Ammonia (used here as a synonym for the sum of NH3 and NH4+) is highly toxic to humans and can cross the blood–brain barrier, which leads to a decrease in cerebral function, neuropsychiatric disorders and death(Reference Felipo and Butterworth1, Reference Wilkinson, Smeeton and Watt2). Ammonia-mediated excitotoxicity has been implicated in the mediation of central nervous system damage(Reference Munoz, Monfort and Gaztelu3, Reference Nybo4).
Data obtained from exercise studies have been used to elucidate the effects of hyperammonaemia. Several investigations have demonstrated that increased ammonaemia occurs during various types of exercise(Reference Wagenmakers, Coakley and Edwards5–Reference Bassini-Cameron, Monteiro and Gomes8). During prolonged submaximal exercise, an increase in ammonaemia (>160 μmol/l) has been observed in various studies(Reference Bassini-Cameron, Monteiro and Gomes8, Reference Graham and MacLean9). The consensus view is that the production of ammonia during exercise occurs via a combination of both AMP deamination and catabolism of amino acids, processes that are activated in an intensity- and duration-dependent manner(Reference Wilkinson, Smeeton and Watt2). It has been suggested that ammonia promotes both central and peripheral fatigue and that better control of ammonia production will improve exercise performance(Reference Nybo4, Reference Banister and Cameron10).
The increase in the ammonia levels in response to exercise can be managed through the use of amino acids or carbohydrates that interfere with ammonia metabolism(Reference Carvalho-Peixoto, Alves and Cameron7). During metabolism, amino acids are deaminated or transaminated to form keto acids via release of the amino group(Reference Furst11). These reactions are reversible, and the use of keto analogues could reduce blood ammonia concentration, resulting in the production of amino acids(Reference Walser12). The long-term use of keto analogues associated with amino acids (KAAA) to provide amino acid supplementation has been described previously(Reference Savica, Santoro and Ciolino13); to our knowledge, the acute use of KAAA has never been studied.
Although ammonia has been shown to be produced by branched chain amino acid catabolism, independent of glycogen availability(Reference van Hall, van der Vusse and Söderlund14), various studies have linked ammonia formation to carbohydrate availability(Reference Nybo4, Reference Carvalho-Peixoto, Alves and Cameron7). Adopting a low-carbohydrate diet (termed a ketogenic diet) combined with physical exercise can reduce glycogen stores before exercise and induce hyperammonaemia(Reference Snow, Carey and Stathis15, Reference Langfort, Czarnowski and Zendzian-Piotrowska16). We used this metabolic effect of a ketogenic diet to enhance the effect of exercise on ammonia production.
In the present study, we evaluated the acute effect of KAAA supplementation on ammonia production during prolonged exercise. We hypothesised that acute KAAA supplementation can prevent the increase in ammonaemia during exercise owing to the function of keto analogues as energetic substrates or as ammonia-chelating agents.
Materials and methods
A total of thirteen male endurance-trained cyclists (28·6 (sem 1·6) years; 68·8 (sem 2·3) kg; 1·77 (sem 0·01) m) with similar exercise training levels (VO2max 52·7 (sem 2·8) ml/kg per min and maximum heart rate 191·4 (sem 1·6) beats/min) participated in the study voluntarily. All subjects had similar physical capabilities and had a minimum of 3 years of training. They had not used ergogenic substances or any other drugs. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Ethics Committee for Human Research at the Federal University of the State of Rio de Janeiro; ethics number: 117/2007. Written informed consent was obtained from all subjects.
