As the incidence of obesity is reaching ‘epidemic’ proportions, there is currently widespread interest in the impact of dietary components on appetite regulation, satiety and energy intake. There is evidence to suggest that constituents of dairy foods such as proteins, lactose and a number of lipid components, including conjugated linoleic acid and medium-chain fatty acids, influence body weight through their effects on the regulation of food intake( Reference Aziz 1 ).
Among the dairy food constituents, proteins have the greatest putative role in appetite control, with several studies showing that caseins, whey and, in particular, whey-derived bioactive peptides, regulate satiety and food intake( Reference Luhovyy, Akhavan and Anderson 2 – Reference Hursel, van der Zee and Westerterp-Plantenga 4 ). Pal & Ellis( Reference Pal and Ellis 5 ) recently showed that men who consumed dried whey as a breakfast preload had lower energy intake at lunch (2950 kJ, P < 0·001) compared with preloads containing egg, turkey or tuna (3535, 3514 and 3275 kJ, respectively). The putative mechanisms underlying the effect of whey on short-term appetite include: (1) increased plasma concentrations of gut hormones such as cholecystokinin, peptide tyrosine tyrosine (PYY) and glucagon-like peptide-1, which are known to reduce gut motility, gastric emptying and appetite( Reference Anderson and Moore 6 , Reference Batterham, Heffron and Kapoor 7 ), (2) an insulinotropic effect of whey bioactive components, such as α-lactalbumin and branched-chain amino acids( Reference Mourao, Bressan and Campbell 8 ), and (3) an increased concentration of plasma total amino acids after digestion( Reference Boirie, Dangin and Gachon 9 ). Furthermore, a Ca-specific mechanism for appetite control has been proposed( Reference Tordoff 10 ), which hypothesised that low concentrations of Ca in the diet may promote a desire to eat foods rich in Ca content( Reference McCaughey, Forestell and Tordoff 11 ). However, there is a lack of plausible mechanisms linking Ca with appetite and inconsistency among the few human studies published to date( Reference Lorenzen, Nielsen and Holst 12 – Reference Major, Alarie and Dore 14 ).
Although the effect of dairy food constituents, and in particular, protein, on satiety or energy intake has been investigated to some degree, studies have often used preloads of 50–70 g of protein which is considerably higher than that found in standard dairy portions (2·3–14·3 g)( Reference Hollis and Mattes 15 ). Additionally, several studies have used dairy products fortified with either whey, fibre, Ca or other satiating factors, which influence the impact of the non-fortified ‘native’ product on subsequent food intake( Reference Hursel, van der Zee and Westerterp-Plantenga 4 , Reference Mourao, Bressan and Campbell 8 , Reference Ruijschop, Boelrijk and te Giffel 16 , Reference Potier, Fromentin and Calvez 17 , Reference Lluch, Hanet-Geisen and Salah 18 ). A few studies have examined the effect of milk v. carbonated beverages or juices on satiety and ad libitum energy intake, the majority of which failed to show an effect of milk on suppressing appetite responses or energy intake( Reference Almiron-Roig and Drewnowski 19 – Reference Soenen and Westerterp-Plantenga 22 ). Other studies( Reference Tsuchiya, Almiron-Roig and Lluch 23 , Reference Hogenkamp, Mars and Stafleu 24 ) have examined the effect of texture, viscosity and energy density of yogurts on appetite responses. Although consumption of semisolid yogurt led to lower appetite ratings or eating rates compared with beverages or liquid yogurts, no energy compensation was observed( Reference Tsuchiya, Almiron-Roig and Lluch 23 , Reference Hogenkamp, Mars and Stafleu 24 ). Two studies have shown that drinking yogurts had higher satiating capacity compared with isoenergetic snacks, such as chocolate bars( Reference Chapelot and Payen 25 ), banana or crackers( Reference Almiron-Roig, Grathwohl and Green 13 ). However, overall, the literature on the impact of dairy products consumed as whole food at physiological intakes on acute appetite and ad libitum food and energy intake is ambiguous.
Furthermore, no study has directly compared individual commercial dairy products. Therefore, although snacking continues to grow in popularity( Reference Bilman, van Trijp and Renes 26 ), there is insufficient evidence to allow identification of the types of commercially available dairy foods which may help regulate food intake. The aim of the present study was to compare the impact of physiologically relevant intakes of three dairy foods consumed as a snack on appetite and ad libitum energy intake and to examine the possible mechanisms underlying any observed effects.
