Introduction
Populations of most vulture species in Africa are in rapid decline, with six of the nine species found on the continent currently categorized as Endangered or Critically Endangered on the IUCN Red List (BirdLife International, 2015). The consequences of these declines for ecosystems are potentially significant because vultures are one of the most important groups of scavengers and play a major role in the consumption of dead animals (Mundy et al., Reference Mundy, Butchart, Ledger and Piper1992), which reduces the potential for disease transmission (Ogada et al., Reference Ogada, Torchin, Kinnaird and Ezenwa2012).
Two major causes of these declines are the intentional and unintentional poisoning of vultures and the unsustainable harvesting of birds for cultural beliefs and the trade in fetish (Ogada et al., Reference Ogada, Shaw, Beyers, Buij, Murn and Thiollay2016b). Vultures are obligate scavengers and are therefore vulnerable to poisoning, particularly at carcasses that are laced with poison deliberately to kill terrestrial carnivores, such as black-backed jackals Canis mesomelas, lions Panthera leo and spotted hyaenas Crocuta crocuta (Kendall & Virani, Reference Kendall and Virani2012; Ogada, Reference Ogada2014), illegally. The gregarious feeding habitats of Gyps vultures in particular, whereby hundreds of individuals can congregate at large carcasses such as those of elephants (Mundy et al., Reference Mundy, Butchart, Ledger and Piper1992), make them susceptible to mass mortalities from poisoned carcasses. In addition to the threat from poisoning, cultural beliefs in many parts of Africa ascribe certain properties (e.g. clairvoyance, good luck and good vision) to vulture body parts (Saidu & Buij, Reference Saidu and Buij2013). As a result, vulture body parts are often in demand and this puts wild populations under additional pressure from unsustainable harvesting (Nikolaus, Reference Nikolaus2001; Groom et al., Reference Groom, Gandiwa, Gandiwa and van der Westhuizen2013; McKean et al., Reference McKean, Mander, Diedrichs, Ntuli, Mavundla, Williams and Wakelin2013). Vultures are long-lived birds with high annual survival of adult age classes (Piper et al., Reference Piper, Boshoff and Scott1999; Monadjem et al., Reference Monadjem, Botha and Murn2013; Chantepie et al., Reference Chantepie, Teplitsky, Pavard, Sarrazin, Descaves, Lecuyer and Robert2016); additional mortality, particularly of adult age classes, has potentially serious negative effects on the demographics of populations with these characteristics (Bennett & Owens, Reference Bennett and Owens1997; Sæther & Bakke, Reference Sæther and Bakke2000).
Vulture mortalities resulting from poisoned elephant carcasses have increased dramatically since 2012 (Ogada et al., Reference Ogada, Shaw, Beyers, Buij, Murn and Thiollay2016b). With a concomitant increase in elephant poaching over the previous decade (Bennett, Reference Bennett2015; Booth & Dunham, Reference Booth and Dunham2016) the illegal ivory trade and poaching of elephants has seen the development of a major threat to vultures and other scavengers: vultures being targeted deliberately by elephant poachers in an attempt to prevent the circling birds alerting authorities to the presence of poachers (Ogada et al., Reference Ogada, Botha and Shaw2016a).
Since early 2015 poisoned carcasses have been used at least three times by poachers to deliberately target and kill vultures in or immediately adjacent to Kruger National Park, the largest national park in South Africa, located in the north-east of the country. Sixty-five vultures are known to have been killed in May 2015 (Botha, Reference Botha2015), 46 in September 2015 (Green, Reference Green2015) and 110 in February 2016 (SANParks, 2016). The first event was suspected to be related primarily to bushmeat poaching, whereas the second was aimed at harvesting vultures. The third event involved a poached elephant Loxodonta africana laced with poison and intended to kill vultures. However, the carcass was discovered and the poison neutralized by field staff (SANParks, 2016), thus minimizing vulture deaths, which can number > 300 at poisoned elephant carcasses that go undiscovered by field staff or rangers (McNutt & Bradley, Reference McNutt and Bradley2014).
