Non-communicable diseases mainly CVD are major causes of adult morbidity and mortality worldwide(Reference Roth Gregory, Mensah George and Johnson Catherine1). In 2019 alone, 18·6 million deaths were due to CVD, predominantly IHD and stroke(Reference Roth Gregory, Mensah George and Johnson Catherine1). The burden of CVD largely varies across time and regions which could be due to demographic and socio-economic changes, epidemiological transitions, and changes in lifestyle-related factors resulting from globalisation and industrialisation(2–Reference Gaziano, Bitton and Anand4).
Unhealthy dietary patterns, along with metabolic and anthropometric determinants, are among the most important behavioural risks of CVD(Reference Roth Gregory, Mensah George and Johnson Catherine1). In 2019, diet-related risks were among the top five risk factors for mortality(Reference Roth Gregory, Mensah George and Johnson Catherine1). Lifestyle modification, particularly targeting dietary risks, is one strategy to prevent cardiovascular events(Reference Brandhorst and Longo5,Reference Rippe6) . Reduction of excess calorie intake, processed food, and increased intake of fruit, vegetables, and wholegrains have been shown to minimise CVD risk(Reference Brandhorst and Longo5,Reference Yu, Malik and Hu7) . Likewise, reduction of saturated fat intake or replacement with polyunsaturated fat and increased intake of fibre are among the dietary recommendations for better heart health(Reference Lichtenstein, Appel and Vadiveloo8).
Several countries and international organisations have established healthy dietary guidelines to prevent non-communicable disease, including CVD. Nevertheless, passive dissemination of dietary recommendations alone is generally considered ineffective in changing the intended behaviour(Reference Brownson, Eyler and Harris9). Multicomponent interventions through active community engagement can improve an individual’s dietary patterns and reduce CVD burden at the population level(Reference Parker and Assaf10,Reference Mensah, Wei and Sorlie11) . Community-based CVD preventive interventions aimed at improving dietary patterns and physical activity have been implemented using various strategies. However, comprehensive evidence on the impact of such interventions in improving dietary patterns is limited. Few reviews have highlighted the effectiveness of interventions on dietary outcome measures; however, such studies are limited to specific regions, contexts(Reference Brown, Smith and Bhopal12–Reference Ndejjo, Hassen and Wanyenze14) or target populations(Reference Walton-Moss, Samuel and Nguyen15,Reference Mohan, Thomson and Leslie16) . In those reviews, details of the intervention components, implementation strategy and their impact on improving specific dietary patterns were not provided. Thus, we systematically reviewed the types and implementation of community-based preventive interventions for CVD and their effectiveness in improving dietary patterns. The evidence from this review is important for practitioners and researchers to design and implement preventive interventions through improvement of dietary patterns.
Methods
This work is part of a systematic review under the SPICES project – Scaling-up Packages of Interventions for CVD in selected sites in Europe and Sub-Saharan Africa (https://www.uantwerpen.be/en/projects/spices/), which aimed to synthesise available evidence on the effect of community-based interventions (CBI) in improving behavioural risks and CVD knowledge. This paper specifically summarises the evidence on the effects of such interventions on various measures of dietary patterns. The protocol for this review is registered in the PROSPERO international prospective register of systematic reviews (Reg. Number: CRD42019119885), and the result is presented in line with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) 2009 guideline(Reference Moher, Liberati and Tetzlaff17). The methodological details are available elsewhere(Reference Hassen, Ndejjo and Musinguzi18), and those relevant to this study are briefly summarised here.
Information sources and search strategy
Initially, MEDLINE, EMBASE, Cochrane Register of Controlled Studies, CINAHL and PSYCINFO were used as the main databases to identify all studies published from 2000 to 2019. Then, the search was updated until June 2022 to include recent results. Other sources, including thesis online, OpenGrey, ProQuest, CHW Central, Google Scholar, ClinicalTrials.gov and the WHO International Clinical Trials Registry, were also searched for more similar articles. After a preliminary keyword search, we developed a systematic search strategy using terms related to population, intervention and outcomes. The details of the search strategy are available elsewhere(Reference Hassen, Ndejjo and Musinguzi18). In addition, more eligible studies were included from reference lists of the included articles.
Study screening
Studies were eligible to be included in this review if they aimed at prevention of CVD and have dietary patterns as one of the outcomes. Studies were eligible if they were individual/cluster randomised controlled trials or controlled quasi-experimental or interrupted time series studies that tested interventions aimed at primordial or primary prevention of CVD. Moreover, studies were included if they involved adult participants aged 18 years or above; and the interventions were based in community and/or primary healthcare settings. Studies were excluded if participants had diagnosed CVD; interventions included clinical and/or pharmacologic components, with sample size below 150, retention rate below 60 % and a follow-up period shorter than 9 months. Studies that were reported in the English language were considered with no limitation on study location.
Endnote files from all databases were checked for duplication, and deduplication was performed using Bramer’s method(Reference Bramer, Giustini and de Jonge19). The deduplicated articles were exported into rayyan.QCRI.org(Reference Ouzzani, Hammady and Fedorowicz20) for further deduplication and screening purposes. We performed double screening (HYH and RN/BGS) on all retrieved titles/abstracts using defined criteria. Then, articles included in full-text review were read thoroughly by two independent reviewers (HYH and BGS), and a final decision for inclusion was made. Disagreements between two reviewers were solved through discussion. The article selection process is outlined in the PRISMA flow chart (see Fig. 1).