Subjects and methods
Healthy male volunteers (n 40) were recruited through advertising at the University of Reading and surrounding areas. Subjects were screened by a detailed health and lifestyle questionnaire and a questionnaire related to eating behaviour (the Three-Factor Eating Questionnaire (TFEQ))( Reference Stunkard and Messick 27 ) and physical activity. All subjects were in good health, normotensive, non-smokers, aged 18–50 years, had a BMI ranging from 25 to 29·9 kg/m2 and had a stable body weight (weight change of < 3 kg during the past 2 months). The exclusion criteria were as follows: food allergies, dislike of the ‘study’ foods, irregular eating patterns, use of any medication which would affect taste, smell, appetite and behaviour, lactose intolerance, athletes in training (trained >10 h/week), protein supplements and excess consumption of alcohol (>4 units/d). Moreover, subjects were excluded if they were cognitively dietary restrained eaters (TFEQ factor 1 >11), non-breakfast consumers or non-snack consumers, had any dietary restrictions or were on a weight-reducing diet. The study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the University of Reading Research and Ethics Committee. Written informed consent was obtained from all subjects before commencing the study.
Study design
A randomised within-subject experimental design was performed, with each subject returning for four separate test sessions, at least 1 week apart. The randomisation method used was the random permuted blocks (size of 4), which ensured treatment group numbers were evenly balanced at the end of each block. Subjects were instructed to refrain from alcohol and organised exercise for 24 h before each test session and consume a standardised meal supplied by the study, which consisted of chicken stew with vegetables (937 kJ; 32·1 % energy from carbohydrates, 50·7 % energy from protein and 17·7 % energy from fat) with as much rice as they liked, on the evening before each visit. It was requested the evening meal to be no later than 20.00 hours and not to change their habitual intake significantly on the day before each study day. On arrival at the Hugh Sinclair Unit of Human Nutrition in the fasted state, subjects were asked to complete a questionnaire before each session to assess their food intake and possible factors over the last 24 h that could have influenced their appetite and food intake, such as exercise and mood. Compliance with the evening meal consumption and adherence to their usual diet was assessed by this questionnaire. If subjects had an atypical diet or exercise day on the day before the study day, their study visit was rescheduled for the following week. After 20 min rest, subjects had anthropometric measures (weight, height and waist circumference) and a fasting blood sample taken and their appetite assessed with a visual analogue scale (VAS). A light breakfast was provided at 09.00 hours (0 min) and appetite was assessed at 10, 60, 115, 125, 145, 165, 185, 205 and 230 min. The start time (0 min) began when the breakfast was provided to the subjects in the rare cases when the study visit was not run in real time. The dairy snacks or the control were administered at 120 min and had to be consumed entirely within 5 min, and the ad libitum lunch provided at 210 min. Meals were consumed in the Nutrition Unit's dining room without the presence of other people. A second blood sample was collected 80 min after the snack (200 min). The experimental day procedure is represented schematically in Fig. 1.
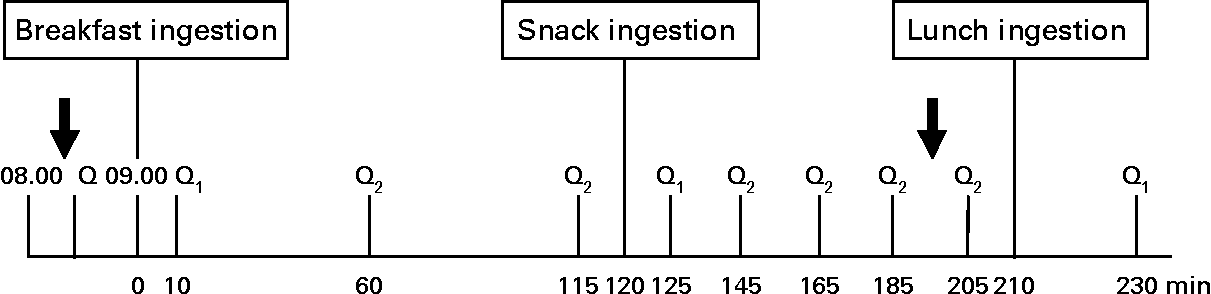
Fig. 1 Diagrammatic representation of the experimental procedure. Q, food intake and recent physical activity questionnaire; Q1, appearance and palatability; appetite and mood questionnaires; Q2, appetite and mood questionnaire.
![]() , Blood collection.
, Blood collection.
The time interval between breakfast, mid-morning snack and lunch was selected based on the typical British eating patterns and previous research( Reference Almiron-Roig and Drewnowski 19 , Reference Tsuchiya, Almiron-Roig and Lluch 23 ). Additionally, whey protein and its bioactive peptides, which are inversely related to food intake, have been found to suppress appetite more than 90 min after their ingestion( Reference Luhovyy, Akhavan and Anderson 2 ). The real aim of the study was masked by emphasising to the subjects that the aim was to assess the effect of consuming a snack on their mood and appetite. The subjects were not informed that energy intake at the lunch meal was assessed and represented a primary study outcome. Whether or not the participants established the ‘purpose’ of the ad libitum lunch was not assessed. Power analysis indicated that a total of forty male subjects should enter the present four-treatment within-subject study. The probability was 80 % that the study would detect a treatment difference at a two-sided 5·0 % significance level, of 418·4 kJ in energy intake between the treatments. This was based on the assumption that the standard deviation of the difference in the response variables was 962 kJ.