As individual events these are all significant mortalities, and such events are not a recent phenomenon; poisoning at various levels of intensity has affected vultures in and around the Park for several decades (van Jaarsveld, Reference van Jaarsveld1986, Reference van Jaarsveld1987; Butchart, Reference Butchart1987). In the context of Kruger National Park such events are extremely serious because the Park contains regionally and internationally important breeding populations of the Critically Endangered white-backed Gyps africanus and white-headed vultures Trigonoceps occipitalis, the Endangered lappet-faced vulture Torgos tracheliotos (Murn et al., Reference Murn, Combrink, Ronaldson, Thompson and Botha2013) and the Critically Endangered hooded vulture Necrosyrtes monachus (Allan, Reference Allan, Taylor, Peacock and Wanless2015; BirdLife International, 2015).
The population-level impact of poison-related mortalities is poorly understood for vultures in Africa. Here we utilize demographic parameters of the white-backed vulture, the most widespread African vulture species, to examine the impacts of poison-related mortalities of various intensities on the persistence of the globally important population of this species breeding in Kruger National Park. We also assess the effects of focused poison response activities on reducing vulture mortalities, and population persistence. Although all vultures feed at large animal carcasses to a lesser or greater extent, we selected the white-backed vulture for analysis because it is the most gregarious species and is well known as a medium- to large-sized carcass specialist (Mundy et al., Reference Mundy, Butchart, Ledger and Piper1992). The species’ feeding ecology has led to it being the most affected by poisoning events.
Methods
We used the population viability analysis software VORTEX v. 10.1.5.0 (Lacy & Pollak, Reference Lacy and Pollak2014) to examine the persistence of the white-backed vulture population in Kruger National Park under various scenarios. VORTEX is a simulation environment that models the effects of deterministic and stochastic (random) forces on wildlife populations and the effects of various extinction vortices that threaten small populations (Lacy, Reference Lacy1993). The scenarios we developed examined the impacts of poison-related mortalities of varying severity on a vulture population of known size and structure.
Population size and structure
We used existing estimates of the breeding population of white-backed vultures in the Park (c. 900 pairs; Murn et al., Reference Murn, Combrink, Ronaldson, Thompson and Botha2013; Murn & Botha, Reference Murn and Botha2016) and added 0.33 additional immature and non-breeding individuals per breeding adult (Mundy et al., Reference Mundy, Butchart, Ledger and Piper1992) to calculate a starting population size of 2,400. Age structure (proportion of juveniles, subadults and adults) was determined based on roadside abundance data of white-backed vultures collected in the Park during 2008–2015 (authors, unpubl. data). The demographic parameters used in the simulations are in Table 1.
Table 1 Demographic parameters of white-backed vultures Gyps africanus used in population viability analysis modelling.
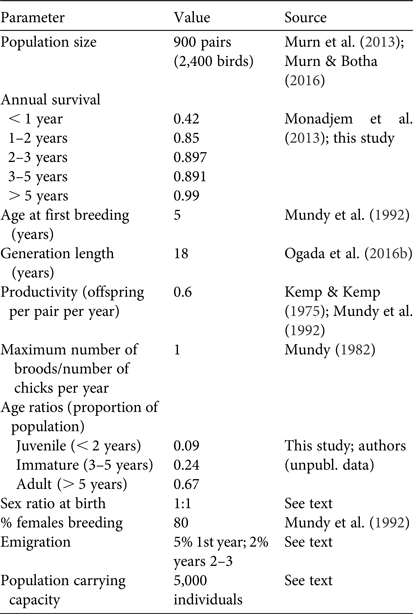
White-backed vultures are sexually monomorphic and the population sex ratio cannot be estimated from field observations; therefore, we assumed an equal sex ratio for the population, and also that the sex ratio at birth was equal. We assumed that land use and habitat availability within the Park remained constant during the simulation period (i.e. no habitat loss, no increased human presence).
Vultures are highly mobile, in particular immature white-backed vultures (Phipps et al., Reference Phipps, Willis, Wolter and Naidoo2013), but the extent to which they exhibit natal philopatry is currently unknown. Thus, we incorporated an estimated background emigration rate of 5% for first-year birds and a 2% emigration rate for 2–3 year old birds and applied this to all scenarios. Given the size and extent of the population in Kruger National Park relative to other protected areas nearby, we treated it as a source population and did not include an immigration component.