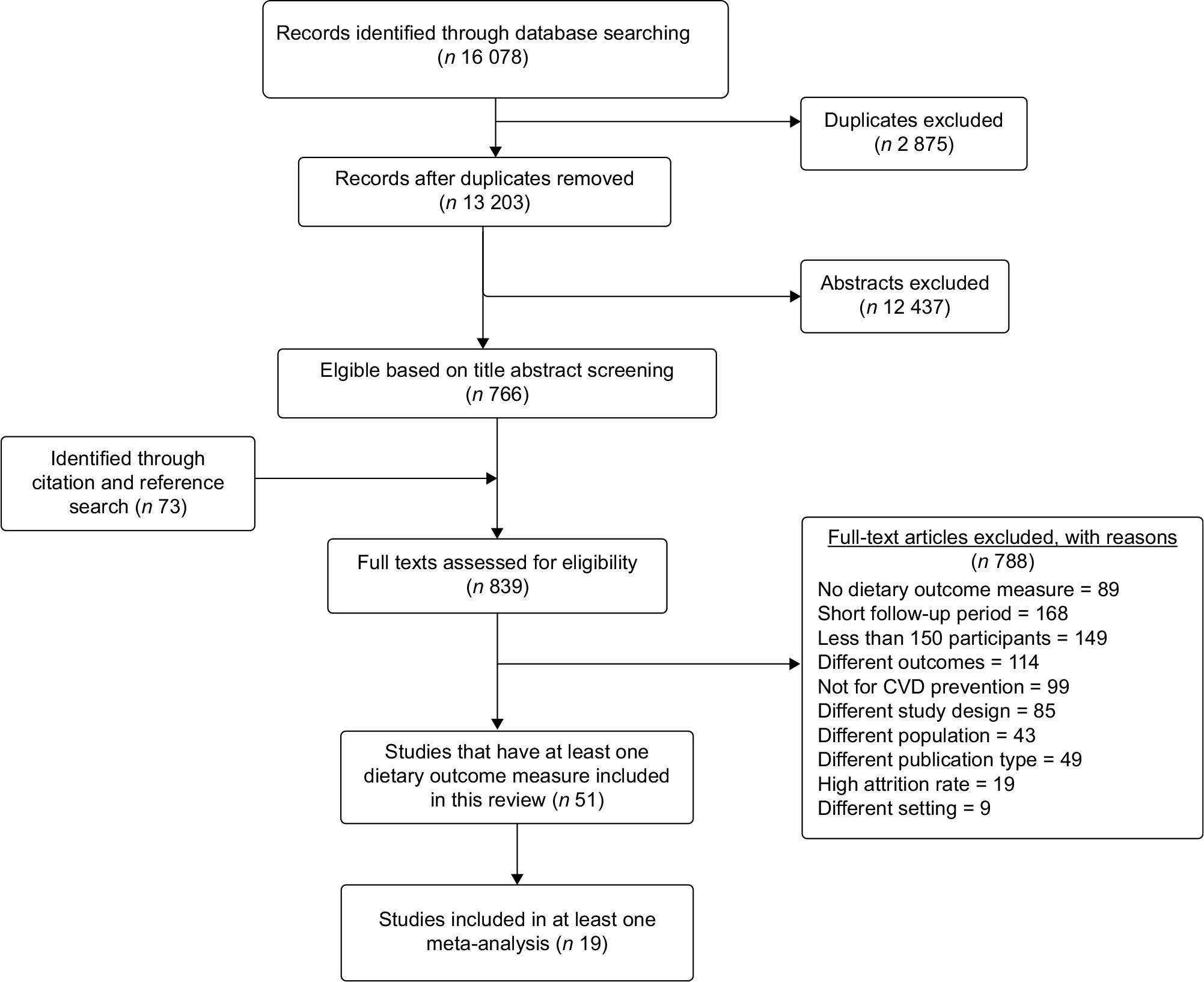
Fig. 1 PRISMA flow chart illustrating the article selection process
Risk of bias assessment and data extraction
For RCT, the revised Cochrane tool for Risk of Bias (RoB2)(Reference Sterne, Savović and Page21), while for NRC studies the Risk of Bias In Non-randomised Studies – of Interventions (ROBINS-I) tool(Reference Sterne, Hernán and Reeves22) were used to assess risk of bias of included studies. Double risk of bias assessment (HYH and BGS/RN) was performed independently, and differences were resolved through consensus.
Relevant information was extracted from included articles by two reviewers (HYH and BGS) independently, and disagreements were resolved through consensus. Data on year and country of study, intervention characteristics (description, setting, approach, duration, etc.), study design, participant characteristics, control group, sample size, attrition rate, outcome measures and summary findings were also captured. Furthermore, the outcome measures, summary measures and effect estimates were extracted. Whenever necessary, authors of included studies were contacted for further information. Results that were presented only graphically were extracted using WebPlotDigitizer(Reference Cramond, O’Mara-Eves and Doran-Constant23).
Data analysis
Findings are descriptively presented and discussed by study design, risk of bias, country and income per capita, intervention approach, and outcome measurements. Whenever needed, tables were used to present data comparing country, year of study, intervention duration, context and outcomes.
We used both narrative and quantitative synthesis to summarise evidence in this review. Studies were evaluated for eligibility to be included in the meta-analysis assessing the homogeneity of intervention and outcome measurements. Studies reported several measures of dietary patterns, and we performed a meta-analysis for any measure with at least two studies. As a result, meta-analysis was performed for intake of energy (MJ/d), fat (% of energy), saturated fat (% of energy), fibre (g/d), and fruit and vegetable (servings/d). Findings from studies without sufficient information on the above-mentioned outcome measures or those with other measures of dietary pattern were summarised narratively.
Meta-analysis
Due to heterogeneity observed in study populations and intervention duration, we expected between study heterogeneity and we performed a random-effects meta-analysis(Reference Borenstein, Hedges and Higgins24) for most of the outcome measures. Mean differences (MD) with 95 % CI were used to summarise continuous outcomes. Whenever needed, standard deviations and/or standard errors for MD were calculated from other reported parameters based on the Cochrane guideline(Reference Higgins, Thomas and Chandler25). We used the I2 statistic to quantify heterogeneity, and we tested the significance thereof using Cochran’s Q statistic(Reference Higgins and Thompson26). We explored the variation in effectiveness across time using subgroup analysis based on follow-up time (9–12 months, 18–24 months, and 36 months and above) and study design for each outcome measure included in the meta-analysis.