Test meals
All foods and snacks were commercially available, retail products. The light breakfast consisted of two oats cereal bars with strawberry filling (Nutrigrain soft baked bars; Kellogg's) and orange juice (250 ml; Sainsbury's), which together had an energy content of 1456 kJ and provided 60·5 g carbohydrate, 3 g protein and 7 g fat. The breakfast provided 15 % of the energy intake of an average UK male( Reference Bates, Lennox and Swan 28 ). The dairy snacks were either isoenergetic semi-skimmed milk (Cravendale; Arla Foods), a natural set biopot yogurt (Dr Oetker) or a mild Cheddar cheese (Sainsbury's). The dairy snacks provided the same energy load (841 kJ) and volume (410 ml). The energy load was selected based on the energy load that other commonly consumed snacks provide( Reference Chapelot and Payen 25 , Reference Bilman, van Trijp and Renes 26 , Reference Holt, Miller and Petocz 29 ) to improve the relevance of the experiment to the mid-morning snacks. The fourth treatment was an isovolumetric serving (compared with milk) of non-carbonated water. Non-carbonated water was ingested separately with cheese and yogurt in order to equate the volume of milk. The content of macronutrients and micronutrients in the dairy snacks was estimated by using Dietplan 6.6 dietary assessment software (Forestfield Software) and information based on the manufacturer's labelling and is given in Table 1. All packaging and labels were removed before serving, with the snacks provided in random order over the four study visits. The lunch test meal was designed to measure short-term energy compensation and not food choice. Therefore, lunch was not a buffet( Reference Blundell, de Graaf and Hulshof 30 ), but consisted of one main course, which was provided in excess (11 247 kJ), composed of pasta, tomato and basil sauce, olive oil and parmesan cheese ((2811·9 kJ/689 g of serving portion with 106 g carbohydrate (63·1 % energy), 21·6 g protein (12·9 % energy) and 11·8 g fat (15·8 % energy)). The subjects were asked to consume as much as they liked until they felt comfortably satisfied.
Table 1 Energy and macronutrient composition of the dairy snacks

* Volume of water consumed separately with the semisolid and solid treatments to match the volume of the liquid.
Visual analogue scales
The appetite profile was assessed using VAS ratings of hunger, fullness, desire to eat, prospective food consumption, thirst, desire for something sweet, savoury, salty and fatty anchored with the terms ‘not at all’ and ‘extremely’( Reference Blundell, de Graaf and Hulshof 30 ). Additionally, subjects' mood was recorded using VAS ratings of sadness, happiness, weariness, calmness, tenseness, sleepiness, effort and mental alertness. Finally, information about the appearance and palatability of the breakfast, lunch and mid-morning snacks was also recorded within 10 min of ingestion. The following scales had to be completed: pleasantness, enjoyment, visual appeal, taste, smell, overall palatability and how hard it was to consume the given amount. The recommended and valid questionnaires used in the present study( Reference Blundell, de Graaf and Hulshof 30 ) were performed electronically in personal laptops using Adaptive VAS software( Reference Marsh-Richard, Hatzis and Mathias 31 ). All the volunteers were trained and familiarised with the software before the study commenced.
Energy intake at lunch
Energy intake was assessed by an ad libitum hot pasta meal provided 90 min after the dairy snack or water control. Subjects were instructed to eat only until they felt comfortably satisfied and were given 20 min to consume the meal. Subjects ate individually in the dining room of the Unit for the entire length of time and ad libitum food intake was monitored by determining total food consumed (g) and energy consumed (kJ).
Anthropometric measurements
Height was measured to the nearest 0·1 cm with a wall-mounted stadiometer (Seca Limited) and weight to the nearest 0·1 kg. Waist circumference (cm) was measured at the site of the smallest circumference between the rib cage and the iliac crest with subjects in the standing position. Body composition was measured during screening and each test day with the bioelectrical impedance method (TANITA, BC-418MA; Marsden), and body weight (kg) and percentage of fat were calculated with the subjects being in a fasted state and wearing light clothes and no shoes. Subjects rested for 15 min and then blood pressure was measured three times, and the average of three was taken. Systolic and diastolic blood pressures were recorded during screening and each test day with an automatic blood pressure monitor (Omron 705CPII; Omron Healthcare Europe).
Psychometric measurements
The validated TFEQ was used to determine the subjects' eating behaviour( Reference Stunkard and Messick 27 ). The scores on cognitive restrained eating (F1), disinhibition of control (F2) and subjective feeling of hunger (F3) are shown in Table 2.
Table 2 Subject characteristics and habitual daily dietary and physical activity characteristics based on a 3 d diary record (Mean values and standard deviations)

TFEQ, Three-Factor Eating Questionnaire.
* Cognitive restraint eating (score range 0–21) cut-off point was 11, with scores above 11 showing cognitive restraint.
† Disinhibition or emotional eating (score range 0–14).
‡ A measure of feeling of hunger (score range 0–14).
