In the 2013 report by the FAO of the United Nations, it was recommended that the digestible indispensable amino acid score (DIAAS) replaces the protein digestibility-corrected amino acid score as the method for protein quality evaluation in foods for humans(1). The growing pig has been recognised as an appropriate model for determining DIAAS(1). To calculate DIAAS, the apparent ileal digestibility of amino acids (AA) is determined and then corrected for the basal endogenous loss of AA and the resulting values are described as standardised ileal digestibility (SID) values(Reference Stein, Sève and Fuller2). These values are equivalent to the true ileal digestibility values that are needed to calculate DIAAS in foods for humans(1,Reference Stein, Sève and Fuller2) .
Dairy proteins have DIAAS that are greater than plant proteins(Reference Rutherfurd, Fanning and Miller3,Reference Mathai, Liu and Stein4) . Other animal proteins, such as fish and animal protein hydrolysates, have greater DIAAS than plant proteins(Reference Shaheen, Islam and Munmun5,Reference Bindari, Lærke and Nørgaard6) , and the DIAAS of beef was recently determined to be greater than that in plant proteins(Reference Hodgkinson, Montoya and Scholten7). Meat is a concentrated source of protein providing a balanced indispensable AA (IAA) pattern in diets for humans, and in most cases, meat is further processed prior to consumption(Reference Seman, Boler and Carr8). Hodgkinson et al. (Reference Hodgkinson, Montoya and Scholten7) observed only minor differences in AA digestibility among beef topside steaks cooked by various techniques and to a common degree of doneness (internal temperature of 71°C). Further processing techniques may increase the digestibility of AA because of changes in the three-dimensional structures of proteins(Reference Tornberg9,Reference Santé-Lhoutellier, Astruc and Marinova10) .
To our knowledge, no DIAAS values have been reported for meat heated to various degrees of doneness as commonly consumed by humans. Furthermore, the influence of processing (i.e. drying, curing or fermenting) on the SID of IAA and DIAAS has yet to be determined. Therefore, the objectives of this research were to test the hypotheses that meat products have DIAAS that are >100 and that cooking of ground or unground meat increases the SID of AA and the DIAAS of the protein.
Materials and methods
The Institutional Animal Care and Use Committee at the University of Illinois reviewed and approved the protocol for this experiment (protocol no. 16113).
Preparation of meat products
Eight meat products were prepared at the Meat Science Laboratory at North Dakota State University, Fargo, ND, USA (Tables 1 and 2). These products included salami, bologna, beef jerky, beef ribeye roast (cooked to three degrees of doneness: 56°C, 64°C or 72°C), raw ground beef and cooked ground beef. All products were obtained from commercial sources that are international suppliers of meat and meat products. Salami, bologna and beef jerky were processed in accordance with Appendix A of the Food Safety and Inspection Services Compliance Guidelines for Meeting Lethality Performance Standards for Certain Meat and Poultry Products(11). The beef ribeye roasts (Institutional Meat Purchase Specifications no. 112A)(12,13) represented three treatments that were cooked at 56°C (medium-rare), 64°C (medium) or 72°C (well-done). The internal temperature of each ribeye roast was monitored using thermocouples placed in the centre of each roast. All roasts were removed from the cooking cycle when the temperature was 5°C less than the desired degree of doneness, and roasts were allowed to rest at room temperature to reach the final internal treatment temperature. The raw ground beef was coarse ground and one portion was uncooked and the remaining ground beef was fully cooked (>72°C), drained of grease and chilled prior to packaging and freezing at North Dakota State University. All meat products were vacuum-packaged before being shipped to the University of Illinois, where they were stored at −20°C until use. At the University of Illinois, the beef jerky was chopped in a four-quart commercial food processor (Waring) and the salami, bologna and ribeye roasts were chopped in an eight-quart bowl chopper (Professional Processor Food Equipment).