We constructed funnel plots to evaluate publication bias graphically, and the significance of symmetry was tested using Egger’s regression test(Reference Egger, Smith and Schneider27). We used the meta-package in the free statistical software package R version 4.0.2 for all the analyses(Reference Schwarzer28). The review results are reported in accordance with the PRISMA 2009 statement(Reference Moher, Liberati and Tetzlaff29), and a completed PRISMA checklist is available in the supplementary material (online Supplementary Table S3).
Results
From all databases, a total of 16 078 titles/abstracts were retrieved (15 885 from initial search and 193 recently updated). Seven hundred and sixty-six articles were retained based on abstract screening, and seventy-three more studies were identified through manual reference searching. Based on the full-text review, fifty-one studies involving 100 746 (56 689 in intervention and 44 057 in control group) reported at least one measure of dietary patterns and were eligible to be included in the narrative synthesis. Of these studies, nineteen were eligible for a meta-analysis with regard to at least one dietary outcome measure. The article screening process is summarised using the PRISMA flow chart (Fig. 1).
Study characteristics
Detailed characteristics of included studies are available in the supplementary material (online Supplementary Table S1). Of fifty-one studies included in this review, twenty-eight focused on high-income countries, specifically twelve in the USA(Reference Wedick, Ma and Olendzki30–Reference Landry, Thomson and Huye41), four in the Netherlands(Reference Kloek, van Lenthe and van Nierop42–Reference van Keulen, van Breukelen and de Vries45), two each in the UK(Reference Davies, Gray and Troughton46,Reference Ashfield-Watt, Welch and Godward47) , Spain(Reference Bóveda-Fontán, Barragán-Brun and Campiñez-Navarro48,Reference Arija, Villalobos and Pedret49) , and Australia(Reference Glasson, Chapman and Wilson50,Reference Lombard, Deeks and Jolley51) , and one each in Japan(Reference Takahashi, Sasaki and Okubo52), Italy(Reference Bo, Ciccone and Baldi53), Denmark(Reference Baumann, Toft and Aadahl54), Germany(Reference Koeder, Husain and Kranz55), Sweden(Reference Törmä, Lundqvist and Eliasson56) and Finland(Reference Lindström, Louheranta and Mannelin57). In contrast, twenty-three were in low- and middle-income countries, particularly five in China(Reference Zhang, Chao and Li58–Reference Lu, Tang and Lei62), four in India(Reference Thankappan, Sathish and Tapp63–Reference Joshi, Chow and Raju66), three in Iran(Reference Mirmiran, Ramezankhani and Hekmatdoost67–Reference Azizi, Mirmiran and Momenan69), two each in Sri Lanka(Reference Chandraratne, Yamaguchi and Indrawansa70,Reference Gunawardena, Kurotani and Indrawansa71) and Kenya(Reference Van de Vijver, Oti and Gomez72,Reference Okube, Kimani and Mirie73) , one each in Bangladesh(Reference Fottrell, Ahmed and Morrison74), Nepal(Reference Neupane, McLachlan and Mishra75), Malaysia(Reference Ibrahim, Ming Moy and Awalludin76), Pakistan(Reference Nishtar, Badar and Kamal77), Thailand(Reference Yokokawa, Yuasa and Nedsuwan78), and Vietnam(Reference Nguyen, Pham and Nguyen79), and one study recruited participants living in China, India and Mexico(Reference Anthony, Dyson and Lv80).
Regarding the study design, thirty-three studies were randomised, of which twenty-one and twelve, respectively, were individual- and cluster-randomised. Whereas, eighteen studies were non-randomised controlled studies. Out of thirty-three randomised studies, eight have low, twenty-two some concerns and three high risk of bias based on the Cochrane RoB2 tool. Of eighteen non-randomised studies, two has low, thirteen moderate and three serious risk of bias. The risk of bias summary tables and figures are presented in the supplementary material (online Supplementary Table S4 and Fig. S1).
Several continuous dietary outcome measures were reported including energy intake (MJ/d), Na intake, salt intake, fat (% of energy), saturated fat (% of energy), fibre (g/d), carbohydrate (% of energy or g/d), protein (% of energy or g/d), frequency of sugary beverages, salty diet, fast and/or fried food, fruit and vegetable (servings per d), number of days eat fruit and/or vegetable, healthy eating index, plant-based diet index and diet score. Categorical measures were also reported such as attainment of the required daily fruit and vegetable intake, recommended level of salt, adherence to dietary advice, vegetable procurement, recommended level of sugar, high salt intake, Mediterranean diet, snacks ≥ twice/d, etc. Details of the outcome measurement for individual studies are presented in the supplementary material (online Supplementary Table S1).
