D-allulose, D-sorbose and D-tagatose are D-fructose isomers that are also called rare sugars because they are rarely found in nature(1). Among the rare sugars, D-allulose, D-sorbose, D-tagatose and D-allose have been studied intensively in terms of biological production and food application as well as physiological effects(Reference Chen, Chen and Zhang2–Reference Yamada, Hayashi and Iida17). D-allulose and D-tagatose have been approved as generally recognised as safe by the USA FDA. The EU introduced D-tagatose as a new food ingredient(Reference Levin18). Many kinds of rare sugar containing foods have already been marketed in several countries, including the USA, EU and Japan(Reference Armstrong, Luecke and Bell19–Reference Daniel, Hauner and Hornef21).
There are several papers with regard to the in vivo kinetics of these rare sugars. Urinary excretion of D-allulose after a single oral administration was 11 to 15 % in rats(Reference Matsuo, Tanaka and Hashiguchi22). Tsukamoto et al. reported that 33 % of orally administrated D-allulose was excreted in urine within 2 h in rats using 14C-labelled D-allulose(Reference Tsukamoto, Hossain and Yamaguchi23). The intestinal absorption rate of D-tagatose was estimated to be 20 % in rats using 14C-labelled D-tagatose(Reference Saunders, Zehner and Levin24). Normen et al. demonstrated that the apparent absorption of 15 g D-tagatose/d was 81 % in an ileostomy study(Reference Normen, Laerke and Jensen25). Urinary excretion of D-sorbose in humans was 24·9 %(Reference Kitagawa, Tanaka and Yoshikawa26). The urinary excretion rate of D-allose was reported to be 91·2 % and 67 % in rats and humans, respectively(Reference Kitagawa, Tanaka and Yoshikawa26,Reference Iga and Matsuo27) .
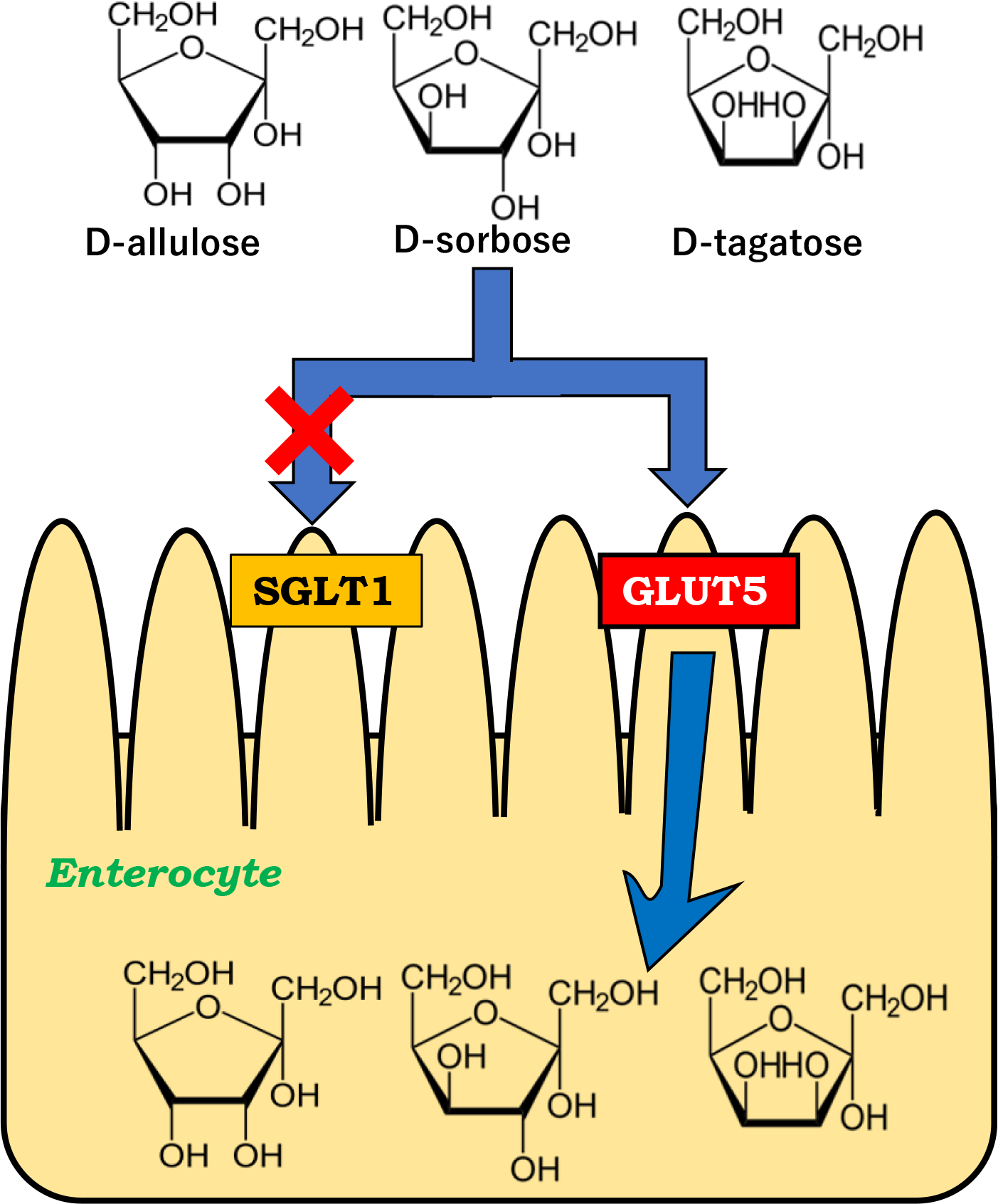
No transporters that mediate the intestinal absorption of rare sugars had been identified until we reported that glucose transporter type 5 (GLUT5) and sodium-dependent glucose cotransporter 1 (SGLT1) mediates intestinal D-allulose and D-allose transport, respectively(Reference Kishida, Martinez and Iida28,Reference Kishida, Iida and Yamada29) . It is essential to clarify which transporters are involved in the intestinal absorption of rare sugars to elucidate their physiological effects as functional food ingredients. In the present study, we focused on three types of D-fructose isomers, D-allulose, D-sorbose and D-tagatose and examined whether their intestinal absorption was mediated via GLUT5 and/or SGLT1 in rats.