Table 1. Cooking procedure for the eight meat products
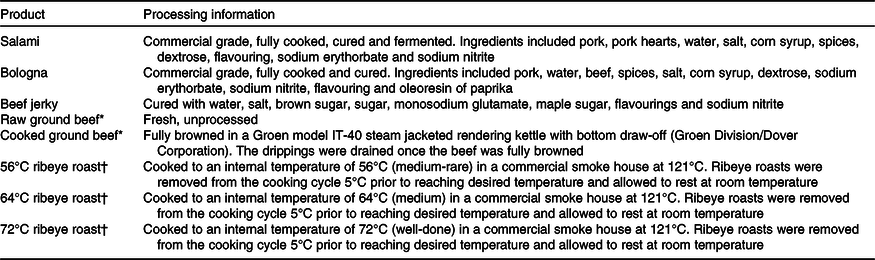
Table 2. Analysed nutrient composition of meat products and the nitrogen-free diet (as-fed basis)
AEE, acid-hydrolysed ether extract; AA, amino acid.
* To convert kcal to kJ, multiply by 4·184.
Diets, animals, housing and feeding
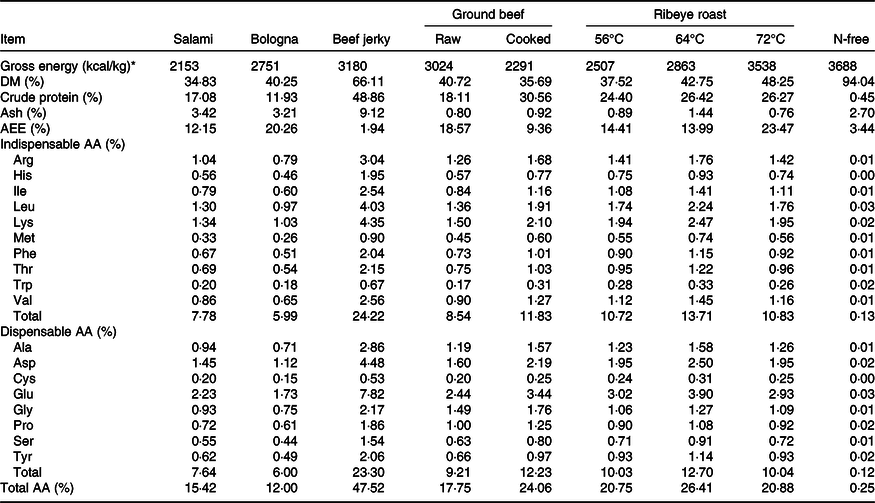
Nine diets were formulated (Tables 3 and 4); eight diets contained a single meat product as the only crude protein (CP) and AA-containing ingredient. A N-free diet was also formulated and used to determine basal endogenous losses of CP and AA. Titanium dioxide was included in the N-free diet at 0·5 % as an indigestible marker. Vitamins and minerals were also included in the N-free diet to meet or exceed currently reported nutrient requirements for growing pigs(14). The meat products were combined daily with sufficient amounts of the N-free diet to provide approximately 16 % CP on an as-fed basis.
Table 3. Ingredient composition of experimental diets (as-fed basis)*
* All diets were formulated to contain approximately 16 % crude protein on an as-fed basis.
† The vitamin–micromineral premix provided the following quantities of vitamins and microminerals per kg of complete diet: vitamin A as retinyl acetate, 3·83 mg; vitamin D3 as cholecalciferol, 0·06 mg; vitamin E as dl-α tocopheryl acetate, 66 mg; vitamin K as menadione dimethylpyrimidinol bisulphite, 1·42 mg; thiamin as thiamine mononitrate, 0·24 mg; riboflavin, 6·59 mg; pyridoxine as pyridoxine hydrochloride, 0·24 mg; vitamin B12, 0·03 mg; d-pantothenic acid as d-calcium pantothenate, 23·5 mg; niacin, 44·1 mg; folic acid, 1·59 mg; biotin, 0·44 mg; Cu, 20 mg as copper sulphate and copper chloride; Fe, 126 mg as ferrous sulphate; iodine, 1·26 mg as ethylenediamine dihydriodide; Mn, 60·2 mg as manganese sulphate; Se, 0·3 mg as sodium selenite and selenium yeast and Zn, 125·1 mg as zinc sulphate.