Interventions
Various strategies were employed to deliver the intervention package to target participants and/or populations. Most of them used various health education and awareness creation activities, including seminars, lectures and workshops as the main components of intervention(Reference Wedick, Ma and Olendzki30,Reference Laska, Lytle and Nanney34,Reference Dirige, Carlson and Alcaraz35,Reference Østbye, Krause and Lovelady37,Reference Havas, Anliker and Greenberg39,Reference Landry, Thomson and Huye41,Reference Davies, Gray and Troughton46–Reference Bóveda-Fontán, Barragán-Brun and Campiñez-Navarro48,Reference Glasson, Chapman and Wilson50,Reference Takahashi, Sasaki and Okubo52,Reference Bo, Ciccone and Baldi53,Reference Koeder, Husain and Kranz55,Reference Törmä, Lundqvist and Eliasson56,Reference Zhang, Chao and Li58–Reference Lu, Tang and Lei62,Reference Daivadanam, Wahlström and Ravindran65,Reference Mirmiran, Ramezankhani and Hekmatdoost67–Reference Azizi, Mirmiran and Momenan69,Reference Van de Vijver, Oti and Gomez72–Reference Yokokawa, Yuasa and Nedsuwan78,Reference Anthony, Dyson and Lv80) . Furthermore, other strategies were also considered including individual-tailored coaching interventions through face-to-face, mHealth or web-based(Reference Woodruff, Haardörfer and Gazmararian31,Reference Alexander, McClure and Calvi33,Reference Elmer, Obarzanek and Vollmer40,Reference van Keulen, van Breukelen and de Vries45,Reference Davies, Gray and Troughton46,Reference Takahashi, Sasaki and Okubo52,Reference Baumann, Toft and Aadahl54,Reference Törmä, Lundqvist and Eliasson56,Reference Chao, Wang and Xu60,Reference Ramachandran, Snehalatha and Ram64–Reference Joshi, Chow and Raju66,Reference Azizi, Mirmiran and Momenan69,Reference Fottrell, Ahmed and Morrison74–Reference Ibrahim, Ming Moy and Awalludin76,Reference Yokokawa, Yuasa and Nedsuwan78,Reference Nguyen, Pham and Nguyen79) , motivational interviewing(Reference Alexander, McClure and Calvi33,Reference van Keulen, van Breukelen and de Vries45,Reference Bóveda-Fontán, Barragán-Brun and Campiñez-Navarro48) , group interactive sessions and/or activities(Reference Wedick, Ma and Olendzki30,Reference Carrasquillo, Lebron and Alonzo38,Reference Elmer, Obarzanek and Vollmer40,Reference Kloek, van Lenthe and van Nierop42,Reference Davies, Gray and Troughton46,Reference Arija, Villalobos and Pedret49,Reference Lombard, Deeks and Jolley51,Reference Bo, Ciccone and Baldi53,Reference Baumann, Toft and Aadahl54,Reference Thankappan, Sathish and Tapp63,Reference Daivadanam, Wahlström and Ravindran65,Reference Azizi, Mirmiran and Momenan69,Reference Gunawardena, Kurotani and Indrawansa71,Reference Fottrell, Ahmed and Morrison74,Reference Ibrahim, Ming Moy and Awalludin76,Reference Yokokawa, Yuasa and Nedsuwan78) , print or electronic materials(Reference Woodruff, Haardörfer and Gazmararian31,Reference Havas, Anliker and Greenberg39,Reference Luten, Reijneveld and Dijkstra43,Reference Wendel-Vos, Dutman and Verschuren44,Reference Koeder, Husain and Kranz55,Reference Chao, Wang and Xu60,Reference Lu, Tang and Lei62,Reference Azizi, Mirmiran and Momenan69) , peer support(Reference Ayala, Ibarra and Cherrington32,Reference Havas, Anliker and Greenberg39,Reference Glasson, Chapman and Wilson50,Reference Thankappan, Sathish and Tapp63) , campaigns and mass media(Reference Wendel-Vos, Dutman and Verschuren44,Reference Glasson, Chapman and Wilson50,Reference Joshi, Chow and Raju66,Reference Van de Vijver, Oti and Gomez72,Reference Nishtar, Badar and Kamal77,Reference Nguyen, Pham and Nguyen79) , and posters, brochures and pamphlets(Reference Luten, Reijneveld and Dijkstra43,Reference Wendel-Vos, Dutman and Verschuren44,Reference Joshi, Chow and Raju66,Reference Mirmiran, Ramezankhani and Hekmatdoost67,Reference Azizi, Mirmiran and Momenan69) . Likewise, health promotion activities through community mobilisation, community networks, structural changes and policy measures(Reference Laska, Lytle and Nanney34,Reference Dirige, Carlson and Alcaraz35,Reference Ashfield-Watt, Welch and Godward47,Reference Lombard, Deeks and Jolley51,Reference Törmä, Lundqvist and Eliasson56,Reference Lv, Liu and Ren59,Reference Sarrafzadegan, Kelishadi and Esmaillzadeh68,Reference Azizi, Mirmiran and Momenan69,Reference Anthony, Dyson and Lv80) were employed. Details of intervention strategies used by each included studies are available in the supplementary material (online Supplementary Table S2).
Eight studies had an intervention duration ranging from 6 to 9 months(Reference Østbye, Krause and Lovelady37,Reference Havas, Anliker and Greenberg39,Reference Landry, Thomson and Huye41,Reference Luten, Reijneveld and Dijkstra43,Reference Arija, Villalobos and Pedret49,Reference Koeder, Husain and Kranz55,Reference Daivadanam, Wahlström and Ravindran65,Reference Van de Vijver, Oti and Gomez72) , eighteen studies for 12 months(Reference Wedick, Ma and Olendzki30–Reference Alexander, McClure and Calvi33,Reference Carrasquillo, Lebron and Alonzo38,Reference van Keulen, van Breukelen and de Vries45,Reference Ashfield-Watt, Welch and Godward47,Reference Bóveda-Fontán, Barragán-Brun and Campiñez-Navarro48,Reference Lombard, Deeks and Jolley51–Reference Bo, Ciccone and Baldi53,Reference Thankappan, Sathish and Tapp63,Reference Chandraratne, Yamaguchi and Indrawansa70,Reference Gunawardena, Kurotani and Indrawansa71,Reference Neupane, McLachlan and Mishra75–Reference Yokokawa, Yuasa and Nedsuwan78) , five studies for 14–18 months(Reference Dirige, Carlson and Alcaraz35,Reference Elmer, Obarzanek and Vollmer40,Reference Chao, Wang and Xu60,Reference Okube, Kimani and Mirie73,Reference Fottrell, Ahmed and Morrison74) , ten studies for 24 months(Reference Laska, Lytle and Nanney34,Reference Ortega, Albert and Sharif36,Reference Kloek, van Lenthe and van Nierop42,Reference Davies, Gray and Troughton46,Reference Zhang, Chao and Li58,Reference Lv, Liu and Ren59,Reference Lu, Tang and Lei62,Reference Ramachandran, Snehalatha and Ram64,Reference Joshi, Chow and Raju66,Reference Anthony, Dyson and Lv80) , six studies for 36–42 months(Reference Glasson, Chapman and Wilson50,Reference Lindström, Louheranta and Mannelin57,Reference Huang, Hu and Chen61,Reference Mirmiran, Ramezankhani and Hekmatdoost67,Reference Azizi, Mirmiran and Momenan69,Reference Nguyen, Pham and Nguyen79) and four for 5 years or above(Reference Wendel-Vos, Dutman and Verschuren44,Reference Baumann, Toft and Aadahl54,Reference Törmä, Lundqvist and Eliasson56,Reference Sarrafzadegan, Kelishadi and Esmaillzadeh68) . The majority of studies followed up participants for outcome measures at 12, 24 and 36 months post-intervention. Most interventions were based in the community-targeting groups of individuals, followed by home-based strategies either face to face or electronically, schools and workplaces or a combination of two or more settings. Trained volunteers, community health workers, peers, healthcare practitioners, nutritionists and other professionals were involved in facilitating the intervention.