Assessment of nutrient intake and physical activity
The subject's habitual diet and physical activity were assessed with the use of a 3 d food and physical activity diary (2 weekdays and 1 weekend day). The Dietplan 6.6 (Forestfield Software) nutrition analysis software package was used to calculate the energy and nutrient composition of the diets with the addition of specific foods using the manufacturer's nutritional information. Physical activity diaries were analysed using the Compendium of Physical Activities( Reference Ainsworth, Haskell and Leon 32 , Reference Ainsworth, Haskell and Whitt 33 ). Energy expenditure in kcal or kcal per body weight was estimated based on subject's body weight, intensity and duration of each specific activity according to the calculation of energy cost by Ainsworth et al. ( Reference Ainsworth, Haskell and Leon 32 ). Both diaries were completed over the same days during the third week of the study and returned as soon as possible after completion, when they were reviewed by the study nutritionist.
Blood sampling and biochemical analysis
At screening, a 12 h fasted blood sample was collected into a serum separation tube to determine the concentrations of glucose, total cholesterol, TAG, aspartate aminotransferase, alanine aminotransferase and γ-glutamyl transferase. During the study days, blood was collected into EDTA-treated tubes to determine the concentrations of active ghrelin and total peptide tyrosine tyrosine (PYY) and in lithium–heparin tubes for amino acid quantification. For active ghrelin measurement, the plasma sample was further acidified with HCl to a final concentration of 0·05 m. All samples were centrifuged at 4°C for 15 min at 2000 g immediately after collection and were separated and stored in cryogenic vials at − 80°C awaiting analysis. All the biochemical parameters for screening purposes were assayed on an ILAB 600 chemistry analyser (Instrumentation Laboratory) using enzyme-based colorimetric tests supplied by Instrumentation Laboratory. For insulin, blood was collected into serum separation tube and serum insulin was measured using a specific ELISA incorporating monoclonal antibodies (Dako Limited). Plasma active ghrelin and total PYY concentrations were measured by ELISA (EZGRA-88K and EZHPYYT66K, respectively; Linco Research, Inc.). The detection limit for total PYY was 10 pg/ml and 10 % of the samples which were below the detection limit were assigned a value of 5 pg/ml as described by Bradford et al. ( Reference Bradford, Harvatine and Allen 34 ). The proportion of samples below the detection limit was not affected by treatment (P = 0·272, Fisher's exact test). Plasma free amino acids and standards were derivatised using the EZ-Faast amino acid analysis kit (Phenomenex). Each derivatised plasma sample was analysed using a gas chromatograph (GC, 3400; Varian, Inc.) equipped with a flame ionisation detector and a Zebron ZB-AAA, 10 m × 0·25 mm capillary GC column (Phenomenex) with hydrogen as the carrier gas. As shown in Table 5, the intra-assay CV were < 10 % in agreement with the manufacturer's data for all the amino acids.
Statistical analyses
The effect of the four test meals on the baseline-adjusted self-reported appetite scores or energy intake and plasma analytes was evaluated by the PROC MIXED procedure (SAS Institute). Subject and subject × time interactions were treated as random effects and treatment (dairy snacks or water) and visit as fixed effects. The models included the effects of time as a repeated effect using the appropriate covariance structure within repeated measurements based on goodness-of-fit criteria. The covariance structures that were evaluated were the variance components, the autoregressive, the first-order autoregressive and the unstructured. The first-order autoregressive covariance structure was selected for the appetite scores and the variance components was selected for the energy intake and plasma biomarkers based on goodness-of-fit criteria. The significant effect of visit, time, treatment × time and treatment × visit interactions was tested and was included as a covariate when it was the case. The analyses were conducted with further adjustment for differences in three factor scores (eating restraint, hunger and disinhibition) as fixed effects (model 1). Model 2 was further adjusted for all the appetite and mood values and model 3 was further adjusted for the appearance and palatability values as fixed effects. Further backward stepwise analysis was conducted by checking the significance of these fixed effects or their interactions and including in model 4 only the significant effects. The percentage of energy compensation at lunch was calculated as described by Rolls et al. ( Reference Rolls, Castellanos and Halford 35 ) by dividing the energy intake at lunch in the control treatment by the sum of energy intake at lunch in dairy snacks including energy from the snacks and multiplying by 100. Values below 100 % specified under-compensation (overeating). Differences in energy compensation scores between the dairy snacks and the control were compared with a two-tailed paired t test. All models were tested for the normality of residuals. In the case of a skewed distribution (amino acids, insulin, ghrelin and PYY), variables were transformed accordingly to meet the normal distribution assumption. The statistical analyses were performed using SAS (release 9.2; SAS Institute, Inc.). Post hoc comparisons of significant effects were carried out with Tukey's honest significance test. Differences were considered statistically significant if P values < 0·05 (two-tailed). Data are presented as means and standard errors unless otherwise indicated.