Population viability analysis
We investigated seven scenarios for the white-backed vulture population, each over a simulation period of 100 years, with 100 iterations per scenario (Table 2). In VORTEX, two additional types of mortality (removal of individuals from the population) can be incorporated: harvesting and catastrophes. We modelled persistent low-level mortality caused by poachers seeking vulture body parts for witchcraft (herein ‘muti’) as a type of harvesting, and a larger poisoning event aimed at maximizing the number of vultures obtained for muti as a catastrophe that occurred with a frequency of once per year. The number of individuals killed per catastrophic event was kept as a proportion of the population and based on recent data on vultures known to be targeted for muti (46 individuals; Green, Reference Green2015); 1.92% of the population (46/2,400 individuals) was killed in each event. Using the demographic parameters in Table 1 (67% of the population are adults and 80% of adults attempt to breed each year) the same event was modelled to disrupt 2.78% (25/900 nests) of breeding attempts that year.
Table 2 Seven 100-year-long scenarios with varying rates of mortality used for simulations of population persistence of white-backed vultures in Kruger National Park, South Africa. Each scenario was run through 100 simulations.
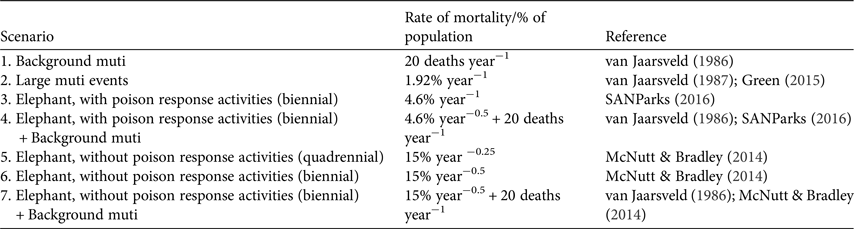
An elephant (or elephants) killed by poachers and laced with poison to kill as many vultures as possible was modelled as a catastrophic event in one of two ways, based on recent reports. Firstly, a poisoned elephant carcass that remained undiscovered by field staff was expected to kill 350 individuals, or 15% of the population (350/2,400 individuals; McNutt & Bradley, Reference McNutt and Bradley2014), and disrupt 21% of breeding attempts (188/900 nests). Secondly, a poisoned elephant carcass discovered by field staff trained and equipped to conduct poison response activities such as site decontamination and avian first aid was expected to kill 110 individuals (SANParks, 2016). The model predicted that such an event would kill 4.6% of the population (110/2,400 individuals; SANParks, 2016) and disrupt 6.5% of breeding attempts (59/900 nests). Two frequency scenarios for the occurrence of poisoned elephant carcasses were modelled: biennial (50% probability of occurrence in any year) and quadrennial (25% probability of occurrence in any year).
VORTEX uses the demographic parameters provided at the input stage to calculate the deterministic and stochastic growth rates for the population under each scenario, as well as the final population size. In the event that one or more of the modelled future populations goes extinct, VORTEX calculates the probability of extinction and the mean time to extinction. The threshold for extinction in all scenarios was set at a population size of < 20.
Prior to modelling the additional mortality scenarios, we tested the background population trajectories by running population viability analysis simulations for 100-year periods, using the parameters in Table 1. These background simulations and the main simulations were run as density-independent models. We also tested a density-dependent model for the background scenario by setting the carrying capacity (K, the upper size limit of the population) in VORTEX at approximately double the existing population estimate (5,000 individuals), and the reduced reproductive output at 0.25 (cf. 0.6 in Table 1) as K was approached with a slope, B, of 2. Although population limitation for raptors is often determined by nest sites or food availability (Newton, Reference Newton1979), and density-dependent effects on productivity have been reported for vultures (Fernández et al., Reference Fernández, Azkona and Donázar1998), we did not emphasize density-dependence in our simulations for two reasons. Firstly, vultures subject to nest-site limitation are cliff-nesting species (Carrete et al., Reference Carrete, Donázar and Margalida2006; García-Ripollés & López-López, Reference García-Ripollés and López-López2011), whereas density-dependent breeding productivity has not been reported for tree-nesting vultures (such as white-backed vultures). Secondly, we assumed Kruger National Park to have an adequate food supply. We did not incorporate an Allee effect because this has not been reported for vultures, although the potential for it exists given that vulture foraging efficiency is theoretically lower when the number of vultures in any given area is reduced (Jackson et al., Reference Jackson, Ruxton and Houston2008).