Studies employed various dietary outcome measures, including total energy intake (per d), fruit and vegetable servings, fat and/or carbohydrate % of energy, fibre intake, soda/sugary beverage consumption, cholesterol, saturated/unsaturated fat intake, salt intake, Mediterranean diet, healthy eating index, diet score, and frequency of fast food and/or snacks.
Meta-analysis
The pooled effects of CBI with respect to selected dietary outcome measures are summarised in Table 1. In total, nineteen studies were included at least once for one of the five dietary outcome measures that were synthesised. Studies that reported a change in total energy intake (MJ/d), fruit and vegetable intake (servings/d), fibre intake (g/d), fat (% of energy) and saturated fat (% of energy) were considered. Based on ten studies, interventions led to a decrease in daily energy intake compared with controls (MD: –0·25; 95 % CI: –0·37, –0·14; number of studies (n) = 10; I2 = 0 %), which is equivalent to 59·8 kilo calories lower intake of energy per d. The pooled results of seven studies showed a 1·1 grams of higher fibre intake per d in the intervention groups compared with controls (MD: 1·08; 95 % CI: 0·39, 1·77; n 6; I2 = 68 %). A pooled analysis of five studies (all RCT) indicate that the decrease in fat % (MD: –1·01; 95 % CI: –1·76, –0·25; n 5; I2 = 66 %) and saturated fat % (MD: –1·54; 95 % CI: –2·01, –1·07; n 2; I2 = 0 %) of daily energy was higher in the intervention group as compared with controls. The increase in fruit and vegetable servings per d was higher in the intervention group compared with control, but the difference was not statistically significant (MD: 0·26; 95 % CI: –0·03, 0·54). Forest plots of all synthesised dietary outcome measures are presented in Fig. 2(a)–(e).
Table 1 Pooled effects of community-based interventions on dietary outcome measures

CI, Confidence interval; MD, mean difference; MJ, mega joule; FU, follow-up; I2, Heterogeneity statistic.
** P < 0·01;
*** P < 0·001.
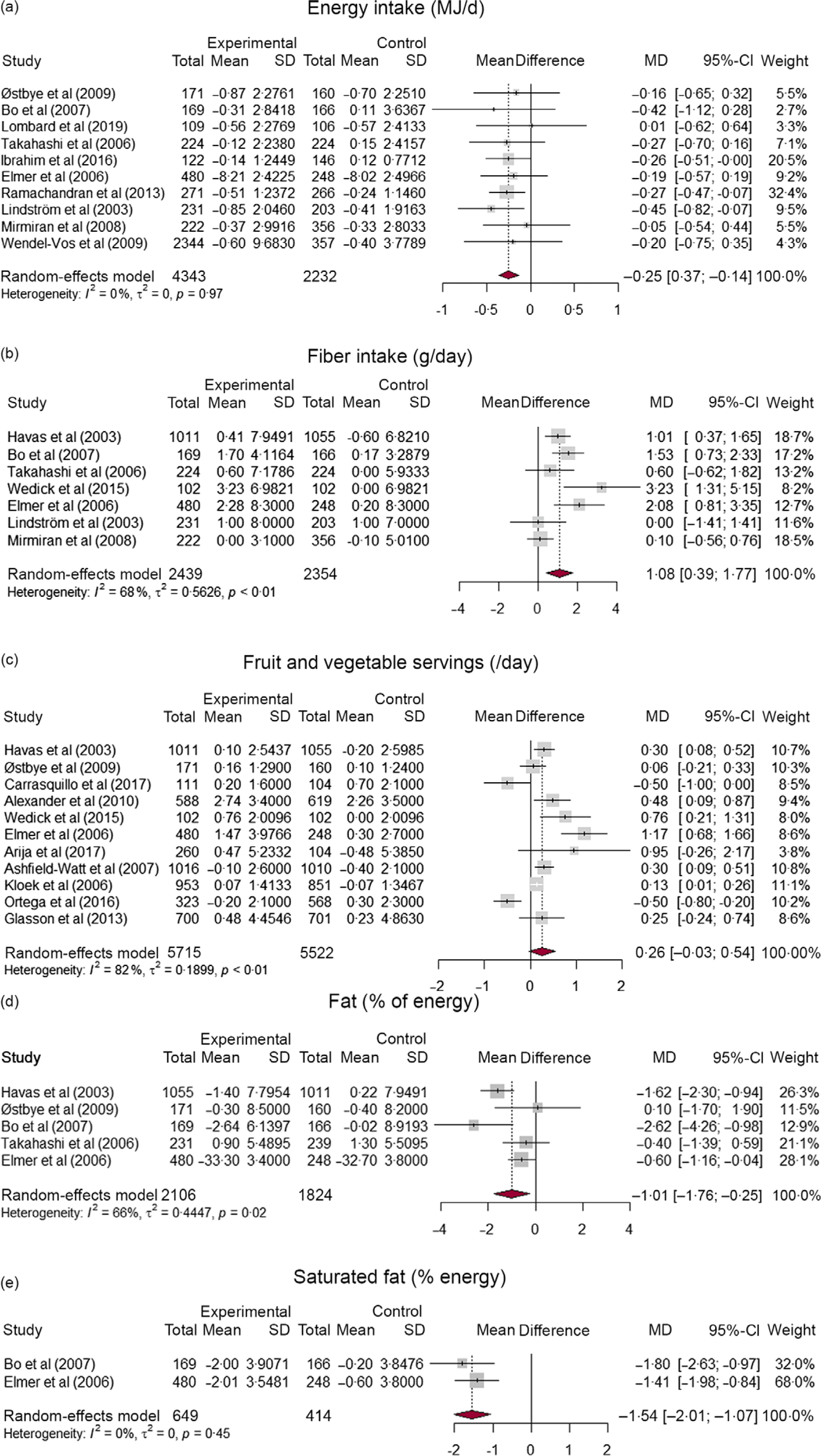
Fig. 2 Forest plots indicating the effect of community-based CVD preventive interventions on (a) energy intake, (b) fibre intake, (c) fruit and vegetable servings per d, (d) fat % of energy, and (e) saturated fat % of energy
The subgroup analysis indicated that higher intervention effect in increasing fibre intake at 9–12 months (MD: 1·29; 95 % CI: 0·71, 1·88) and 18–24 months (MD: 2·08; 95 % CI: 0·81, 3·35) of follow-up compared with ≥ 36 months (MD: 0·08; 95 % CI: –0·52, 0·68), with statistically significant subgroup difference (P < 0·01). The decrease in fat percent of energy was higher at 9–12 months (MD: –1·16; 95 % CI: –2·20, –0·12) than at 18–24 months (MD: –0·60; 95 % CI: –1·16, –0·04), but the subgroup difference is not statistically significant (P = 0·36). No time trend was observed in the remaining outcome measures. Forest plots of subgroup analysis are available in the supplementary material (online Supplementary Fig. S2–S5). Further subgroup analysis by study design showed that RCT showed a larger decrease in energy intake (MD: –0·28; 95 % CI: –0·42, –0·14) than NRC studies (MD: –0·21; 95 % CI: –0·42, 0·00), but the subgroup difference is not statistically significant (P = 0·61). The increase in fibre intake was slightly higher for RCT (MD: 1·28; 95 % CI: 0·63, 1·93) than NRC studies (MD: 0·10; 95 % CI: –0·56, 0·76), with significant subgroup difference (P = 0·01). Likewise, the increase in fruit and vegetable intake was higher in RCT (MD: 0·41; 95 % CI: –0·00, 0·82) than NRC studies (MD: 0·04; 95 % CI: –0·32, 0·41), with no statistically difference between subgroups (P = 0·19) (online Supplementary Fig. S6–S8).
