Evidence exists for an increased incidence of aggressive behaviour in schizophrenia, estimated at two to 10 times that of the general population (Reference Hafner and BokerHafner & Boker, 1982; Reference WesselyWessely, 1997). Causes of aggressive behaviour are complex and multi-factorial, but studies by Strous et al (Reference Strous, Bark and Parsia1997) and Lachman et al (Reference Lachman, Nolan and Mohr1998) reported associations between aggression in schizophrenia and the lowactivity allele of the catechol-O-methyl-transferase (COMT) gene, whereas Kotler et al (Reference Kotler, Barak and Cohen1999) found a non-significant trend in the same direction. The COMT gene inactivates catecholamines (including noradrenaline and dopamine) and a common polymorphism at this gene results in a three— to four-fold difference in enzyme activity. These differences are due to a G to A transition that results in a valine to methionine substitution, forming corresponding high— and low-activity enzyme variants (Reference Lachman, Papolos and SaitoLachman et al, 1996). In this study we investigate the association between COMT genotype and aggression in schizophrenia using a larger sample than previous studies, and using the Overt Aggression Scale (OAS; Reference Yudofski, Silver and JacksonYudofski et al, 1986) as an objective measure of the degree of aggression.
METHOD
Subjects
The sample consisted of 180 unrelated individuals diagnosed as having DSM—IV schizophrenia (American Psychiatric Association, 1994). All patients were Caucasian, with both parents born in the UK or Eire. The sample was recruited from both community and hospital settings and consisted of 136 (75.6%) males and 44 (24.4%) females. Patients were assessed either by a psychiatrist or a psychologist trained in using the Schedules for Clinical Assessment in Neuropsychiatry (SCAN; Reference Wing, Babor and BrughaWing et al, 1990), which is a semi-structured diagnostic interview. Diagnoses were made by consensus of two independent raters, based on all available clinical information including the SCAN interview, examination of case records and information from relatives and mental health professionals. All subjects satisfied DSM—IV criteria for a consensus lifetime diagnosis of schizophrenia. The duration of illness for each individual was recorded and was a measure of the period between first contact with psychiatric services and date of SCAN interview. In view of the known association between substance misuse and aggressive behaviour (Krakowski, 1986; Rasanen, 1998), we also recorded a lifetime history of alcohol and substance misuse in these individuals.
Controls
We also genotyped 173 unaffected controls to establish COMT genotype distribution in the general population. The controls were recruited from volunteers attending the Blood Transfusion Service donor sessions in South Wales, and were matched for age, gender and ethnicity.
Measurements
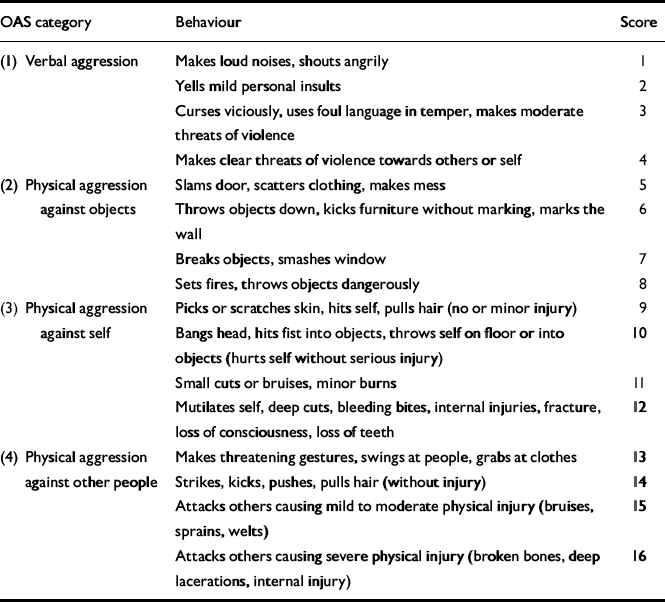
The OAS was used to rate episodes of aggression into four main categories representing escalating violent behaviour (Table 1). This scale was designed initially for rating episodes of in-patient aggression and has been shown to have an intraclass correlation coefficient of 0.87 (Reference Yudofski, Silver and JacksonYudofski et al, 1986).