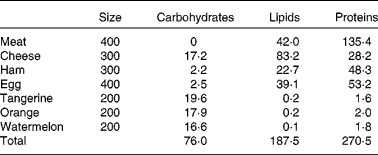
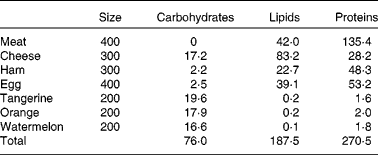
Athletes reported to the laboratory 3 d before the start of the experiment to become familiar with the cycle ergometer and to optimise the power output. Subjects received an individualised ketogenic diet (Table 1; 12·9 (sem 0·4) MJ; 35 % of the recommended energy intake from protein, 3·8 (sem 0·1) g/kg; 55 % from lipids, 2·7 (sem 0·1) g/kg; and less than 10 % from carbohydrates, 1·1 (sem 0·1) g/kg; see Westman et al. (Reference Westman, Feinman and Mavropoulos17) for a review of the effects of a low-carbohydrate diet on metabolism) for 2 d before the experiment and during the day of the experiment.
Subjects were asked to maintain their normal training schedule (approximately 70 km/d) and to follow the ketogenic diet up to 48 h (normal training and a ketogenic diet were used to reduce muscle glycogen stores and to induce a higher increase in ammonaemia) before the day of the experiment. On the day of the experimental period, the subjects reported to the laboratory in a fasting state and received breakfast and a light lunch. At 1 h after lunch, the subjects received either five tablets of a KAAA mixture (experimental group (KEx), n 6; Ketosteril®; Fresenius, Bad Homburg, Germany) or five 200 mg tablets of lactose (control group (LEx), n 7; Via Farma, São Paulo, Brazil) in a randomised double-blind manner. The composition of the KAAA mixture/tablet was as follows: α-keto analogues of isoleucine (335 mg), leucine (505 mg), phenylalanine (430 mg) and valine (340 mg); α-hydroxy analogue of methionine (295 mg); l-lysine acetate (75 mg l-lysine); l-threonine (265 mg); l-tryptophan (115 mg); l-histidine (190 mg); l-tyrosine (150 mg). Both supplements were provided in indistinguishable capsules.
Before the experimental trial, initial stretching was followed by a warm-up of 10 min at 50 % of the maximum heart rate. The experiment began 1 h after supplementation, and athletes cycled indoors for 2 h at 80 rpm according to a metronome in a room with constant temperature and relative humidity (23 ± 2°C and 60 ± 5 %, respectively). The subjects' heart rates were recorded continuously throughout the exercise period using a heart rate monitor (Polar CS200, Kempele, Finland). The power output was modified for each individual every 5 min so that the athletes maintained 75–85 % of their estimated maximum heart rate (approximately 156·0 (sem 2·8) beats/min with a work of 180·0 (sem 1·4) W, respectively).
A catheter was placed into the median cubital vein. At 1 h after the supplementation, blood samples were obtained at rest and at 30 min intervals throughout the exercise period. Finally, blood samples were collected during a 1 h recovery period at 30 min intervals. Athletes received water ad libitum during the trial.
Blood samples were analysed after collection. To avoid the loss of volatile compounds, blood samples were immediately centrifuged, and the serum was separated, frozen in liquid N2 and stored at − 70°C for subsequent biochemical analysis in a 24 h period. Biochemical determination of glucose, urea and urate concentrations was performed in serum using commercially available spectrophotometric assays (Labtest, Minas Gerais, Brazil). Lactate and ammonia concentrations were measured using an enzymatic UV method (Randox, Crumlin, UK) on a Dade Model Dimension RXL Automated Chemistry Analyzer (Dade Behring, Eschborn, Germany).
Statistical analyses were performed using SigmaStat version 3.5 for Windows (Systat Software Inc., San Jose, CA, USA). To decrease individual variability, data were normalised to 0 min values. After testing for normality (Kolmogorov–Smirnov) and equality test variance (Levene median), the changes in the variables between time points were analysed by a one-way ANOVA, and the group changes were evaluated by a two-way ANOVA for repeated measures. Significance (P < 0·05) was confirmed using the Tukey post hoc test. Data are presented as means with their standard errors. The area under the curve for blood ammonia data for each individual in each treatment was determined using the following equation:
assuming that the ammonia level at baseline corresponds to the resting ammonia level and where AUC is the area under the curve, A is ammonia and T is time.
