anti-atherosclerotic and hypolipidaemic effects were considered separately in a study on hamsters supplemented with red wine or dealcoholised red wine(Reference Vinson, Teufel and Wu58); it was observed that the polyphenols present in these products exhibited an anti-atherosclerotic effect over and above their effects on the lipid profile. Also, these effects on the development of atheromatous plaque were presumably due not only to their antioxidant effects, but also to inhibition of cell proliferation in smooth muscle, regulation of adhesion molecules and reduction of the flux of atherogenic molecules from the endothelium to the arterial wall(Reference Freedman, Parker and Li52, Reference Boban, Modun and Musc53).
To the authors' knowledge, studies in animals have not specifically addressed the possible atherosclerotic effect of grape skins and grape seeds.
Although many of these assays cannot be performed on human subjects, some studies have examined the effect of wine intake on monocyte–endothelial cell adhesion, an early event in atheromatous plaque formation. Results show that white and red wine intakes reduced monocyte adhesion to TNF-α-stimulated endothelial cells by 51 and 89–96 % respectively(Reference Badía, Sacanella and Fernández-Solá16, Reference Sacanella, Vázquez-Agell and Mena17). Since the effect achieved by gin was 39 %, the polyphenols present in wine presumably contributed significantly to this effect, which may be the result of down-regulation of some monocyte adhesion molecules, especially very light-appearing antigen 4, on the monocyte surface.
Effects on LDL oxidation
The oxidative hypothesis of atherosclerosis suggests that LDL oxidation could be a first step in the development of atheromatous plaque. For instance, several studies have considered the effects of grape polyphenols on different parameters related to LDL oxidation.
A decrease in the concentration of oxidised LDL or an increase in the lag-phase in the oxidation of LDL has been observed after red wine or grape supplementation to apoE-deficient mice or to cholesterol-fed rabbits(Reference Yamakoshi, Kataoka and Koga63, Reference Hayek, Fuhrman and Vaya70, Reference Benteon, Skovenborg and Hansen85). Interestingly, supplementation with wine polyphenols in ethanol to rats reduced LDL oxidation, but polyphenols in water had no effect and ethanol alone increased it, indicating a possible contribution of ethanol to the metabolism of polyphenols(Reference Xia, Allenbrand and Sun87).
In studies in human subjects, the intake of red wine, red wine polyphenols, white wine enriched with red wine polyphenols, grape juice or concentrated grape juice by healthy subjects or haemodialysed patients had positive effects on LDL oxidation(Reference Pignatelli, Ghiselli and Buchetti21, Reference Castilla, Echarri and Davalos65, Reference Tsang, Higgings and Duthie77, Reference Avellone, Di Garbo and Campisi78, Reference O'Byrne, Devaraj and Grundy80, Reference Nidgikar, Williams and Griffin88). The effect of supplementation to human subjects with grape pomace on this parameter has not been considered.
However, other similar studies reported no such effects(Reference De Rijke, Demacker and Assen25, Reference Vinson, Proch and Bose33). It is interesting that in one of these studies(Reference Chou, Keevil and Aeschlimann47), the authors explained that the storage conditions of the tested product had not been the same as in a previous one where they had observed a decrease in LDL oxidation. This shows the importance of storage conditions for polyphenol-rich products, since it is well known that the antioxidant capacity of polyphenol-rich products decreases during storage at room temperature.
Castilla et al. (Reference Castilla, Echarri and Davalos65) looked for a possible correlation between oxidised LDL and LDL concentration after the intake of grape juice by human subjects for 4 weeks; they did not find any, which suggests that besides a reduction in total LDL (the hypolipidaemic effect of polyphenols noted earlier), there may be other mechanisms – mainly antioxidants – that would explain the reduction in oxidised LDL.
Effects on inflammation markers
Inflammation and CVD are closely linked, since oxidised LDL are taken up by monocytes, starting the process that produces the foam cells characteristic of the onset of atherosclerosis.