Table 1 The Overt Aggression Scale (OAS), which rates the degree of aggression into four main categories
| OAS category | Behaviour | Score |
|---|---|---|
| (1) Verbal aggression | Makes loud noises, shouts angrily | 1 |
| Yells mild personal insults | 2 | |
| Curses viciously, uses foul language in temper, makes moderate threats of violence | 3 | |
| Makes clear threats of violence towards others or self | 4 | |
| (2) Physical aggression against objects | Slams door, scatters clothing, makes mess | 5 |
| Throws objects down, kicks furniture without marking, marks the wall | 6 | |
| Breaks objects, smashes window | 7 | |
| Sets fires, throws objects dangerously | 8 | |
| (3) Physical aggression against self | Picks or scratches skin, hits self, pulls hair (no or minor injury) | 9 |
| Bangs head, hits fist into objects, throws self on floor or into objects (hurts self without serious injury) | 10 | |
| Small cuts or bruises, minor burns | 11 | |
| Mutilates self, deep cuts, bleeding bites, internal injuries, fracture, loss of consciousness, loss of teeth | 12 | |
| (4) Physical aggression against other people | Makes threatening gestures, swings at people, grabs at clothes | 13 |
| Strikes, kicks, pushes, pulls hair (without injury) | 14 | |
| Attacks others causing mild to moderate physical injury (bruises, sprains, welts) | 15 | |
| Attacks others causing severe physical injury (broken bones, deep lacerations, internal injury) | 16 |
Each category (labelled OAS 1, 2, 3 and 4) represents a graded spectrum of aggression consisting of: verbal aggression (OAS 1, score 1-4), physical aggression against objects (OAS 2, score 5-8), physical aggression against self (OAS 3, score 9-11) and physical aggression against other people (OAS 4, score 12-16). Each episode of aggression was given a rating between 1 and 16, and the category of aggression (OAS 1-4) also was recorded. All episodes of aggression since the onset of schizophrenia were rated. A record was made of:
-
(a) total OAS score (sum of the scores of all episodes of aggression);
-
(b) highest OAS score (highest individual episode score, 0-16);
-
(c) the OAS category (the percentage of people scoring within each category);
-
(d) number of episodes (none, single or multiple episodes of aggression).
Two raters blind to the genotype carried out the ratings of aggression. Interrater reliability was assessed on 20 cases. The intraclass correlation for total OAS score was 0.92.
The genotyping was done by polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP) assay, and was performed blind to history of aggressive behaviour.
Statistics
Analysis was performed using the Statistical Package for the Social Sciences (SPSS). Kruskal—Wallis (a non-parametric analysis of variance) and χ2 contingency analyses were applied to the OAS distributions. The power of this study to detect an odds ratio of 2 for aggressive behaviour between the low-activity homozygote and the other genotypes was 0.8.
RESULTS
There were 136 males and 44 females in this study. The frequencies of the high-activity homozygote, heterozygote and low-activity homozygote genotypes in males were 25%, 48% and 27%, respectively, whereas for females the frequencies were 20.5%, 59% and 20.5%. There was no significant difference in the distribution of genotypes between males and females: χ 2=1.72, d.f.=1, P=0.423.
The mean duration of illness for the whole sample was 19.88 years (s.d.=12.27). There was no significant difference in duration of illness compared across the genotypes (Kruskal—Wallis test: χ 2=4.461, d.f.=1, P=0.107) or across gender (Kruskal—Wallis test: χ2=0.001, d.f.=1, P=0.969). There was no significant association between genotype and alcohol misuse (χ2=0.735, d.f.=2, P=0.963) or substance misuse (χ2=1.830, d.f.=2, P=0.400).