We explored the potential of publication bias using Egger’s test of symmetry and funnel plots. Based on Egger’s test, the null hypothesis of symmetry was not rejected at 5 % significance level for energy intake (P = 0·392), fibre intake (P = 0·332), fruit and vegetable intake (P = 0·485) and fat percentage of energy (P = 0·855), indicating that no substantial publication bias was observed. Due to a small number of studies included in the meta-analysis, the statistical power of Egger’s test might not be sufficient to detect considerable bias. However, visual inspection of funnel plots of standard errors against observed effect sizes showed no large deviation from symmetry. Funnel plots of all outcome measures are available in the supplementary material (online Supplementary Fig S2(a)–(e)).
Narrative synthesis
Besides meta-analyses, a narrative synthesis was also employed to incorporate studies not included therein due to different outcome measures. Overall, out of fifty-one studies, thirty-seven studies (twenty-one from high-income countries and sixteen from low- and middle-income countries) found statistically significant differences in at least one dietary outcome measure favouring the intervention group. Whereas fourteen studies (nine from high-income countries and five from low- and middle-income countries) found no statistically significant difference in various dietary outcome measures across intervention and control groups(Reference Ayala, Ibarra and Cherrington32,Reference Alexander, McClure and Calvi33,Reference Østbye, Krause and Lovelady37,Reference Carrasquillo, Lebron and Alonzo38,Reference Kloek, van Lenthe and van Nierop42,Reference Luten, Reijneveld and Dijkstra43,Reference Ashfield-Watt, Welch and Godward47,Reference Arija, Villalobos and Pedret49,Reference Lombard, Deeks and Jolley51,Reference Lv, Liu and Ren59) . Of thirty studies that measured fruit and vegetable consumption, ten (33·3 %) found no significant difference across intervention groups. One study(Reference Luten, Reijneveld and Dijkstra43) found a significant increase in vegetable consumption but not fruit intake. A study by Baumann et al. (Reference Baumann, Toft and Aadahl54) indicated that the improvement in fruit and vegetable intake in the intervention group compared with the control group was greatest at 5 years of follow-up, but at 10 years the difference across groups was not significant. A study in Sweden(Reference Törmä, Lundqvist and Eliasson56) found no significant difference across intervention groups in most dietary measures, including percentage of energy from fat, carbohydrates, and protein, intake of fruits, vegetables, wholegrain, fish, sweetened beverages or fried potatoes, and overall diet quality (assessed by Healthy Diet Score). However, men in the intervention county decreased intake of sweets to a greater extent than those in control(Reference Törmä, Lundqvist and Eliasson56).