Supplementation to apoE-deficient mice did not modify the levels of monocyte chemo-attractant protein 1 (MCP-1)(Reference Waddington, Puddey and Croft84). However, studies in human subjects have mainly reported positive results regarding these parameters. The intake of red wine, but not of alcohol, reduced the expression of lymphocyte function-associated antigen 1, Mac-1, very light-appearing antigen 4 and MCP-1 in monocytes, and also the expression of vascular cellular adhesion molecule 1 and intercellular adhesion molecule 1 and the concentration of fibrinogen in lymphocytes(Reference Sacanella, Vázquez-Agell and Mena17, Reference Avellone, Di Garbo and Campisi78, Reference Estruch, Sacanella, Badia and Antunez89). Interestingly, one of these studies(Reference Sacanella, Vázquez-Agell and Mena17) reported these results in women who were supplemented with 20 g ethanol per d; although women's threshold of moderate ethanol consumption is lower than that of men, this dose was associated with beneficial effects in inflammation markers similar to those observed in men consuming doses of 30 g/d. Also, the intake of concentrated red grape juice by patients affected by end-stage renal disease treated with haemodialysis (with an increased oxidative stress) led to a reduction in plasma concentrations of MCP-1 and of NADH oxidase activity, a main source of superoxide radicals(Reference Castilla, Dávalos and Teruel24).
A significant increase was reported in IL-6 at 6 h after the intake of a meal accompanied by red or white wine, greater than the increase observed when the meal was accompanied by a control beverage(Reference Williams, Sutherland and Whelan20). The authors suggested that this increase was due more to a response to the hepatic damage induced by alcohol than to an activation of the inflammation response, since there was no change in the levels of cell adhesion molecules. Moreover, this increase in plasma IL-6 did not prevent a substantial improvement in endothelial function with wine intake.
However, other biomarkers have been proposed as being better suited to study the relationship between inflammation and CVD than cell adhesion molecules, specific cytokines or fibrinogen, which may be associated with any kind of inflammatory situation. The most important of these biomarkers are C-reactive protein and homocysteine(Reference Ducros, Demuth and Sauvant90, Reference King91). A significant decrease in C-reactive protein has been observed after the intake of red wine(Reference Avellone, Di Garbo and Campisi78, Reference Estruch, Sacanella, Badia and Antunez89), but not of freeze-dried grapes(Reference Zern, Wood and Greene68). These studies did not express the content of polyphenols of the tested samples in the same way, which makes difficult a direct comparison between the results and to arrive at a possible explanation of the different effects observed. This disparity in the expression of polyphenol content is common in the reviewed trials and is a severe drawback when trying to compare them.
The results on homocysteine have also been contradictory; a non-significant decrease has been observed after the intake of red wine(Reference Tsang, Higgings and Duthie77), while other studies have reported a significant undesired increase(Reference Van der Gaag, Ubbink and Sillanaukee92). However, in this trial the subjects drank four glasses of wine daily, which is more than what is usually considered moderate.
In any event there is a need for more studies on the effects of grapes and derived products (particularly grape skins and grape seeds, products on which no study has been developed in relation to inflammation) on inflammation markers, particularly C-reactive protein and homocysteine.
Effects on oxidative stress
There is a close link between a prolonged situation of oxidative stress and CVD, and dietary antioxidants may play a role in the prevention of the overproduction of free radicals. There are several ways to evaluate in vivo antioxidant status, including measurements of plasma antioxidant capacity, oxidation biomarkers, antioxidant compounds and evaluation of the activity of hepatic enzymes. Table 5 summarises the results of the trials reviewed in these parameters. It is interesting to note that the effect of all the grape-derived products considered in the present review on oxidative stress has been tested using more than one of the different common ways to evaluate it.
Table 5 Summary of observed effects of grapes, wine and derived products on oxidative stress

* Negative effects.