The mean total OAS was higher in males than females (19.3 v. 9.2), and this difference was statistically significant (Kruskal—Wallis: χ 2=5.921, d.f.=2, p=0.015). The mean highest OAS scores for males and females were 7.7 and 5.8, respectively. For purposes of the analysis, the highest OAS scores (0-16) were divided into four categories (0-4, 5-8, 9-12, 13-16). Analysis of the highest OAS by gender showed no ordinal by ordinal association: Kendall's tau-b=-0.089, P sim=0.204 (P value obtained from 106 Monte-Carlo simulations).
The percentage of males having recorded episodes of verbal aggression (OAS category 1) was 52%, aggression against objects (OAS category 2) was 39%, aggression against self (OAS category 3) was 23% and aggression against other people (OAS category 4) was 39%. The corresponding percentages for females were 46%, 25%, 9% and 34%, and there was no significant association between gender and OAS category scores (χ2=2.55, d.f.=3, P=0.473).
The percentage of males having no recorded episodes of aggression was 23%, those having only one recorded episode was 26% and those having more than one episode was 52%. The corresponding percentages for females were 36%, 30% and 34%, and there was no significant association between gender and number of episodes (χ2=3.45, d.f.=3, P=0.36).
Out of our sample of 180 individuals, 43 (24%) were high-activity homozygote genotype, 91 (50%) were heterozygotes and 46 (26%) were low-activity homozygotes (see Table 2). The distribution of genotypes was in Hardy—Weinberg equilibrium (χ2=0.02, d.f.=1, P=0.88). Within our unaffected control group, 37 (21%) were high-activity homozygote genotype, 84 (49%) were heterozygotes and 52 (30%) were low-activity homozygotes. There was no significant difference in genotype frequencies between our sample group and our control group (χ2=0.121, d.f.=2, P=0.94).
Table 2 Distribution of Overt Aggression Scale (OAS) scores and genotype (n=180)

| Genotype | n (%) | Total OAS mean (range) [95% Cl] | Highest OAS mean (range 1-16) | OASI (%) | OAS 2 (%) | OAS 3 (%) | OAS 4 (%) | Violence | ||
|---|---|---|---|---|---|---|---|---|---|---|
| None (%) | Single (%) | Multiple (%) | ||||||||
| 1-1 (high) | 43 (24) | 23.51 (0-95) [15.9-31.1] | 9.1 | 20 (46.5) | 19 (44.2) | 11 (25.6) | 22 (51.2) | 10 (23.3) | 8 (18.6) | 25 (58.1) |
| 1-2 | 91 (50) | 12.32 (0-140) [8.2-16.4] | 6.0 | 48 (52.7) | 27 (29.7) | 14 (15.4) | 28 (30.8) | 26 (28.6) | 30 (33) | 35 (38.4) |
| 2-2 (low) | 46 (26) | 19.7 (0-92) [12.8-26.5] | 7.9 | 23 (50.0) | 18 (39.1) | 10 (21.7) | 18 (39.1) | 11 (23.9) | 10 (21.7) | 25 (54.4) |
| Total | 180 | 16.9 (0-140) [13.6-20.1] | 7.2 | 91 (50.6) | 64 (35.6) | 35 (19.4) | 68 (37.8) | 47 (26.1) | 48 (26.7) | 85 (47.2) |
The mean total OAS score for the high-activity homozygote was 23.5 (range 0-95), for the heterozygote was 12.3 (range 0-140) and for the low-activity homozygote was 19.7 (range 0-92). There was a statistically significant effect of genotype on total OAS score (Kruskal—Wallis: χ2=8.31, d.f.=2, P=0.016). When the genotype groups were compared individually using Dunn's multiple comparison procedure, the only significant difference was between the high-activity homozygote and the heterozygote (P=0.025).