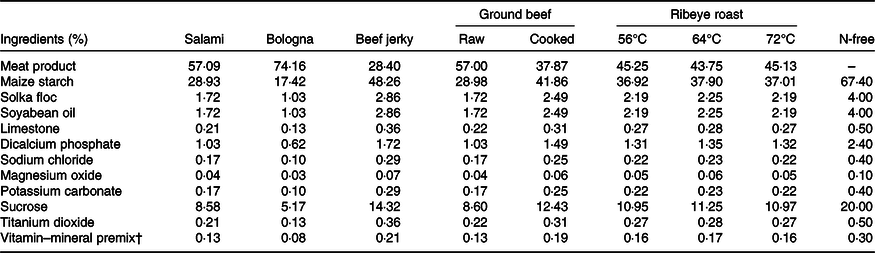
Table 4. Calculated nutrient composition of experimental diets (DM basis)
AA, amino acid.
* Nutrient composition of the N-free diet was analysed.
† Diets were formulated to contain approximately 16 % crude protein on an as-fed basis.
Nine growing female pigs (Line 2, Pig Improvement Company) with an initial body weight of 35·50 (sd 3·77) kg were surgically fitted with a T-cannula in the distal ileum using procedures adapted from Stein et al. (Reference Stein, Shipley and Easter15). Following surgery, pigs were allowed a 7-d recovery period and then allotted to a 9 × 8 Youden square design with nine diets and eight 7-d periods. All pigs received each diet only once during the experiment; therefore, there were eight replicate pigs per treatment. With eight replicate pigs per treatment, we expected to be able to detect differences in SID values among ingredients of 2–5 %, depending on AA(Reference Rojas, Venyeta and Stein16). Pigs were housed in individual pens (2 × 3 m) that had partially slatted concrete flooring in an environmentally controlled room. Each pen was equipped with smooth plastic siding, a feeder and a nipple drinker. At the conclusion of the experiment, pigs were approximately 18 weeks of age and had a body weight of 100·38 (sd 4·41) kg.
Pigs were fed a daily amount of feed equivalent to 4 % of body weight in two equal meals that were provided at 08.00 and 17.00 hours. All pigs were weighed at the beginning of each period to calculate the feed allowance during the following period, and all pigs were weighed at the conclusion of the experiment. Water was available at all times throughout the experiment.
Sample collection
The initial 5 d of each period was considered the adaptation phase to the diets with the following 2 d being used for ileal digesta collection. Ileal digesta collection began at 08.00 hours and ceased at 17.00 hours each day following procedures explained by Stein et al. (Reference Stein, Shipley and Easter15). In brief, cannulas were opened and cleaned, a 232 ml capacity plastic bag was attached to the cannula barrel and secured by a cable tie and ileal digesta flowing into the bag were collected. Bags were removed when they were filled with ileal digesta, or at least once every 30 min, and immediately stored at –20°C to prevent bacterial degradation of AA in the ileal digesta.
Chemical analysis
At the conclusion of each experimental period, ileal digesta samples were thawed at room temperature and mixed within animal and diet, and a subsample was collected. A subsample of each source of protein was collected at the start of the experiment, and a sample of the N-free diet was collected at the time of mixing. Ileal digesta and meat product samples were lyophilised and finely ground prior to chemical analysis. Meat products were analysed in duplicate for DM (method 930.15)(17), ash at 600°C for 12 h (method 942.05)(17) and AA (method 982.30 E (a, b, c))(17). Meat products were also analysed in triplicate for CP using the Kjeldahl method by quantifying N and using a conversion factor of 6·25 to calculate CP (method 984.13)(17) on a KjeltecTM 8400 (FOSS Inc.), for GE using an isoperibol bomb calorimeter (model 6400; Parr Instruments) with benzoic acid as the standard for calibration, and for acid hydrolysed ether extract using the acid hydrolysis filter bag technique (Ankom HCl Hydrolysis System; Ankom Technology) followed by crude fat extraction using petroleum ether (AnkomXT15 Extractor; Ankom Technology). The N-free diet and ileal digesta samples were analysed in duplicate for DM and AA as explained for the meat products. CP was determined in the N-free diet and in ileal digesta samples by the combustion procedure (method 990.03)(17) using a LECO FP628 Analyzer (LECO Corp.). The N-free diet and ileal digesta samples were also analysed in duplicate for Ti(Reference Myers, Ludden and Nayigihugu18).
Calculations
The apparent ileal digestibility of CP and AA was calculated for all diets as previously explained by Stein et al. (Reference Stein, Sève and Fuller2). Values for apparent ileal digestibility of CP and AA were corrected for the basal endogenous loss of CP and each AA to calculate the SID of CP and AA(Reference Stein, Sève and Fuller2). The SID of CP and AA calculated in each diet also represented the SID of the meat product.