Studies that showed a significant improvement in dietary outcomes involved various intervention components, including tailored individual lifestyle coaching and interactive sessions by trained professionals mainly dieticians, health education individually or in group, health promotion activities, community engagement activities and/or structural and system changes such as improving access to healthy food. More specifically, effective interventions consisted of one or more of the following intervention components: individual lifestyle coaching based on risk level and using motivational change tools; counselling by trained professionals besides primary care physicians either in practice or home; customised advice, motivational interview and feedback; and visual demonstrations on food portions. In contrast, interventions through mobile text messages alone, written health pamphlets, brochures and booklets, and postal healthy lifestyle guides were relatively less or not effective. At group level, interventions involving regular interactive group sessions and community lifestyle activities were effective. Furthermore, structural changes such as ensuring healthy foods during organisational meetings/events and increasing availability of affordable fresh fruits and vegetables in corner stores were also effective in improving healthy eating among participants. However, healthy cooking interventions in restaurants and cafeterias were not effective. Further details of intervention strategies and direction of effects for included studies are available in the supplementary material (online Supplementary Table S2).
Discussion
This review summarises the available evidence on the approach, strategies and effectiveness of community-based CVD preventive interventions in improving healthy dietary patterns, which would contribute to halting the burden of CVD and associated premature mortality. We reviewed fifty-one eligible studies, thirty-three RCT and eighteen NRC studies, exploring the intervention components, duration, outcome measures and their effect on dietary patterns. We also conducted meta-analyses for studies with similar dietary outcome measures. Overall, the findings support that energy intake and fat percentage of energy, particularly saturated, could potentially be reduced through CBI targeting both general and high-risk populations. The mean daily fibre intake was also significantly improved in the intervention group compared with the controls. Intervention strategies involving lifestyle coaching, health education, health promotion activities, community engagement activities, and/or structural and systemic changes demonstrated more pronounced effects. Furthermore, the subgroup analysis showed that relatively higher effects on fibre intake were observed at 12 and 24 months than at 36 months and longer, with significant subgroup differences across time.
Excess energy intake is associated with weight gain, which may increase the risk of CVD incidence and mortality(Reference Jayedi, Rashidy-pour and Soltani81,Reference Dehghan, Mente and Zhang82) . By suppressing atherosclerosis and protecting heart cells against ischemic damage, energy restriction is associated with a lower rate of CVD events(Reference Mattson83). Thus, decreasing energy intake is one of the required outcomes of preventive interventions for CVD. Most of the studies in this review measured energy intake to evaluate the effectiveness of the intervention, and the majority indicated that CBI are effective in decreasing total daily energy intake, which is also supported by our meta-analysis. On average, participants in the intervention group had 59·8 kcal (250·2 kJ) lower energy intake per d compared with controls. The average recommended daily calorie intake of an adult ranges from 2000 to 2500 kcal(84). Thus, CBI decrease daily energy intake of participants by 2·5 % to 3·0 % as compared with controls, which is a significant percentage towards weight reduction provided that the intervention effect is sustained in the long run. Since calorie restriction favourably affects cardiac function(Reference Han and Ren85), CVD preventive interventions should incorporate strategies to limit an individual’s total calorie intake to the required level that is sufficient for energy balance. Nevertheless, energy restriction interventions require self-monitoring of intake and loss through active weight and food measurements. Training and demonstration of participants on self-monitoring of diet and body weight could be vital components of such interventions.
Healthy dietary guidelines recommend a reduction in dietary saturated fat and replacement with polyunsaturated and monounsaturated fat to lower the risk of CVD(Reference Sacks, Lichtenstein and Wu86). Our review and meta-analysis showed that interventions were effective in reducing percent of energy from fat, particularly saturated fat. Overall, interventions led to a 1·1 % decrease in percent of daily energy from fat. Nevertheless, crude assessment of ‘fat percentage of energy’ might not be an appropriate measure of healthy dietary pattern, rather, qualitative identification of specific fat type is more informative. Findings on the association between saturated fat intake and heart disease are inconsistent, which would most probably be due to the variation in comparison groups(Reference Zong, Li and Wanders87). Replacing saturated fats with polyunsaturated fats is strongly associated with a lower risk of CHD(Reference Hooper, Martin and Jimoh88). However, replacing saturated fats with refined low-quality carbohydrates results in cardiometabolic disorders, including obesity and diabetes, which increase the CVD risk(Reference Hu89,Reference Forouhi, Krauss and Taubes90) . Thus, the superficial use of phrases such as ‘fat intake reduction’ as a dietary intervention might be practically misleading. A few studies included in this review measured percent of energy from saturated fat and the meta-analysis showed that interventions decreased percent of daily energy from saturated fat by 1·5 %. Thus, rolling out such CBI would decrease percent of energy from saturated fat. Interventions should explicitly describe the reduction of saturated fats and their replacement with healthier polyunsaturated fats rather than processed carbohydrates.
Increasing consumption of fibre is also recommended to minimise the risk of a range of diseases, including heart diseases and diabetes(Reference Threapleton, Greenwood and Evans91–Reference James, Muir and Curtis93). A few studies included in our review evaluated the effects of interventions on fibre intake. Overall, our meta-analysis showed that interventions were effective in increasing daily fibre intake by approximately 1·1 g than controls. Compared with the recommended daily intake of 25–30 g of fibre, interventions led to a decrease by 3·3–4·0 %. Including fibre intake improvement as a dietary intervention strategy could be helpful for the primary prevention of CVD.