† Numbers correspond to refernces in the Reference section.
Plasma antioxidant capacity
The antioxidant capacity of plasma has been measured in different grapes and derived product supplementation studies in animals. An increase in the ferric-reducing antioxidant power value was observed in several studies after the intake of red wine or dealcoholised red wine by rats; the increase was greater with red wine(Reference Araya, Rodrigo and Orellana64, Reference Rodrigo, Rivera and Orellana93). Similarly, the intake of grape polyphenols by hamsters increased plasma antioxidant capacity measured by 2,2′-azino-bis(3-ethylbenz-thiazoline-6-sulfonic acid); this increase was greater when polyphenols were dissolved in ethanol than when they were dissolved in water(Reference Auger, Caporiccio and Landrault59). These results suggest that the bioavailability of polyphenols is enhanced in ethanol media, and hence in wine.
It has also been reported that there was an increase in the antioxidant capacity of rat and chicken faeces after supplementation with a grape product rich in both dietary fibre and polyphenols, and an increase in the excretion of polyphenols, particularly proanthocyanidins(Reference Goñi and Serrano94–Reference Brenes, Viveros and Goñi96).
However, other authors have obtained results that contradict these. For example, one study found that the intake of red wine by apoE-deficient mice did not modify plasma antioxidant capacity(Reference Benteon, Skovenborg and Hansen85). The same happened after supplementation to hamsters with different extracts from grapes(Reference Freedman, Parker and Li52) or to rats with grape pomace. In this last case, there was actually a significant loss of antioxidant capacity in the liver(Reference Bobek, Ozdin and Hromadova26). These differences in results of similar assays may be related to the various limitations that have been reported in the determination of plasma antioxidant capacity(Reference Wirleitner, Schröecksnadel and Winkler97), as well as to differences in the designs of the studies (duration of treatment, doses employed).
In the case of human subjects, although some studies reported no effect on plasma antioxidant capacity after supplementation with grape-derived products(Reference Simonetti, Ciapellano and Gardana98), most of the trials did find some effect; for instance, increased plasma antioxidant capacity was observed after the intake of polyphenol extracts from red wine(Reference Carbonneau, Leger and Monnier32), from grapes(Reference Vinson, Proch and Bose33), grape juice(Reference Castilla, Echarri and Davalos65), concentrated grape juice(Reference O'Byrne, Devaraj and Grundy80), grape pomace(Reference Pérez-Jiménez99) and red wine(Reference Tsang, Higgings and Duthie77).
Also, studies on the possible effect of grape polyphenols in the postprandial phase have reported that after a meal accompanied by red wine, plasma antioxidant capacity by the total radical-trapping antioxidant parameter assay was sustained in type 2 diabetic subjects, whereas after the same meal without wine there was a significant decrease in antioxidant capacity(Reference Ceriello, Bortolotti and Motz100).
A correlation was found between plasma antioxidant capacity and plasma cholesterol and TAG(Reference Benteon, Skovenborg and Hansen85, Reference Ceriello, Bortolotti and Motz100). This is quite surprising, since subjects with higher plasma lipids and hence a higher risk of CVD would be expected to have lower values of plasma antioxidant capacity. This aspect requires further study, since it could be related to the transport of polyphenols within the bloodstream, a process that is not yet fully understood.
Oxidation biomarkers
Oxidation biomarkers are molecules that are directly affected by the oxidation process and hence furnish a more specific measurement of oxidative status.