Results
Subjects and baseline characteristics
Of the fifty-five subjects screened, thirteen were excluded (BMI outside range (n 4), cognitive restraint eating (n 2), smoker (n 1), elevated plasma analytes (n 3), medication (n 1) and unavailable by the time the study commenced (n 2)) and forty-two subjects were randomised, with two withdrawals due to personal reasons. The remaining forty overweight men completed the study and their baseline, habitual dietary and physical activity characteristics are shown in Table 2. The blood biomarkers were within the right range (data not shown).
Evaluation of the treatments and test meal
Table 3 reports the appearance and palatability ratings for the breakfast and lunch meals of the four study days and the four treatments. All of the appearance and palatability variables for the breakfast and lunch meals were relatively good, without significant differences between the four study days. However, differences in these variables were observed between the snack treatments (P < 0·001). The taste rating of yogurt was 29·7 mm lower than milk, 29·5 mm lower than cheese and 15·2 mm lower than water (P < 0·05). Similarly, overall palatability of yogurt was 27·2 mm lower relative to milk and cheese and 15·2 mm lower than water (P < 0·05). The given amount of yogurt was the most difficult to consume (52·1 (se 3·2) mm) between the different treatments (P < 0·05).
Table 3 Appearance and palatability of the breakfast, lunch-meal and the four snack treatments as assessed by repeated visual analogue scale ratings in mm (Mean values with their standard errors)

a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* Average ratings over the four study days.
† How hard was it to consume the given amount of food.
Self-reported appetite profile
Baseline appetite ratings (hunger, desire to eat, fullness and prospective food consumption) were not significantly different between the treatments. The subjective appetite responses measured by VAS are shown in Fig. 2 (data on thirst, desire for something sweet, savoury, salty or fatty are not shown). For hunger, desire to eat, fullness and prospective food consumption, there was an effect of treatment and treatment × time interaction observed (P < 0·001). The means of all appetite ratings over the full experiment following the intake of the different snacks are shown in Table 4. In model 1, baseline values, treatment, visit, time × treatment interaction, and the three factors of the TFEQ were used as covariates. The results showed that among the milk products, yogurt had the greatest suppressive effect on appetite. Hunger rating was 8, 10 and 24 % (P < 0·01) lower after the intake of yogurt compared with the intake of cheese, milk and water, respectively. Similar results were observed for ratings of desire to eat (P < 0·01). Fullness rating was 9 % (P = 0·019) and 30 % (P < 0·001) higher after the intake of yogurt compared with the intake of milk and water, respectively, although there was no difference relative to cheese (P = 0·550). Similarly, prospective food consumption did not differ between cheese and yogurt (P = 0·090), although it was 7 % (P = 0·003) lower after the intake of milk. In model 2, when further adjustment for the other appetite and mood variables was made, no significant changes in levels of significance or relative differences were observed. In model 3, when further adjustment for the appearance and palatability variables was made, there were no significant differences between the three dairy snacks for ratings of all appetite responses. Model 4 was a backward stepwise analysis of model 3 by excluding all the variables without a significant effect. There were no changes for ratings of fullness and desire to eat. Prospective food consumption was 4 % (P = 0·048) lower after the intake of yogurt relative to milk but not relative to cheese (P = 0·718), while hunger ratings were 7 % (P = 0·011) and 8 % (P = 0·008) lower compared with the intake of milk and cheese, respectively (Table 4).

Fig. 2 Subjective visual analogue scale (VAS) ratings for (A) hunger (how hungry do you feel?), (B) desire to eat (how strong is your desire to eat?), (C) fullness (how full do you feel?), (D) prospective food consumption (how much do you think you could eat right now?) throughout the study following the intake of semi-skimmed milk (
![]() ), cheddar cheese (
), cheddar cheese (
![]() ), natural yogurt (
), natural yogurt (
![]() ) or water (
) or water (
![]() , control). The time × treatment interaction was significant for all appetite responses (P < 0·001). Values are means of forty overweight men, with their standard errors represented by vertical bars. * Mean values of water were significantly different from those of milk, cheese and yogurt (P < 0·05). † Mean values of yogurt were significantly different from those of both milk and cheese (P < 0·05). ‡ Mean values of yogurt were significantly different from those of milk but not cheese (P < 0·05). When the statistical model was corrected for multiple testing (Tukey's P values) there was no significant differences remaining between the dairy snacks at the individual time points.
, control). The time × treatment interaction was significant for all appetite responses (P < 0·001). Values are means of forty overweight men, with their standard errors represented by vertical bars. * Mean values of water were significantly different from those of milk, cheese and yogurt (P < 0·05). † Mean values of yogurt were significantly different from those of both milk and cheese (P < 0·05). ‡ Mean values of yogurt were significantly different from those of milk but not cheese (P < 0·05). When the statistical model was corrected for multiple testing (Tukey's P values) there was no significant differences remaining between the dairy snacks at the individual time points.
Table 4 Mean subjective appetite responses using repeated visual analogue scale ratings in mm over the study day following the intake of semi-skimmed milk, cheddar cheese, natural yogurt or water (control) (Mean values with their standard errors)

a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* Model 1 was adjusted for baseline values, treatment, visit, time × treatment interaction, factor 1: cognitive restraint eating, factor 2: disinhibition and factor 3: feelings of hunger.