Comparison of total OAS scores between the heterozygotes versus both high— and low-activity homozygotes grouped together resulted in significantly lower aggression scores (Kruskal—Wallis: χ2=7.682, d.f.=1, P=0.006). When the high-activity homozygotes were compared with the two other genotypes, a significantly higher total OAS score was seen (χ2=4.939, d.f.=1, P=0.026). This indicated a statistically significant association between the high-activity COMT homozygote and higher total OAS scores. This mainly reflected the difference between the high-activity homozygote and the heterozygote genotypes.
Analysis of allelic association with total OAS score was non-significant (Kruskal—Wallis: χ2=0.521, d.f.=1, P=0.471).
The association between COMT genotype and highest OAS score also was investigated. The mean highest OAS score for the high-activity homozygote was 9.1, for the heterozygote was 6.0 and for the low-activity homozygote was 7.2. Analysis of highest OAS score against genotype using an ordinal by ordinal association test was non-significant (Kendall's tau-b=-0.59, P sim=0.376). There was no association between genotype and ratings for OAS category or number of episodes.
Logistic regression was used to see if OAS category could predict the genotype. The item that best predicted the high-activity genotype was OAS 4: physical aggression against other people (P=0.04). The odds ratio for aggression against others (OAS 4) comparing the high-activity homozygote with the other genotypes was 2.07, with a 95% CI of 1.03-4.15. The odds ratio for aggression against others (OAS 4), comparing the heterozygote with the other genotypes, was 0.54, with a 95% CI of 0.30-1.00.
When analysing the genders separately, the association between genotype and total OAS score remained statistically significant in males (Kruskal—Wallis: χ2=9.346, d.f.=2, P=0.009) and comparing the high-activity variant with other genotypes in males resulted in χ 2=3.818, d.f.=1 and P=0.051. There were no significant associations found between genotype and either highest OAS, OAS category or number of episodes in males. For females no significant difference was found between genotype and any of the measures of aggression.
DISCUSSION
Main findings
The results from our study suggest an association between the high-activity COMT homozygote and an increased reported rate of aggression in schizophrenia. The significant association between genotype and total OAS score is not present in females alone, but remains significant for males. This may be owing to lack of power, given the smaller number of females in our sample.
It is interesting that a significant difference was found between the homozygote high-activity genotype and the heterozygote, but not between the high— and low-activity homozygotes. If the high-activity genotype is indeed a risk factor for aggression, we would expect individuals with two copies of this allele to have a higher risk than those with one copy, and an even higher risk relative to those with none. One possible explanation of our findings is that heterozygosity confers some kind of protection against aggressive behaviour. This would be an example of the phenomenon of heterosis that has been observed in other situations (Reference Falconer and MackayFalconer & Mackay, 1996). The lack of a significant allelic association with total OAS score in our study lends further support to the possible existence of heterosis.
Comparison with previous studies
Our results apparently contradict the findings of previous authors. Lachman et al (Reference Lachman, Nolan and Mohr1998) studied a sample of patients with DSM—IV schizophrenia or schizoaffective disorder and compared those with a history of multiple physical assaults against those with no history of violence, based on a review of all the information available. Any subjects showing intermediate levels of violence were excluded from the study, resulting in a sample size of 55. Their results showed a significant association between homozygosity for the low-activity COMT genotype and the violent group. Strous et al (Reference Strous, Bark and Parsia1997) studied a sample of 37 patients of mixed ethnicity and found that low-activity homozygotes were more likely to be judged by their psychiatrist to be at a higher risk for aggressive and dangerous behaviour. Kotler et al (Reference Kotler, Barak and Cohen1999) compared the COMT genotype across three groups: a group with schizophrenia who had committed homicide (n=30), a group with schizophrenia who were non-violent (n=62) and a non-violent unaffected control group (n=415). They found a significant excess of the low-activity genotype in the homicidal schizophrenia group compared with the controls, but the difference between the homicidal and non-violent schizophrenia groups did not achieve statistical significance.