Malondialdehyde (MDA) is a biomarker of lipid oxidation. The MDA concentration decreased in plasma, liver and kidney of rats supplemented with red wine for 6 months; in the group supplemented only with ethanol, MDA decreased in plasma in response to a higher consumption of vitamin E(Reference Roig, Cascón and Arola101). Similarly, freeze-dried grape supplementation to rats reduced MDA concentration in the heart(Reference Cui, Cordis and Tosaki10), polyphenol-enriched red wine and white wine supplementation to hamsters reduced plasma MDA(Reference Auger, Rouanet and Vanderlinde61), and red wine and dealcoholised red wine supplementation to rats reduced the concentration of MDA in the renal cortex and renal papilla(Reference Rodrigo, Rivera and Orellana93). Grape extract supplementation to rabbits did not modify plasma MDA levels but did modify levels in the aorta(Reference Yamakoshi, Kataoka and Koga63). Also, a reduction of MDA has been reported in breast and thigh meat from chicken supplemented with concentrated grape pomace(Reference Goñi, Brenes and Centeno95, Reference Brenes, Viveros and Goñi96).
Thiobarbituric acid-reactive substances (TBARS) are another marker of lipid oxidation, although less specific. A decrease in the velocity of generation of TBARS was observed after supplementing rats with wine or with ethanol(Reference Cestaro, Simonetti and Cervato62). Similarly, grape seed polyphenol supplementation to rats reduced this marker by 33 %(Reference Nakamura and Tonogai72). However, red wine supplementation did not modify this value in apoE-deficient mice(Reference Hayek, Fuhrman and Vaya70).
In human studies, TBARS were reduced after the intake of white wine, white wine enriched with polyphenols and red wine polyphenols(Reference Nidgikar, Williams and Griffin88), although De Rijke et al. (Reference De Rijke, Demacker and Assen25) reported no effect on this value after the intake of white or red wine. These differences may be due to the dose of polyphenols administered in each case; in the case of De Rijke et al. (Reference De Rijke, Demacker and Assen25), the subjects drank more wine than in Nidgikar et al. (Reference Nidgikar, Williams and Griffin88) (550 v. 375 ml/d), but different polyphenol contents may have affected the results. It is not possible to determine this, since Nidgikar et al. (Reference Nidgikar, Williams and Griffin88) reported the total polyphenol content while De Rijke et al. (Reference De Rijke, Demacker and Assen25) determined the content of only some particular polyphenols (catechins, quercetin, etc) in the wines tested, by HPLC. Many of the reviewed trials express the polyphenol contents in the tested substances differently, which makes it difficult to compare them directly.
Another suggested marker of lipid oxidation is F2-isoprostanes, although the results reported by different authors for this marker have been contradictory. It has been reported that there was a significant decrease in plasma and urine isoprostanes after the intake of red wine by male smokers(Reference Cacetta, Burke and Mori67), of freeze-dried grapes by pre- and postmenopausal women(Reference Zern, Wood and Greene68) and of white and red wine by healthy subjects(Reference Pignatelli, Ghiselli and Buchetti21). Nevertheless, Waddington et al. (Reference Waddington, Puddey and Croft84) found no change in plasma isoprostanes or in hydroxyeicosatrenoic acids after red wine supplementation to apoE-deficient mice. Similarly, there was no change in this biomarker after the intake of concentrated grape juice(Reference O'Byrne, Devaraj and Grundy80) or grape seed polyphenols(Reference Ward, Hodgson and Croft46) by human subjects. Once again, these differences may be due to the different doses employed or to the phenolic composition of the tested products.
In contrast, biomarkers of protein and DNA oxidation have proven to be useful tools for evaluating the effect of supplementation with grape-derived products, and although not many studies have used them, the results tend to agree. A decrease in protein oxidation has been observed after polyphenol-enriched red wine and white wine supplementation to hamsters with an atherogenic diet(Reference Auger, Rouanet and Vanderlinde61) and after concentrated grape juice supplementation to human subjects(Reference O'Byrne, Devaraj and Grundy80). As regards DNA, a decrease in damage to neuronal DNA was observed after supplementing gerbils with freeze-dried grapes(Reference Wang, Simona and Li102). In trials in human subjects, there was a decrease in the 8-oxo-deoxygunaosin:deoxiguanosin ratio after the intake of procyanidins from grape seeds, sustained for 1 week after cessation of the treatment(Reference Simonetti, Ciapellano and Gardana98), and after supplementation with red wine to patients with acute coronary syndrome(Reference Guarda, Godoy and Foncea103).