† Model 2 was further adjusted for all the appetite and mood variables.
‡ Model 3 was further adjusted for the appearance and palatability variables
§ Model 4 was model 3 excluding the variables without a significant effect.
Energy intake at the lunchtime meal
The mean ad libitum energy intake as a lunchtime meal was 11, 9 and 12 % (P < 0·02) lower after the intake of yogurt, cheese and milk, respectively, compared with water (4301 (se 226) kJ; Fig. 3(A)). However, there were no significant differences between the dairy snacks. When the energy load of the dairy snacks was added to the energy intake at the lunchtime meal, subjects consumed more energy when they consumed the dairy snacks (milk: 4646 (se 226) kJ, cheese: 4796 (se 226) kJ and yogurt: 4690 (se 226) kJ) compared with the control (P < 0·05; Fig. 3(B)). There was no difference between the energy compensation after the intake of milk (91·3 %), cheese (90·4 %) or yogurt (90·7 %), relative to water (100 %; P = 0·021, P = 0·015 and P = 0·007, respectively).

Fig. 3 (A) Mean energy intake at an ad libitum lunch meal following each of the four snacks (n 40) and (B) mean energy intake at lunch meal including the energy content of the treatment (dairy snacks or water). Values are means, with their standard errors represented by vertical bars. a,b Mean values with unlike letters were significantly different (P < 0·05).
![]() , Milk;
, Milk;
![]() , cheese;
, cheese;
![]() , yogurt;
, yogurt;
![]() , water.
, water.
Plasma concentration of glucose, insulin, ghrelin, peptide tyrosine tyrosine and amino acids
There were no differences between baseline plasma concentrations of all hormones and amino acids. Table 5 reports the baseline adjusted plasma concentrations of these biomarkers 80 min after the intake of the snacks and immediately before the lunch. Glucose concentrations were not different among the four treatments. Although there were no significant differences in hormonal responses between the dairy snacks, insulin and PYY concentrations were higher after the intake of dairy compared with the control and ghrelin was lower after the intake of milk and yogurt compared with the control (P < 0·05). Considering the amino acid profile, the concentration of all amino acids other than α-aminobutyric acid, cystine, glutamic acid, glycine and histidine was higher after the intake of dairy compared with the control (P < 0·05). Comparisons of adjusted least-squares means between the dairy snacks showed that alanine and isoleucine concentrations were increased to a greater degree after the intake of yogurt than after the intake of milk or cheese. Adjusted baseline plasma concentrations of valine, leucine, methionine, threonine, tyrosine and phenylalanine were not significantly different between cheese and yogurt, but were increased more after the intake of yogurt than milk (P < 0·05). Finally, the amino acids serine, proline and asparagine were higher after the intake of yogurt and cheese than after the intake of milk (P < 0·05).
Table 5 Baseline adjusted plasma or serum concentrations of glucose, hormones and amino acids 80 min after the intake of the snack treatments (dairy snacks or water) (Mean values and 95 % confidence intervals)

PYY, peptide tyrosine tyrosine; GHR, active ghrelin; Aba, α-aminobutyric acid; C–C, cystine.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* Average plasma or serum concentration over the four study days after an overnight fasted condition.
† Branched-chain amino acids.
Discussion
To our knowledge, this is the first study to directly compare the satiating capacity of physiologically relevant intakes of isoenergetic servings of a number of commercially available dairy products. The main findings were as follows: (1) hunger rating was 8, 10 and 24 % lower after the intake of yogurt compared with the intake of cheese, milk and water, respectively, (2) there was no difference in energy intake at the subsequent lunch between the dairy snacks, although energy intake was 11, 9 and 12 % lower after the intake of yogurt, cheese and milk, respectively, compared with water, and (3) there were no differences in the postprandial responses of glucose, insulin, PYY or ghrelin to dairy product consumption, but alanine and isoleucine concentrations were higher after the intake of yogurt than after the intake of cheese or milk.
The acute effect of the consumption of dairy products on the subjective appetite ratings or energy intake has been investigated by several studies and the results are equivocal( Reference Dougkas, Reynolds and Givens 36 ). The majority of studies examined the effect of milk on appetite or energy intake relative to isoenergetic and isovolumetric servings of fruit juices or carbonated beverages, with the energy provided by the test beverage being between 900 kJ and 1·5 MJ and subsequent energy intake being assessed between 30 min and 4 h after milk consumption( Reference Almiron-Roig and Drewnowski 19 , Reference Harper, James and Flint 21 , Reference Soenen and Westerterp-Plantenga 22 , Reference Dove, Hodgson and Puddey 37 ). In agreement with the present findings, the majority of the studies failed to show an effect of milk on suppressing appetite responses or on energy intake at the next meal. Only Dove et al. ( Reference Dove, Hodgson and Puddey 37 ) showed that consumption of 600 ml skimmed milk relative to a fruit drink (1062 kJ) at breakfast increased satiety and reduced energy intake 4 h later in overweight men and women. The authors noted that the time lapse between the preload and the subsequent meal may be crucial, since evidence suggests that protein enhances satiety over several hours( Reference Fischer, Colombani and Wenk 38 ). On the other hand, there is a paucity of evidence of the effect of cheese consumption on appetite responses( Reference Potier, Fromentin and Calvez 17 , Reference Bates, Lennox and Swan 28 ). Potier et al. ( Reference Potier, Fromentin and Calvez 17 ) showed that regular consumption of a moderate-energy cheese (836 kJ per portion) was partially compensated at lunch 1 h after the ingestion but fully compensated over the whole day. Similar to the present findings, there was a partial compensation of the dairy snacks by participants eating less at lunch (provided at 1 h 30 min after the snack) compared with the control. It is unclear whether full compensation over the whole day would have been achieved if this had been a full-day study.