Results
A 120 min cycling session was employed to evaluate the effect of KAAA on blood ammonia concentration. The resting ammonaemia, before exercise and after supplementation, was elevated (approximately 90 μmol/l) in both groups, and ammonia concentration increased up to a maximum of 35 % above baseline levels in response to exercise in the LEx group (normalised values) at 60, 90 and 120 min (P < 0·001). In contrast, ammonia concentration was not increased (approximately 17 %) in the KEx group at 60, 90 or 120 min. The KEx group actually experienced an approximately 18 % decrease in ammonia concentration at 60 min (P = 0·040) and 90 min (P = 0·049) compared with the LEx group. The area under the curve of the KEx group was 12 % smaller than that of the LEx group. Ammonia concentrations in the LEx group returned to baseline levels at 150 min (30 min into the recovery period). The KEx group demonstrated a greater decrease in ammonaemia (approximately 20 %) compared with the LEx group at 150 min, reaching levels significantly lower than those of the control after 1 h of recovery (P = 0·022; Fig. 1(a)).

Fig. 1 Acute keto analogue and amino acid (KAAA) supplementation affects the ammonia and urate levels but not the urea level, the glucose level or lactate metabolism. Athletes exercised for 2 h after KAAA supplementation (experimental group (KEx), ○) or control supplementation (LEx, ●). Note that the lactate values were KEx (□) or LEx (■). (a) Ammonia: resting values were KEx 94·31 (sem 6·86) μmol/l and LEx 84·36 (sem 9·06) μmol/l; (b) urea: resting values were KEx 17·25 (sem 1·94) mmol/l and LEx 16·47 (sem 1·79) mmol/l; (c) urate: resting values were KEx 295·51 (sem 20·47) μmol/l and LEx 293·25 (sem 19·24) μmol/l; (d) glucose: resting values were KEx 4·72 (sem 0·17) mmol/l and LEx 5·24 (sem 0·18) mmol/l; lactate: resting values were KEx 1·70 (sem 0·08) mmol/l and LEx 1·92 (sem 0·15) mmol/l. The 0 min values for the five metabolites did not differ between the two groups. Values are means, with standard errors represented by vertical bars; data were normalised to individual 0 min values (100 %). * Mean values were significantly different from 0 min within the group. † Mean values were significantly different from 30 min within the group. ‡ Mean values were significantly different from 150 min within the group. § Mean values were significantly different from 180 min within the group; ∥ Mean values were significantly different between treatments (P < 0·05).
To evaluate the effect of KAAA on urea synthesis, we measured the blood urea concentration. Both groups had a significant increase in blood urea levels in response to exercise (approximately 35 % at 120 min; P < 0·001). Even after a 60 min recovery period, the levels of urea remained constant (Fig. 1(b)).
To differentiate the ammonia produced by AMP deamination from that produced by amino acid deamination, we measured blood urate levels. Resting urate levels were equivalent in both groups and remained statistically unchanged in the KEx group up to 90 min. In contrast, the blood urate levels increased by approximately 16 % at 90 and 120 min in the LEx group. Urate levels remained constant from the end of the exercise period throughout the recovery period (Fig. 1(c)).
To understand the role of KAAA in gluconeogenesis, we measured the blood glucose level during the exercise and recovery periods. No observable difference in glucose levels was found during the trial time between the different groups. An increase in the lactate level was observed during the first 30 min of exercise in both groups, with no difference between the groups (Fig. 1(d)).
Discussion
For several years, it has been accepted that KAAA are able to prevent nephrotoxicity, delaying the necessity for dialysis in patients with chronic nephropathies(Reference Savica, Santoro and Ciolino13). Therefore, we investigated the effect of KAAA on exercise-induced ammonia production.