Results
In six of the seven scenarios the white-backed vulture population declined, and in three scenarios the probability of population extinction increased (Table 3). Low levels of mortality from harvesting for muti did not result in a negative growth rate and the population appeared to be resilient to the additional deaths in this scenario. The addition of low-level mortality from muti harvesting to the mortalities in the poisoning scenarios (Scenarios 4 and 7) did not affect the deterministic population growth rates and had only a slight effect on the stochastic growth rate, which highlights the minimal impact of 20 additional deaths per year on the population. The impact of larger muti events, however, was significant and led to a dramatic decrease in population size (Table 3).
Table 3 Viability analysis and population projections of white-backed vultures subject to poison-related mortalities, breeding in Kruger National Park, South Africa, with deterministic and stochastic annual growth rates, probability of extinction within 100 years, mean time to extinction, and remaining population size. Each scenario was run through 100 simulations.
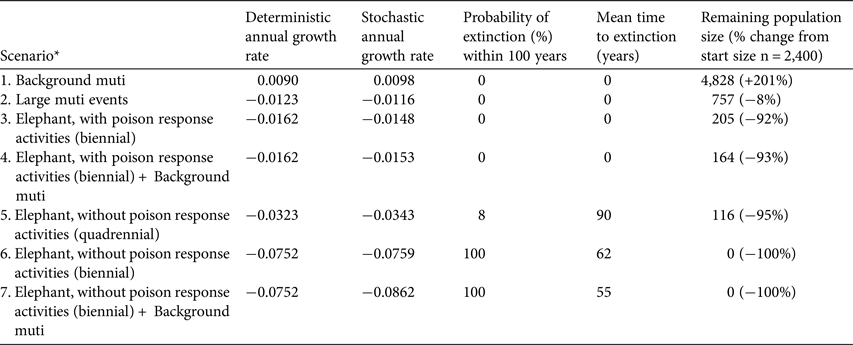
* Muti, persistent low-level mortality caused by poachers seeking vulture body parts for witchcraft
The results from the population viability analysis clearly demonstrate that poisoning from unattended elephant carcasses is the biggest threat to the white-backed vulture population in Kruger National Park (Scenarios 5–7; Fig. 1), almost irrespective of the intensity of harvesting for muti. Uncontrolled (i.e. no poison response activities) poisoned elephant carcasses occurring c. once every 2 years are predicted to cause the extinction of the population after c. 50 years. Scenarios 3 and 4 highlight that despite population declines exceeding 90% over the simulation period, the effective intervention of poison response activities reduces the probability of population extinction within 100 years to zero (Fig. 1; Table 3).

Fig. 1 Mean (± SD) population size and 100-year trends for white-backed vultures Gyps africanus in Kruger National Park subject to poison-related mortalities from elephant Loxodonta africana carcasses deliberately poisoned by poachers. Curves are derived from VORTEX models run through 100 simulations, and describe two scenarios: (a) one elephant with 50% annual probability of occurrence remaining undetected by field staff and causing 350 deaths (solid line); (b) one elephant with 50% annual probability of occurrence located by field staff and neutralized by poison response activities (PRA) and causing 110 deaths (dotted line).
Discussion
We present the first estimates of threats to the white-backed vulture population in Kruger National Park from poisoning, based on recent rates of mortality and the historical context, and highlight the remedial effects of poison response activities. As VORTEX models stochastic processes, the outcomes of each population viability analysis will be slightly different for each simulation. However, although the input permutations in VORTEX are virtually limitless and it is possible to adjust parameters and explore outcomes under a wide variety of scenarios, the unavoidable conclusion is that regardless of the frequency at which they occur, poisoned elephant carcasses that are not found and neutralized by poison response activities will have a dramatic negative impact on the Park's vulture population. Unchecked, this impact is likely to cause the disappearance of the population within the next three generations, or c. 60 years. Similar outcomes are likely in other areas of Africa where elephant poaching occurs.