Materials and methods
Chemicals
D-allulose, D-sorboseand D-tagatose were obtained from Matsutani Chemical Industry Co., Ltd. (Hyogo, Japan), and their purity was 99·5 %. All other chemicals were purchased from Sigma-Aldrich Co. LLC (St. Louis, MO, USA) or FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). KGA-2727, a selective SGLT1 inhibitor(Reference Shibazaki, Tomae and Ishikawa-Takemura30), was obtained from Kissei Pharmaceutical Co., Ltd. (Nagano, Japan).
Animals and feedings
Sixteen male Sprague–Dawley rats (5 weeks old) were purchased from Kiwa Laboratory Animals Co. Ltd. (Wakayama, Japan). Ten portal vein cannulated rats (8 weeks old, male, Sprague–Dawley) were purchased from Charles River Laboratories Japan, Inc. (Kanagawa, Japan). The rats were singly housed in cages and were allowed free access to a standard laboratory chow (MF, Oriental Yeast Co., Ltd.). The rats were maintained at 22°C ± 3°C on 12 h light and dark cycles. The present study was approved by the Animal Care Committee of Kindai University (permit number KABT-2022–005), and the animals were maintained in accordance with the guidelines.
Examination of intestinal absorption via glucose transporter type 5
The experimental condition was performed according to our previous report(Reference Kishida, Martinez and Iida28,Reference Kishida, Iida and Yamada29) . The rats were separated into two groups. One group (n 4) was given a high-fructose diet for 1 week to increase the abundance and activity of GLUT5 in the small intestine(Reference Kishida, Martinez and Iida28,Reference Kishida, Iida and Yamada29) . The other group (n 4) was given a high-glucose diet for 1 week and served as a control. The composition of diets is shown in Table S1 in Supplementary Material. Both groups of rats were fasted for 16 h and D-allulose, D-sorbose or D-tagatose was orally administered (2 g/kg body weight (BW)). The rare sugars were dissolved in Milli-Q water and administered at a volume of 10 ml/kg BW. Blood samples were taken from the tail vein and collected into heparinised tubes at 0, 30, 60, 90, 120 and 180 min after administration. The plasma sugar concentrations were determined by HPLC system (JASCO corporation) according to our previous publication(Reference Kishida, Martinez and Iida28,Reference Kishida, Iida and Yamada29) .