Ammonia production in muscle may be due to the depletion of glycogen stores and the deamination of both AMP and amino acids(Reference Snow, Carey and Stathis15, Reference Ogino, Kinugawa and Osaki18). A low-carbohydrate diet increases the production of ammonia during exercise(Reference Wagenmakers19). Furthermore, it is acknowledged that excessive protein intake leads to increased ammonaemia, and ammonia is metabolised to urea by the liver(Reference Felipo and Butterworth1, Reference Shawcross, Olde Damink and Butterworth20). In the present study, we used a ketogenic diet (with 10 % of energy from carbohydrates and 35 % from protein) to decrease the availability of glycogen in the liver and muscle and to increase the availability of amino acids to supply energy. Exercise intensity is the key point in this type of metabolism because increases in exercise intensity increase the rate of AMP deamination, leading to the release of more ammonia into the bloodstream.
Although both studied groups were ammonaemic at baseline due to the ketogenic diet (approximately 90 μmol/l), and although we know that ammonia is critical to the pathogenesis of hepatic encephalopathy and brain oedema, clinical observations have not shown a consistent correlation between the concentration of ammonia in the blood and symptoms of hepatic encephalopathy(Reference Shawcross, Olde Damink and Butterworth20, Reference Zwingmann, Chatauret and Leibfritz21).
NH3 concentration increased in response to exercise in the LEx group, and this effect was reduced by the administration of KAAA. The supplement also kept the blood ammonia level lower during the recovery period. These effects may be due to the anaplerotic action of KAAA entering directly into the Krebs cycle as intermediates or due to the chelation of ammonia by the keto analogues. Additionally, KAAA may increase glucose availability via gluconeogenesis. In the present study, we did not detect any changes in glucose levels during exercise or as a result of KAAA supplementation. In addition, blood lactate concentration fluctuated similarly in both groups. These data may suggest that ammonia production related to the ingestion of KAAA is not decreased by gluconeogenesis.
Previous studies have shown that KAAA supplementation can effectively decrease blood urea levels after long-term usage(Reference Savica, Santoro and Ciolino13, Reference Walser22). In the present study, in which acute supplementation of KAAA was used in the presence of a high basal concentration of urea, exercise increased blood urea levels similarly in both groups. During a low-energetic state, the ATP:ADP ratio decreases in muscle, and myokinase is activated to synthesise ATP. This process leads to an increase in both AMP deamination and urate synthesis rates(Reference Sahlin, Tonkonogi and Söderlund23). However, while AMP deamination appears to be inhibited during the initial phase of intense exercise, it is pronounced during the recovery period(Reference Hellsten, Richter and Kiens24). In the present study, acute KAAA supplementation delayed the increase in blood urate concentration during prolonged exercise. Our data suggest that the effect of KAAA during exercise is not primarily due to ammonia removal via the synthesis of urea but rather to the carbon bodies used to produce ATP.
The KAAA supplement is a mixture of glucogenic and ketogenic amino acids. Thus, this supplement may promote anaplerosis via different Krebs cycle intermediates. In addition, it has been described previously that KAAA supplementation does not increase the insulin response, an important goal in exercise supplementation(Reference Dalton and Chantler25).
In a recent study, we employed KAAA as a supplement to modify amino acid metabolism and ammonia biogenesis during resistance exercise in an animal model(Reference Almeida, Prado and Llosa26). In the present study, we confirmed that when using a ketogenic diet to promote metabolic stress, the acute use of a mixture of amino acids and keto acids in acute supplementation can diminish the increase in ammonaemia caused by endurance exercise in humans. Due to the high amount of amino acids in the diet used in the present study, this effect seems to be due more to the presence of keto acids in the supplement than to a contribution from an acute intake of additional amino acids.
Acknowledgements
The present study was supported in part by the Institute of Technology and Research – Tiradentes University. E. S. P. and L.-C. C. performed the design; E. S. P., J. M. d. R. N., M. G. D. d. M., R. D. d. A. and L.-C. C. were involved in the data collection and interpretation; E. S. P., R. D. d. A., J. M. d. R. N. and L.-C. C were involved in the manuscript writing. The authors have no conflicts of interest to declare.