The almost certain extinction of this population in the face of poisoned elephant carcasses occurring with a relatively low annual probability of only 50% is alarming and highlights the urgent need for conservation managers to prepare for and be able to act against events of this type in particular. Field staff trained in poison response activities and with access to relevant equipment have already demonstrated the ability to reduce poison-related mortalities (SANParks, 2016) and this significantly reduces the extinction risk for the population. Extending these techniques to other areas is likely to minimize poison-related damage elsewhere, not only for vultures but also for other species that are susceptible to being poisoned whilst scavenging, such as spotted hyaenas, lions and black-backed jackals (Ogada, Reference Ogada2014).
Compared to the catastrophic impact of one poisoned elephant carcass left untreated and exposed to vultures, other causes of mortality, such as low-level muti harvesting, electrocution (Anderson & Kruger, Reference Anderson and Kruger1995) or limited availability of food (Kane et al., Reference Kane, Jackson, Monadjem, Colomer and Margalida2014), are of relatively limited concern. Although the effects of additional mortality from background muti harvesting appear to be small, we caution against indifference towards this cause of mortality, as in other areas with smaller vulture populations unsustainable levels of harvesting are likely to lead to local extinctions if existing trends continue (McKean et al., Reference McKean, Mander, Diedrichs, Ntuli, Mavundla, Williams and Wakelin2013).
Additionally, poisoning for muti is not a new development for the vultures of Kruger National Park (van Jaarsveld, Reference van Jaarsveld1986, Reference van Jaarsveld1987) and the population trend of white-backed vultures in the Park suggests a long-term decline as a result of additional mortality, possibly from this source. Recent estimates (Murn et al., Reference Murn, Combrink, Ronaldson, Thompson and Botha2013) have suggested that the population may have decreased by as much as 50% since the early 1980s; the VORTEX simulations here support that assertion if the rate of additional deaths from poisoning for muti (Scenario 2) have been maintained at the rate described by reports from the 1980s (c. 50 birds year−1). Prior to 2011 there were no dedicated surveys of vultures in the Park but they were counted opportunistically during aerial censuses of large mammals (Herholdt, Reference Herholdt, Boshoff, Anderson and Borello1997); thus it is not possible to estimate how many vultures once nested there. However, by projecting historically the rate of population change calculated in VORTEX for Scenario 2, the 2015 estimate of 900 pairs (2,400 individuals) could have been as high as 1,240 pairs in 1985, which is comparable to the estimate of c. 1,400 pairs in the late 1970s/early 1980s (Tarboton & Allan, Reference Tarboton and Allan1984). It is therefore possible that the vultures have been suffering the effects of poisoning for muti over several decades, with vulture deaths also occurring outside the boundaries of the Park (Butchart, Reference Butchart1987), possibly at a scale similar to Scenario 2 (40–50 deaths per year).
The scenarios considered did not incorporate the impact of a worsening poison situation but it is clear that any increase in the rate at which poisoning occurs will have negative impacts. Poaching of rhinoceroses and elephants has increased in recent years (Emslie, Reference Emslie2013; Bennett, Reference Bennett2015), and with an increased risk from poaching-related poisoned carcasses (Ogada et al., Reference Ogada, Botha and Shaw2016a) the damage to a variety of species is potentially high. As the most significant cause of additional mortality in large ungulates such as rhinoceroses, increased poaching has direct consequences for the demography of target species (Wittemyer et al., Reference Wittemyer, Daballen and Douglas-Hamilton2013; Ferreira et al., Reference Ferreira, Greaver, Knight, Knight, Smit and Pienaar2015). Similarly, the effect of additional mortality from poisoning can have a negative effect on the demography of vulture populations (Margalida et al., Reference Margalida, Colomer and Oro2014). Innovative and high-intensity management interventions, such as real-time tags (O'Donoghue & Rutz, Reference O'Donoghue and Rutz2016) or remote-controlled aircraft (Mulero-Pázmány et al., Reference Mulero-Pázmány, Stolper, van Essen, Negro and Sassen2014), may be needed to help address increased rates of poaching of large mammals. High-technology solutions may be effective in reducing mortality but they will also facilitate detailed monitoring that may prevent carcasses being poisoned deliberately in the event of an animal being killed by poachers.