To verify the gene expression levels of GLUT5, glucose transporter type 2 (GLUT2) and SGLT1 in the small intestine, the rats were sacrificed by exsanguination from the abdominal aorta under isoflurane anesthesia after the experiment. The duodenum, jejunum and ileum were collected and subjected to RNA extraction using Qiazol and RNeasy Mini kit (Qiagen, Hilden, Germany) followed by quantitative reverse transcription PCR (RT-qPCR) using iScript™ Reverse Transcription Supermix for RT-qPCR and SsoFast™ EvaGreen® Supermix (BioRad, Hercules). The RT-qPCR was performed as described elsewhere(Reference Kishida, Martinez and Iida28).
Examination of intestinal absorption via sodium-dependent glucose cotransporter 1
The experimental condition was performed according to our previous report(Reference Kishida, Martinez and Iida28,Reference Kishida, Iida and Yamada29) . The normal rats (n 4, each) were fasted for 16 h, after which, each of the rare sugars (2 g/kg BW) with or without KGA-2727 (0·3 mg/kg BW) was orally administered. The cannulated rats (n 5, each) were fasted for 16 h, after which, each of the rare sugars (0·3 g/kg BW) with or without KGA-2727 (0·3 mg/kg BW) was orally administered. The rare sugars and KGA-2727 were dissolved in Milli-Q water and administered at a volume of 10 ml/kg BW. Blood samples were taken from the tail vein in the normal rats at 0, 30, 60, 90, 120 and 180 min after administration and from the portal vein in the cannulated rats at 0, 15, 30, 60 and 90 min after administration. Samples were collected into heparinised tubes. The plasma sugar concentrations were determined by HPLC system as described previously(Reference Kishida, Martinez and Iida28,Reference Kishida, Iida and Yamada29) .
Statistical analyses
All data were expressed as means ± se for each group. Statistical analysis was conducted using an unpaired Student’s t-test, and the data were analysed using IBM SPSS statistics software, version 19·0 (IBM Co.). P values of < 0·05 were considered statistically significant.
Results and discussion
mRNA expression of sugar transporters in the small intestine
We fed the rats a high-fructose diet to induce intestinal GLUT5 expression and enable examination of whether D-allulose, D-sorbose and D-tagatose are transported by GLUT5. mRNA expression of sugar transporters in the small intestine is displayed in Fig. 1. The rats fed the high-fructose diet showed significantly higher GLUT5 mRNA expression in the duodenum, jejunum and ileum while GLUT2 and SGLT1 mRNA expressions were not changed compared with those in the rats fed the high-glucose diet. The abundance of GLUT5 mRNA expression indicates that sugar transport mediated by GLUT5 is enhanced, as reported previously(Reference Ferraris, Choe and Patel31,Reference Patel, Douard and Yu32) . Thus, the experiments were performed under the condition that the rats fed the high-fructose diet exhibited enhanced transport by GLUT5.

Fig. 1. Relative gene expression of sugar transporters in the duodenum, jejunum and ileum in the rats fed the high-glucose or high-fructose diet. Values are expressed as means ± se (n 4). All samples were standardised to b-actin expression. Each gene expression was normalised to that in the glucose-fed rats. Statistical analyses were performed using an unpaired Student’s t test. *P < 0·05, ***P < 0·001 compared with each control.
Intestinal absorption of D-allulose, D-sorbose and D-tagatose via glucose transporter type 5
Figure 2 and Table 1 show the peripheral concentrations and pharmacokinetic parameters of D-allulose, D-sorbose and D-tagatose in the glucose-fed and fructose-fed rats orally administered each rare sugar alone (2 g/kg BW). D-allulose levels were significantly higher at 30, 60, 90 and 120 min in the fructose-fed rats than those in the glucose-fed rats (Fig. 2(a)). Cmax and AUC0–180 min values were significantly higher in the fructose-fed rats than those in the glucose-fed rats (2·98 ± 0·36 mM v. 1·52 ± 0·22 mM, 375 ± 46 mM·min v. 194 ± 26 mM·min, respectively, Table1). These results are in agreement with those of our previous study(Reference Kishida, Martinez and Iida28). Similarly, D-sorbose levels are significantly higher at 30, 60, 90 and 120 min in the fructose-fed rats than those in the glucose-fed rats (Fig. 2(b)). Cmax and AUC0–180 min values were significantly higher in the fructose-fed rats