Measurement of antioxidant compounds
Several studies have dealt with the evolution of plasma polyphenols after the intake of grapes and derived products. The bioavailability of dietary polyphenols has been reviewed elsewhere(Reference Manach, Williamson and Morand104, Reference Williamson and Manach105), so we will only refer here to observations after chronic and not after acute intakes, which have tended to produce positive results. Plasma polyphenols increased after the intake of red wine, polyphenol-enriched white wine or red wine polyphenols(Reference Shahidi and Naczk15, Reference Pignatelli, Ghiselli and Buchetti21, Reference Nidgikar, Williams and Griffin88). Similarly, an increase was observed in the urinary excretion of 4-o-methyl-gallic acid after the consumption of red wine and dealcoholised red wine(Reference Cacetta, Burke and Mori67), in the urinary excretion of four metabolites from flavan-3-ols after the intake of red wine(Reference Tsang, Higgings and Duthie77) and in the urinary excretion of resveratrol metabolites after the intake of red and white wine(Reference Sacanella, Vázquez-Agell and Mena17).
Vitamins C and E are plasma constituents that exhibit antioxidant capacity and whose plasma concentrations may be affected by polyphenol metabolism. Because of this, some intervention studies with antioxidants have evaluated the evolution of the concentration of these compounds.
In studies in animals, the intake of red wine and ethanol caused a significant increase in plasma vitamin C(Reference Cestaro, Simonetti and Cervato62) in rats, and concentrated grape pomace supplementation increased liver vitamin E(Reference Goñi, Brenes and Centeno95) in chickens, while dealcoholised red wine supplementation did not modify plasma levels of vitamins C and E in apoE-deficient hamsters(Reference Stocker and O'Halloran86).
In the case of human subjects, no modification in plasma antioxidant vitamin concentrations has been observed after the intake of white wine, red wine, dealcoholised red wine or grape procyanidins(Reference De Rijke, Demacker and Assen25, Reference Cacetta, Burke and Mori67, Reference Simonetti, Ciapellano and Gardana98). However, some interesting observations regarding vitamin E should be noted: the intake of red wine polyphenols increased vitamin E content in LDL by 15 %(Reference Carbonneau, Leger and Monnier32); the intake of grape procyanidins did not modify vitamin E levels in plasma, but did increase them in erythrocytes(Reference Simonetti, Ciapellano and Gardana98); although there was no increase in plasma vitamin C after the intake of concentrated grape juice, when its concentration was normalised dividing it between plasma cholesterol, there was a significant increase due to the treatment(Reference Castilla, Echarri and Davalos65). These data show that grape polyphenols do not have the same effect on the plasma levels of vitamin C and of vitamin E, and also that in the latter case polyphenols would contribute to its regeneration, as noted earlier(Reference Zhou, Wu and Yang106).
Hepatic enzymes
Aerobic organisms possess enzymic systems that counteract the effect of free radicals. The activity of these systems may be reduced as a consequence of stress situations, and increased after the intake of polyphenol-rich products. Some trials on animals to determine the effects of grapes and derived products have measured the activity of these enzymes.
Roig et al. (Reference Roig, Cascón and Arola101) observed an increase in the activities of superoxide dismutase and glutathione peroxidase in rats 45 d after supplementation with red wine; however, this was not sustained over 6 months of intake, at the end of which a reduction was observed in the levels of MDA in plasma and other tissues. The authors concluded that the intake of red wine initially activated antioxidant enzymes, but the long-term effect was to reduce lipid peroxidation. Similarly, red wine and ethanol supplementation caused an increase in the activities of glutathione peroxidase in the renal cortex and renal papilla of rats(Reference Rodrigo, Rivera and Orellana93). Also, an increase in hepatic glutathione peroxidase activity after the intake of grape pomace by rats was observed, although there was no change in any of the other hepatic enzymes, and there was a significant decrease in the activities of these enzymes in the erythrocytes. A similar tendency was observed for tomato and apple pomace(Reference Bobek, Ozdin and Hromadova26).