Considering the effect of yogurt consumption on appetite, the present findings give evidence that yogurt intake suppressed hunger more than isoenergetic and isovolumetric servings of milk or cheese. The low palatability ratings of yogurt could partly influence this hunger rating as there were no differences between the snacks when hunger was adjusted for all the appearance and palatability variables. However, the effect remained significant even when sensory rating was added to the model (model 4) as a covariate. Although several studies have demonstrated the higher satiating capacity of yogurt relative to fruit drinks( Reference Tsuchiya, Almiron-Roig and Lluch 23 ) or snacks such as fruits, chocolate bars and crackers( Reference Almiron-Roig, Grathwohl and Green 13 , Reference Chapelot and Payen 25 , Reference Rolls, Fedoroff and Guthrie 39 , Reference de Graaf and Hulshof 40 ), the majority of the yogurts tested were enriched with either fibre or protein; therefore, the impact of yogurt per se could not be delineated. The relatively stronger hunger suppression of yogurt compared with cheese or milk could be attributed to a combination of factors including energy density( Reference Poppitt and Prentice 41 ), macronutrient composition( Reference Prentice and Poppitt 42 ), physical form( Reference Mourao, Bressan and Campbell 8 ), viscosity or texture( Reference Mattes and Rothacker 43 ), manner of consumption( Reference Hogenkamp, Mars and Stafleu 24 ), gastric emptying rate( Reference Marciani, Gowland and Spiller 44 ) or sensory characteristics( Reference Yeomans, Gray and Mitchell 45 ). Comparing the energy density of the snacks, milk and yogurt had lower energy density (2·1 and 3·0 kJ/g, respectively) than cheese (17·2 kJ/g) placing both milk and yogurt in the low-energy density class( Reference Tsuchiya, Almiron-Roig and Lluch 23 ). Cheese is a solid food and there is some evidence that solid foods are more satiating than liquids( Reference Mourao, Bressan and Campbell 8 , Reference Mattes and Rothacker 43 , Reference Stull, Apolzan and Thalacker-Mercer 46 ) due to the slower gastric transit time, which could influence appetite as a result of the different time that nutrients are exposed to and interact with nutrient receptors in the gastrointestinal tract( Reference Schwartz and Moran 47 ). However, there are studies that have shown solid food to have lower( Reference Rolls, Fedoroff and Guthrie 39 , Reference Santangelo, Peracchi and Conte 48 ) or equivalent( Reference Almiron-Roig, Flores and Drewnowski 49 , Reference Tieken, Leidy and Stull 50 ) satiating power to liquids, thus the influence of the physical form on appetite remains an issue of uncertainty. Additionally, the suppressive effect of yogurt on hunger may be due to constituents found mainly in yogurt, such as the metabolic products of fermentation, live cultures and coagulated or intact protein, yet whether these constituents affect appetite, and to what extent, remains unknown( Reference Tsuchiya, Almiron-Roig and Lluch 23 ).
Can sensorial differences between the snacks explain the differences in appetite responses? The sensory properties and palatability differed between the treatments, with yogurt being the least palatable and the given amount being the hardest to consume. Although pre-testing of yogurt was not performed, all volunteers found the proposed dairy products and lunch acceptable during the screening procedure. However, since yogurt was natural and not fruit-flavoured or containing cereal, which are the most common forms of consumption in the UK( Reference Staff 51 ), this might explain the low palatability scores. Palatability and sensory properties of the treatments could influence satiety and subsequent energy intake, with evidence showing that highly palatable food increases energy intake both during the meal and in the short-term postprandial period( Reference Yeomans, Blundell and Leshem 52 ). Based on this, it would be predicted that milk and cheese would lead to greater food intake, yet this was not the case. The effect of palatability on appetite and energy intake remains a subject of discussion( Reference Sorensen, Moller and Flint 53 ), with De Graaf et al. ( Reference De Graaf, De Jong and Lambers 54 ) showing that the palatability of the food influences more satiation (physiological factors that promote meal termination) than satiety (factors that influence the time interval between meals). Altogether, the fact that there was no difference in energy intake 90 min after consumption of the test products suggests that sensory characteristics may have had limited influence on the study findings.