than those in the glucose-fed rats (2·63 ± 0·18 mM v. 1·11 ± 0·037 mM, 304 ± 21 mM·min v. 146 ± 6·3 mM·min, respectively, Table 1). Tmax value was 67·5 ± 7·5 min in the fructose-fed rats, which was significantly lower than that in the glucose-fed rats (Table 1). D-tagatose levels are significantly higher at 30 and 60 min in the fructose-fed rats than those in the glucose-fed rats (Fig. 2(c)). Cmax and AUC0–180 min values were significantly higher in the fructose-fed rats than those in the glucose-fed rats (0·357 ± 0·010 mM v. 0·224 ± 0·0086 mM, 40·2 ± 1·8 mM·min v. 29·5 ± 3·8 mM·min, respectively, Table 1). These results show that more D-allulose, D-sorbose and D-tagatose were rapidly absorbed via highly expressed GLUT5 in the fructose-fed rats, suggesting that GLUT5 likely mediates the intestinal absorption of these rare sugars. Cmax and AUC0–180 min values of D-tagatose were lower than those of D-allulose and D-sorbose. Previous studies reported that urinary excretion rates of D-allulose, D-sorbose and D-tagatose were similar and range from approximately 10 to 30 % although the value of D-sorbose was obtained from humans(Reference Matsuo, Tanaka and Hashiguchi22–Reference Saunders, Zehner and Levin24,Reference Kitagawa, Tanaka and Yoshikawa26) . The reason for the lower plasma concentrations, Cmax and AUC0–180 min values of D-tagatose could be that D-tagatose is catabolised in the same way as D-fructose in contrast to D-allulose and D-sorbose(Reference Guerrero-Wyss, Duran Aguero and Angarita Davila3,Reference Roy, Chikkerur and Roy14) . Since GLUT5 mRNA and protein expressions are upregulated by the GLUT5-mediated transport and ketohexokinase-mediated phosphorylation of D-fructose(Reference Patel, Douard and Yu32), intake of D-allulose, D-sorbose and D-tagatose may induce GLUT5 expression, which needs to be elucidated.

Fig. 2. Peripheral concentrations of D-allulose (a), D-sorbose (b) and D-tagatose (c) in rats orally administered each rare sugar alone (2 g/kg BW) in rats fed the high-glucose or high-fructose diet. Values are expressed as means ± se (n 4). Statistical analyses were performed using an unpaired Student’s t test. *P < 0·05, **P < 0·01, ***P < 0·001 compared with each control.
Table 1. Pharmacokinetic parameters of D-allulose, D-sorbose and D-tagatose in rats orally administered each rare sugar alone (2 g/kg BW or 0·3 g/kg BW) under GLUT5 induction or SGLT1 inhibition

Values are expressed as means ± se (n 4 or 5). Cmax: maximum plasma concentration, Tmax: time to reach Cmax.
AUC: area under the plasma concentration–time curve in 180 min (peripheral) or 90 min (portal).
Statistical analyses were performed using an unpaired Student’s t test.
* P < 0·05.
** P < 0·01.
*** P < 0·001 compared with each control.
Figure 3 shows the peripheral D-glucose concentrations when these rare sugars were orally administrated. After D-allulose administration, the D-glucose level at 30 min was significantly higher in the fructose-fed rats than that in the glucose-fed rats (Fig. 3(a)). The reason for the increased glucose level remains unclear since D-allulose is not a substrate for gluconeogenesis(Reference Iida, Hayashi and Yamada33,Reference Matsuo, Suzuki and Hashiguchi34) . D-glucose levels did not differ between the diet groups after D-sorbose administration (Fig. 3(b)). To the best of our knowledge, D-sorbose has never been examined as a substrate for gluconeogenesis, while L-sorbose, the C-5 epimer of fructose, is partly metabolised like fructose(Reference Burns, Mosbach and Schulenberg35). The result suggests that D-sorbose does not produce energy directly like D-allulose. When D-tagatose was administrated, D-glucose levels were significantly higher at 30, 60, 90 and 120 min in the fructose-fed rats than those in the glucose-fed rats (Fig. 3(c)). This means that the fructose-fed rats absorbed more D-tagatose, which induced gluconeogenesis because D-tagatose is known to be a gluconeogenic substrate(Reference Rognstad36,Reference Rognstad37) .

Fig. 3. Peripheral D-glucose concentrations after oral administration of D-allulose (a), D-sorbose (b) and D-tagatose (c) (2 g/kg BW) in rats fed the high-glucose or high-fructose diet. Values are expressed as means ± se (n 4). Statistical analyses were performed using an unpaired Student’s t test. *P < 0·05, **P < 0·01, ***P < 0·001 compared with each control.