The effects of population supplementation were not considered in any of the scenarios, despite population reintroduction and supplementation methods having been successful for vulture populations in other settings. In Europe the bearded vulture Gypaetus barbatus was extirpated from the Alps as a result of poisoning and persecution (Mingozzi & Estève, Reference Mingozzi and Estève1997) and remained negatively affected by human persecution into the 20th century (Margalida et al., Reference Margalida, Heredia, Razin and Hernandez2008). However, the species is approaching demographic sustainability again following the removal of the causes of mortality, and a concerted reintroduction programme (Schaub et al., Reference Schaub, Zink, Beissmann, Sarrazin and Arlettaz2009). However, supplementation was not considered to be appropriate for the population of white-backed vultures in Kruger National Park for a number of reasons. Firstly, such an intervention would not limit or reverse the negative population trends caused by poison-related mortalities, which is reflected by the general resilience of the population to additional mortalities of 20–50 individuals per year, as seen in Scenario 1 and to a lesser extent in Scenario 2. Secondly, even if birds were available or in the unlikely event that a release project was possible, releasing 50+ white-backed vultures in the Park every year is unrealistic. Thirdly, such releases would be in contravention of existing guidelines for reintroductions and translocations (IUCN/SSC, 2013), which emphasize that the causes of population declines must be removed before releases aimed at supplementing or re-establishing wild populations take place.
Although vultures range much more widely than the protected areas in which they breed (Phipps et al., Reference Phipps, Willis, Wolter and Naidoo2013), the most substantial populations are found in large protected areas (Thiollay, Reference Thiollay2006; Murn et al., Reference Murn, Combrink, Ronaldson, Thompson and Botha2013, Reference Murn, Mundy, Virani, Borello, Holloway and Thiollay2016), which emphasizes the need to protect them there. Furthermore, the task of training and equipping field personnel over the entire African range of six vulture species is beyond available resources and would necessitate areas of focused activity. Our results indicate that significant resources should be directed towards reducing poison-related mortalities in protected areas because it is clear that the rapid implementation of poison response training for relevant field personnel is an important conservation activity that yields positive results.
The scenarios modelled here emphasize that effective poison response activities can reduce the probability of extinction for breeding populations of Critically Endangered vultures, but in reality these are symptomatic responses; deeper efforts are needed to address the underlying systemic problem. Illegal poaching of elephants persists despite a trade ban on ivory, which gives illegal traders a monopoly (Conrad, Reference Conrad2012). The use of poisons to kill elephants adds another dimension to the process; a dimension that is legal but poorly regulated. There is an opportunity for regulation of chemicals, and perhaps disruption of the supply lines that provide them for illegal activities. Thus, in addition to the training and equipping of field personnel to deal with poisoning events, we advocate tighter regulation of chemical supplies and the phasing out of the chemicals most commonly used as poisons, such as carbofuran and aldicarb. Finally, the places where effective poison response activities take place and are combined with direct anti-poaching efforts that reduce (or prevent increases in) mortalities of large mammals offer the best opportunities for breeding populations of vultures and other scavengers to persist.
Acknowledgements
Comments from Rhys Green, Sam Ferreira, Darcy Ogada and an anonymous reviewer improved this article.
Author contributions
CM and AB conceived the study. CM conducted data analysis and wrote and edited the article. AB collected data and edited the article.
Biographical sketches
Campbell Murn is a conservation biologist who works on birds of prey and in particular on vultures in Africa and south Asia. André Botha manages the Birds of Prey Programme of the Endangered Wildlife Trust, South Africa, and is also the Co-chair of the Vulture Specialist Group of the IUCN Species Survival Commission.