The concentration of SID AA (g/kg) in each meat product was calculated by multiplying the SID value (%) for each AA by the concentration (g/kg) of that AA in the product, and this value was divided by the concentration of CP in the product to calculate digestible indispensable AA content (mg) in 1 g protein(Reference Mathai, Liu and Stein4,Reference Cervantes-Pahm, Liu and Stein19) . The digestible indispensable AA reference ratios were calculated for each meat product using the following equation(1) (1):
 $$\eqalign{\rm{Digestible\ indispensable\ AA\ reference\ ratio =} \cr \hskip -180pt\rm{digestible\ indispensable\ AA\ content\ in\ 1\ g\ protein\ }\cr \hskip -180pt \rm of\ food\ (mg)/mg\ of\ the\ same\ dietary\ indispensable\cr \ \hskip -183pt \rm AA\ in\ 1\ g\ of\ reference\ protein.}$$
$$\eqalign{\rm{Digestible\ indispensable\ AA\ reference\ ratio =} \cr \hskip -180pt\rm{digestible\ indispensable\ AA\ content\ in\ 1\ g\ protein\ }\cr \hskip -180pt \rm of\ food\ (mg)/mg\ of\ the\ same\ dietary\ indispensable\cr \ \hskip -183pt \rm AA\ in\ 1\ g\ of\ reference\ protein.}$$Separate ratios were calculated using the reference protein for children from 6 months to 3 years, and children older than 3 years, adolescents and adults(1). The DIAAS were calculated using the following equation(1) (2):
 $${\rm{DIAAS}}\;(\% ) = {\mkern 1mu} 100 \times {\rm{lowest}}\;{\rm{value}}\;{\rm{of}}\;{\rm{digestible}}\;{\rm{indispensable\backslash crAA}}\;{\rm{reference}}\;{\rm{ratio}}.$$
$${\rm{DIAAS}}\;(\% ) = {\mkern 1mu} 100 \times {\rm{lowest}}\;{\rm{value}}\;{\rm{of}}\;{\rm{digestible}}\;{\rm{indispensable\backslash crAA}}\;{\rm{reference}}\;{\rm{ratio}}.$$Statistical analysis
Studentised residuals from each analysis were used to test normality of data. Outliers were removed until the Shapiro–Wilk’s test reached P > 0·05, and studentised residuals were within ±3 sd. Data were analysed using PROC MIXED of SAS (SAS Institute Inc.) in a randomised complete block design with the pig as the experimental unit. The statistical model to determine differences in SID of AA among meat products included diet as the main effect and pig and period as random effects. Treatment means were calculated using the LSMEANS statement, and if significant, means were separated using the PDIFF option of the MIXED procedure. Significance and tendencies were considered at P < 0·05 and 0·05 ≤ P < 0·10, respectively.
Results
All pigs remained healthy throughout the experiment and readily consumed their daily feed allowance. Data from eight replications were used for the apparent ileal digestibility, SID and DIAAS calculations for the following treatments, bologna, cooked ground beef, 56°C ribeye roast and 72°C ribeye roast. Data from seven replications were used for beef jerky and raw ground beef, and data from six replications were used for salami and 64°C ribeye roast.
Standardised ileal digestibility
Among salami, bologna and beef jerky, the SID of all AA did not differ (Table 5). Ribeye roast heated to 56°C had greater (P < 0·05) SID of most AA compared with ribeye roast heated to 64°C with the exception that no differences were observed for the SID of isoleucine, methionine, tryptophan, asparagine, cysteine and glutamate. Ribeye roast heated to 72°C had greater (P < 0·05) SID of arginine, threonine, tryptophan, alanine, glycine and serine than 64°C ribeye roast, but the SID of all AA, except asparagine, did not differ between ribeye roasts heated to 56 and 72°C. Raw ground beef had greater (P < 0·05) SID of histidine, leucine, lysine, methionine, phenylalanine, asparagine, cysteine and tyrosine than cooked ground beef, but cooked ground beef had greater (P < 0·05) SID of arginine than raw ground beef.