It is evident that fruit and vegetable intake is associated with reduced CVD risk, showing a clear dose–response relationship(Reference Roth Gregory, Mensah George and Johnson Catherine1,Reference Aune, Giovannucci and Boffetta94) . Most of the studies included in our review measured fruit and vegetable intake as one of the outcomes. Our narrative synthesis indicated that most studies found a significant improvement in fruit and vegetable consumption measured in various ways. Our meta-analysis specifically on daily fruit and vegetable servings indicated that there was an increase in the average servings per d by 0·26, but the difference was not statistically significant between intervention and control groups. A previous review also found a similar result, that is, the effectiveness in improving fruit and vegetable servings is minimal(Reference Ashton, Sharkey and Whatnall95). A change in fruit and vegetable intake can be hampered by several factors, including the access and affordability of fruits and vegetables. Participants’ socio-economic status and environmental conditions, including access to healthier food, determine the effectiveness of lifestyle interventions(Reference McGill, Anwar and Orton96). However, inaccurate measurement of portion size might also be a reason for the insignificant association.
Overall, effective interventions mostly employed tailored individual lifestyle coaching, stage-matched strategies and interactive sessions by professionals, such as dieticians, health education individually or in groups, community engagement activities, health promotion activities, and/or structural and system changes. One study(Reference Okube, Kimani and Mirie73) demonstrated the recommended portions to participants using diagrams of full platter and found significant improvements in all dietary measures in the intervention group compared with controls. Furthermore, interventions that involve multiple components are likely to be more effective than those that use one or two strategies. A review by Crane et al. also showed that individual-tailored interventions are the most effective behavioural interventions(Reference Crane, Halloway and Walts97). Thus, tailoring interventions to individual needs and readiness to change involving professionals and practical demonstrations is vital for improving effectiveness.
In general, CBI delivered through various strategies have demonstrated effectiveness in improving various measures of dietary pattern; however, studies have focused on high-income countries. Despite measurement of dietary behaviour being complex, consistent changes were observed following the interventions. Nevertheless, interventions need to emphasise practical demonstrations of dietary intake measurements, including portions of food and energy balance, to observe the intended behavioural change. Our review focused on interventions that measured effectiveness beyond 9 months to depict intermediate- and long-term effects and found significant differences between persons who were subject to CBI and those who were not in most dietary outcome measures. Thus, integrating dietary components along with other lifestyle interventions such as physical activity, cessation of smoking and alcohol consumption could help to reduce the burden of CVD and risk factors at the population level(Reference Hassen, Ndejjo and Musinguzi18,Reference Hassen, Ndejjo and Van Geertruyden98) .
Methodological considerations
We assessed the risk of bias of studies using tools from the Cochrane Collaboration. However, this quality assessment was hampered by inadequate reporting of each component, particularly reporting of bias from the intended intervention and bias due to missing outcome data. For randomised studies, sequence generation, methods of allocation concealment and blinding were not well described in some of the eligible studies. For non-randomised studies, an inadequate description of study participant selection and insufficient list of confounders and how they were adjusted were among the issues that affected the risk of bias assessment. We recommend the use of standard guidelines to accurately report methodological processes to ensure appropriate interpretation of results and to provide replicable methods for future similar studies.
Furthermore, we considered individual RCT, cluster RCT and NRC studies in the analysis. The unit of randomisation and sampling is different for these study designs, and thus the CI for the effect size might be narrow because clustering would not be taken into account. Nevertheless, we used a Hartung–Knapp–adjusted Sidik–Jonkman method to estimate CI, which is a conservative approach, and the results are less likely to be biased.
Limitations
By using a systematic approach and two independent reviewers throughout the process, our methodology was strengthened. Nevertheless, interpretation of findings from this review should consider the following limitations. First, restriction of articles to only the English language might have resulted in language bias. Second, owing to the heterogeneity in outcome measurement techniques and inconsistent reporting, we could not perform a meta-analysis for some of the outcomes. Nevertheless, these outcomes were summarised using narrative synthesis. Third, the observed effects of a few outcome measures seems heterogeneous. However, we constructed CI using the Hartung–Knapp-adjusted Sidik–Jonkman method, which resulted in more conservative intervals in case of a small number of studies and large heterogeneity(Reference Sidik and Jonkman99).
Conclusions
This review shows that community-based CVD preventive interventions have the potential of improving dietary patterns and, in turn, CVD risk profiles among adults. Interventions appear to decrease individuals’ daily energy intake, fat and saturated fat percentage of energy, and increase intake of fibre, fruits, and vegetables. A decline in effect size was observed at a longer follow-up, indicating low sustainability after the intervention duration. Intervention components with tailored lifestyle coaching, individual and/or group health education, community-wide health promotion activities, and/or structural and systemic changes such as improving availability of affordable fresh fruits and vegetables in corner stores demonstrated more pronounced effects. Thus, development and implementation of context-specific preventive intervention is beneficial to improve dietary factors, which in turn decrease morbidity and mortality associated with CVD and other non-communicable diseases. Furthermore, favourable intervention effects need to be sustained for longer through linkages with existing primary care centres or community organisations.
Acknowledgements
Acknowledgements: The authors would like to thank the European Commission Horizon 2020 research and innovation, for funding this work. Financial support: This work is supported by the SPICES project which received funding from the European Commission through the Horizon 2020 research and innovation action grant (No: 733356). The funder had no role in the design, decision to publish or preparation of the manuscript. Authorship: H.Y.H., H.B. and S.A. conceived the review. H.Y.H. and R.N. was responsible for conducting the electronic searches and abstract screening. H.Y.H. and B.G.S. independently screened full texts, made final decisions on included papers and extracted data. H.B., S.A. and JV worked closely with H.Y.H. to plan the data analysis. H.Y.H. and B.G.S. performed the analysis, and H.Y.H. wrote the original draft of the manuscript. H.B., S.A., JV, DL and G.M. have provided supervision throughout the review. All other authors reviewed and edited the manuscript for intellectual content. All authors read and approved the final version of the manuscript. Ethics of human subject participation: Not applicable
Conflicts of interest:
We declare no competing interests.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S1368980023000976