The differences in appetite ratings after the consumption of dairy snacks may have been too small to be followed by differences in energy intake at lunch. This is in agreement with a number of studies that failed to detect differences in energy intake at the subsequent lunch despite a significant suppressive effect on appetite ratings( Reference Dougkas, Reynolds and Givens 36 ). It has been proposed that snacks or preloads with an energy content below 900 kJ to 840 kJ are considered insufficient to induce energy compensation in the subsequent meal( Reference Almiron-Roig, Grathwohl and Green 13 , Reference Harper, James and Flint 21 ), and that timing is a key factor to detect differences in food intake. Thus, energy consumption and timing of assessment relative to snack consumption provide an explanation of the inconsistency among studies( Reference Veldhorst, Nieuwenhuizen and Hochstenbach-Waelen 55 ). However, Chapelot & Payen( Reference Chapelot and Payen 25 ) have recently reported that mealtime is usually fixed in the studies that examine the relationship between satiety ratings and food intake and have questioned the appropriateness of assessing the satiating power of a food by the size of the subsequent meal. This is because the process that controls the termination of eating and consequently the meal size and energy intake is satiation and not satiety( Reference Blundell, de Graaf and Hulshof 30 ); thus, reduction in energy intake at a fixed time should not always be expected after consumption of a food.
In addition, the present study investigated some possible mechanistic bases for any effects seen on appetite and subsequent ad libitum food intake. Dairy products are considered to be insulinotropic( Reference Ostman, Liljeberg Elmstahl and Bjorck 56 ) and high insulin responses have been related to suppressed appetite and food intake( Reference Flint, Gregersen and Gluud 57 ). Higher concentration of insulin after consumption of the dairy snacks than after water was associated with lower appetite ratings and energy intake at lunch compared with water. However, there were no differences in the concentration of insulin between the dairy snacks. Similarly, the differences in appetite ratings observed between the dairy snacks were not followed by differences in the gastrointestinal hormones. This is in agreement with a limited number of studies which showed that there is no mathematical association between satiety hormones and self-reported satiety( Reference Diepvens, Haberer and Westerterp-Plantenga 58 , Reference Veldhorst, Nieuwenhuizen and Hochstenbach-Waelen 59 ), and that the regulation and concentration of these gut hormones depends not only on the macronutrient composition of the food, but also on neuroendocrine factors( Reference Coll, Farooqi and O'Rahilly 60 ).
Generally, all the amino acids other than isoleucine reflected the amino acid content of the dairy products used. Isoleucine and alanine were increased to a greater degree after the intake of yogurt than after the intake of cheese or milk. According to Mellinkoff et al. ( Reference Mellinkoff, Frankland and Boyle 61 ) aminostatic hypothesis, elevated concentrations of plasma amino acids, which cannot be channelled into protein synthesis, may suppress appetite. Isoleucine is one of the three branched-chain amino acids which control the synthesis and degradation of protein and the secretion of insulin, processes that could influence appetite( Reference Veldhorst, Nieuwenhuizen and Hochstenbach-Waelen 59 ). However, the physiological and molecular mechanisms underlying the impact of amino acids on satiety and food intake are not clear and need to be further elucidated.
One limitation of the study is that the present findings cannot be generalised as the dairy snacks were only consumed by overweight men as a mid-morning snack. Furthermore, although the present study was not designed to investigate the contribution of specific macronutrients, the influence of energy density or sensory characteristics on appetite ratings, some differences in the sensory characteristics and in the composition of the dairy snacks could have confounded the outcome. There is evidence that the beliefs about the energy or fat content in the food influence satiety( Reference Caputo and Mattes 62 , Reference Shide and Rolls 63 ), thus another limitation of the study is that visual or cognitive cues related to the weight of the dairy snacks could also influence the results.
In conclusion, all dairy products consumed as mid-morning snacks reduced appetite and lunch intake compared with water. Yogurt suppressed subjective hunger ratings compared with isoenergetic and isovolumetric servings of milk and cheese, but subsequent food intake was not affected. However, these results should be interpreted with some caution due to the low sensory characteristics of yogurt. The impact of dairy snacks in other times of the day and in different population subgroups needs further investigation. Furthermore, longer-term well-controlled studies are needed to investigate whether dairy snacks, and the type of yogurt in particular, could affect appetite, and whether this results in a difference in overall daily energy intake. In these studies, the measurement of the circulating concentration of gut hormones, insulin, amino acid profile and any other potential regulators of appetite and food intake, would provide a mechanistic insight into any observed effects.
Acknowledgements
The present study was funded jointly by the Barham Benevolent Trust Studentship, DairyCo UK and The Dairy Council UK. The authors thank Paul Chatfield for his valuable statistical help and advice. A. D. conducted the study, the data analysis and drafted the manuscript. All authors contributed to the design of the study and manuscript, and approved the final version of the manuscript. The authors declare no conflict of interest.