There are several reasons why our findings may appear to contradict those reported previously. First, the different studies have taken samples from very different patient groups with different levels of aggressive behaviour. It is perhaps of note that two of the studies reporting associations with the low-activity genotype studied patients with extreme levels of violence (homicide and multiple history of physical assault), whereas our patients were unselected for forensic history and were rated across a wide spectrum of aggressive behaviour. It is possible therefore that whereas heterozygosity reduces the risk of aggressive behaviour, as indicated by our study, low-activity homozygotes are at greatest risk of extreme aggression, a finding that our study did not have significant power to detect. Second, the results of previous studies may have been false positives owing to small sample size and/or population stratification due to poor ethnic matching of cases and controls.
Finally, given the difficulties in interpreting the results of genetic association studies, the conflict between our findings and those of other studies may well reflect the fact that no real relationship exists between aggressive behaviour and COMT genotype in schizophrenia.
Ethical issues and future implications
There are a number of ethical issues raised by findings such as these, which are worthy of wider debate. The discovery of any risk factors for aggression might have implications for clinical management and detention of patients in the future. It is widely known that prediction of aggressive behaviour is, at best, little better than chance (Reference MonahanMonahan, 1984). There are advocates of an actuarial approach involving the use of statistically weighted risk pro formas, and the genotype of an individual (being a fixed biological marker) potentially may be of use in this approach. However, it should be noted that our results are preliminary and need to be replicated, given that our findings differ from those of previous studies and given the high rate of false positive results in genetic studies. Also, even if our result can be confirmed, the effective size is relatively small and it is unlikely to be useful for predictive testing. The public view of people with mental illness is of individuals affected by loss of reason, unaccountable behaviour and dangerousness (Reference Lawrie, Martin and McNeilLawrie et al, 1998). Those with mental illness are subject to stigmatisation and results such as these may serve to increase that stigmatisation, although clearly it is not our intention to do so. Indeed, COMT genotype may be a risk factor for aggression in the general population and not just in those with schizophrenia.
Replicated findings of genetic associations are necessary steps towards determining the aetiology of schizophrenia and towards understanding the behaviours such as aggression that are associated with this disorder. Catechol-O-methyltransferase is an enzyme that is involved in the degradation of catecholamines such as dopamine, noradrenaline and adrenaline. It is known that catecholamines have an effect on the limbic system and variation in COMT activity may be one of the mechanisms mediating aggressive behaviour. Indeed, a mutation in the gene coding for monoamine oxidase A (an enzyme also involved in the metabolism of catecholamines) has been associated with aggressive and impulsive behaviour in a large Dutch kindred (Reference Brunner, Nelen and BreakefieldBrunner et al, 1993), lending further support for the theory that catecholamines may play an important role in this behaviour. However, translating functional studies of polymorphisms associated with aggression into the mechanism that brings about this behaviour is clearly complex and likely to involve several interactions, with other genes as well as with environmental factors. An understanding of these processes also will be essential for the future development of pharmacological agents that might influence phenotypic variables associated with schizophrenia, including that of aggressive behaviour.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
• There is current political interest in identifying people who are at risk of aggressive behaviour. This association could be regarded as a potential biological marker of aggression.
-
• If catechol-O-methyltransferase (COMT) is indeed involved in determining risk of aggression, then further investigation of this biochemical pathway would potentially allow the development of pharmacological interventions in the future.
-
• These findings question to what degree aggressive behaviour is attributable to biological rather than non-biological factors. There may be wider ethical and forensic implications if this association between COMT genotype and aggression were replicated within the general population.
LIMITATIONS
-
• It is likely that there is an underreporting of aggression in this study. However, each of the genotype groups would have been subject to this bias.
-
• Recruitment of the sample from hospitals and the community probably has excluded those who are seriously violent, because it is likely that they would be either in a secure treatment facility or within the penal system.
-
• More work needs to be done in examining rates of aggression in relation to COMT genotype, both in other psychiatric disorders and in the general population, before further inferences can be drawn.
Acknowledgement
This work was supported by a Medical Research Council programme grant.





eLetters
No eLetters have been published for this article.