Effects on glycaemia
In a trial in fructose-fed rats, a model of insulin resistance without obesity, when the rats were supplemented with grape polyphenols, there was no change in plasma glucose or insulin, but there was a significant reduction in the homeostasis model assessment of insulin resistance (HOMA:ir) index, which measures insulin resistance(Reference Al-Awwadi, Bournet and Azay43).
As regards studies in human subjects, some trials have produced positive results. In those that reported no effect, this may be related to the design of the study. Williams et al. (Reference Williams, Sutherland and Whelan20) observed a decrease in plasma glucose 6 h after intake of red wine or white wine by coronary artery disease patients, and Gin et al. (Reference Gin, Rigalleau and Caubet107) reported that maximum glucose excursion after an acute intake of red wine or tannic acid (phenolic present in red wine) was significantly lower than after the intake of water or ethanol alone.
Two trials reported no effect on plasma glucose, insulin, insulin sensitivity or HOMA:ir after supplementation with red wine or dealcoholised red wine(Reference Cordain, Melby and Hamamoto66, Reference Naissides, Mamo and James69). However, one of these studies was performed in moderately obese women(Reference Cordain, Melby and Hamamoto66); the authors discussed the possibility of an interaction between the alcohol dose and the body composition that could have masked potential beneficial changes in insulin sensitivity, since it is known that the metabolic response of overweight subjects, in terms of glycaemia and insulin sensitivity, differs from the response of normal-weight subjects(Reference Gin, Rigalleau and Caubet107).
Other authors have suggested that, although polyphenols do not have a direct effect on postprandial glycaemia, they may affect other related parameters. For instance, Ceriello et al. (Reference Ceriello, Bortolotti and Motz100) found no difference in plasma glucose in type 2 diabetic patients after a meal accompanied by red wine as compared with a control group. Nevertheless, this study found that the intake of wine had a beneficial effect on postprandial oxidative stress, a key step in the generation of free radicals in diabetic patients(Reference Simonetti, Ciapellano and Gardana98).
Conclusions
From the in vivo studies performed during the last 13 years addressing the effects of grape products on different parameters related to CVD that were reviewed, the following effects have been observed:
(1) A hypotensive effect, observed in several studies in animals and in human subjects, mainly due to an increase in the release of NO.
(2) A hypolipidaemic effect, reducing levels of plasma total cholesterol, LDL-cholesterol and TAG. The results in human subjects suggest that these effects are more pronounced the higher the plasma lipids at baseline are. The hypolipidaemic effect of polyphenols would be related to the fact that these compounds may absorb cholesterol, bile acids and other dietary lipids and increase their faecal excretion.
(3) An anti-atherosclerotic effect in the early stages of development of atherosclerosis, observed as a reduction in atheromatous plaque and in LDL oxidation. This effect appears to be due to the inhibition of cell proliferation in smooth muscle and to antioxidant protection of LDL.
(4) An improvement in antioxidant status measured in terms of plasma antioxidant capacity, oxidation biomarkers, antioxidant compounds and antioxidant enzymes.
The presence of dietary fibre in some of the tested products may enhance some of the above effects.
Differences in the design of the studies and in the composition of the tested products (not always provided) can explain the different results produced by some of these assays. The relative contribution of major constituents such as polyphenols, ethanol and dietary fibre remains to be elucidated.
Acknowledgements
The present review was performed under the financial support of the Spanish Ministry of Education and Science (project AGL 2004-07579-C04-01/ALI). None of the authors had a conflict of interests. J. P.-J. and F. S.-C. prepared the manuscript.