D-fructose is a physiological substrate for GLUT5. D-allulose and D-tagatose are C3- and C4-epimers of D-fructose, respectively. D-sorbose is a C-3 and C-4 diastereomer of D-fructose. From this structural similarity, it is conceivable that these rare sugars are transported via GLUT5 in the small intestine. Our previous study has revealed that intestinal D-allose transport was not changed in the fructose-fed rats compared with the glucose-fed rats(Reference Kishida, Iida and Yamada29), indicating that D-allose is a C3-epimer of D-glucose and thus did not become a substrate for GLUT5 due to the difference in chemical structure.
No effect of sodium-dependent glucose cotransporter 1 inhibitor on intestinal absorption of D-allulose, D-sorbose and D-tagatose
Figure 4 shows the peripheral and portal concentrations of D-allulose, D-sorbose and D-tagatose in the normal and cannulated rats, respectively. Table 1 shows the pharmacokinetic parameters. The rats were orally administered each rare sugar alone (2 g/kg BW or 0·3 g/kg BW) with or without KGA-2727, a selective SGLT1 inhibitor (0·3 mg/kg BW). In the normal rats, no significant differences in the peripheral concentrations were observed between the groups after administration of D-allulose, D-sorbose and D-tagatose (Fig. 4(a)–(c)). We further examined the intestinal absorption of these rare sugars using portal vein cannulated rats. KGA-2727 did not block the increase in portal vein concentrations of D-allulose, D-sorbose and D-tagatose, as shown in Fig. 4(d)–(f). As shown in Table 1, Cmax, Tmax and AUC0–90 min values of D-allulose, D-sorbose and D-tagatose did not change compared with those in each control in both the normal and cannulated rats. These results suggest that D-allulose, D-sorbose and D-tagatose are not likely transported via SGLT1 in rat small intestine.

Fig. 4. Peripheral (a)–(c) and portal (d)–(f) concentrations of D-allulose (a), (d), D-sorbose (b), (e) and D-tagatose (c), (f) in rats orally administered each rare sugar alone (2 g/kg BW or 0·3 g/kg BW) with or without KGA-2727 (0·3 mg/kg BW). Values are expressed as means ± se (n 4 or 5).
The physiological substrates for SGLT1 are D-glucose and D-galactose. SGLT1 also shows transport activity for 3-O-methyl-D-glucoside and α-methyl-D-glucopyranoside(Reference Wright, Loo and Hirayama38). We have previously reported that KGA-2727 completely blocked D-allose absorption, suggesting that D-allose absorption is mediated by SGLT1(Reference Kishida, Iida and Yamada29).
Conclusions
We have demonstrated that the D-fructose isomers, D-allulose, D-sorbose and D-tagatose, are transported via GLUT5 but not SGLT1 in rat small intestine. The strength of this study is the application of KGA-2727. Although phlorizin is a commonly used SGLT1 inhibitor, it is hydrolysed to phloretin and inhibits the transport by GLUT2 expressed on the basolateral membrane(Reference Blaschek39). Therefore, phlorizin administration can create a condition in which both SGLT1 and GLUT2 are inhibited, which prevents the examination of the absorption via SGLT1. A selective SGLT1 inhibitor, KGA-2727, allowed us to evaluate the transport of rare sugars by SGLT1. A different approach using SGLT1-KO mice would confirm and strengthen the findings. There are some limitations for the examination of absorption by GLUT5. We induced intestinal GLUT5 by feeding a high-fructose diet. Since a high-fructose diet can induce adverse metabolic effects such as insulin resistance and hypertriglyceridaemia, sugar absorption in the small intestine could be somewhat altered. The use of GLUT5-KO mice and/or GLUT5-specific inhibitors (if available) would advance our findings.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0007114523001113
Acknowledgements
The authors are grateful to Kissei Pharmaceutical Co., Ltd. for providing KGA-2727.
K. K.: Conceptualisation, methodology, software, validation, Ddata curation, formal analysis, investigation, validation, visualisation, writing – original draft, supervision. T. I.: Validation, resources, writing – review and editing. T. Y.: Validation, resources, writing – review and editing. Y. T.: Methodology, software, validation, data curation, formal analysis, investigation, validation, writing – review and editing.
The authors declare the following financial interests/personal relationships that may be considered as potential competing interests: T. Iida and T. Yamada are employed by Matsutani Chemical Industry Co., Ltd. This work was partly supported by funding from Matsutani Chemical Industry Co., Ltd.