Table 5. Standardised ileal digestibility of amino acids (AA) in products*†
(Mean values and pooled standard errors)
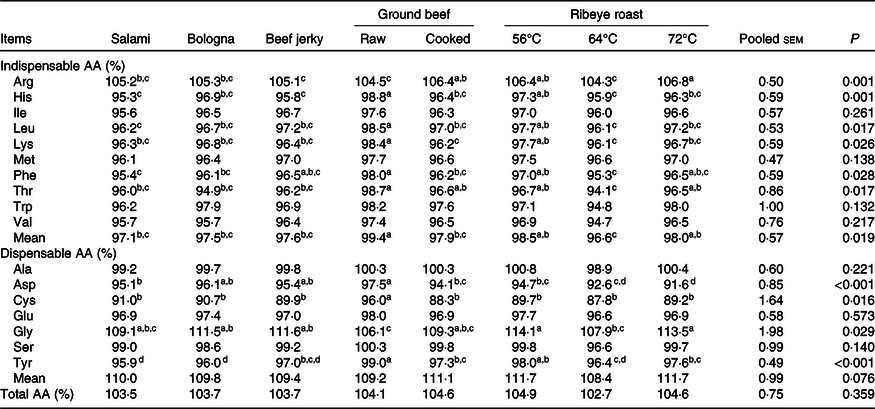
a,b,c,d Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* Data are least squares means of eight observations per treatment except for beef jerky and raw ground beef that have seven observations per treatment and for salami and 64°C ribeye roast that have six observations per treatment.
† Standardised ileal digestibility values were calculated by correcting values for apparent ileal digestibility for the basal ileal endogenous losses. Endogenous losses (g/kg of DM intake) AA were as follows: crude protein, 25·83; arginine, 1·16; histidine, 0·26; isoleucine, 0·35; leucine, 0·61; lysine, 0·58; methionine, 0·09; phenylalanine, 0·37; threonine, 0·69; tryptophan, 0·14; valine, 0·58; alanine, 0·84; asparagine, 1·00; cysteine, 0·28; glutamine, 1·13; glycine, 2·43; proline, 9·66; serine, 0·65; tyrosine, 0·29.
Raw ground beef had the greatest (P < 0·05) SID of cysteine among all meat products. The SID of threonine and tryptophan was greater (P < 0·05) in raw and cooked ground beef than in ribeye roast heated to 64°C. Raw ground beef and 56°C ribeye roast also had greater (P < 0·05) SID of histidine, leucine, methionine and phenylalanine than salami, and raw ground beef had greater (P < 0·05) SID of histidine, leucine and lysine than all other products except ribeye roast heated to 56°C. The SID of arginine and histidine was greater (P < 0·05) in ribeye roast heated to 56°C than in beef jerky, and the 56°C ribeye roast had greater (P < 0·05) SID of histidine, leucine, methionine, phenylalanine and tyrosine than salami, whereas the only difference between ribeye roast heated to 56°C and bologna was the SID of tyrosine. The SID of all AA, except tryptophan, did not differ among salami, bologna, beef jerky and ribeye roast heated to 64°C. Raw ground beef had greater (P < 0·05) SID of total IAA than all other products except ribeye roast heated to 56 and 72°C. The SID of total dispensable AA was greater (P < 0·05) in ribeye roast heated to 56 and 72°C than in raw ground beef and greater (P < 0·05) in cooked ground beef than in the 64°C ribeye roast. The SID of total AA was greater (P < 0·05) in ribeye roast heated to 56°C than in ribeye roast heated to 64°C.
Digestible indispensable amino acid scores
For children from 6 months to 3 years (Table 6), ribeye roast heated to 64°C and bologna had the greatest (P < 0·05) DIAAS and cooked ground beef had the lowest (P < 0·05) DIAAS. The DIAAS for raw ground beef was lower (P < 0·05) than that for ribeye roast heated to 64°C and bologna, but greater (P < 0·05) than for all other products, whereas the DIAAS for salami was less (P < 0·05) than that for ribeye roast heated to 64°C, bologna and raw ground beef. Beef jerky and ribeye roast heated to 56°C were not different, but both had greater (P < 0·05) DIAAS than the 72°C ribeye roast and cooked ground beef, and the DIAAS for ribeye roast heated to 72°C was greater (P < 0·05) than that for cooked ground beef.
Table 6. Digestible indispensable amino acid scores (DIAAS) for the eight meat products*
(Mean values and pooled standard errors)
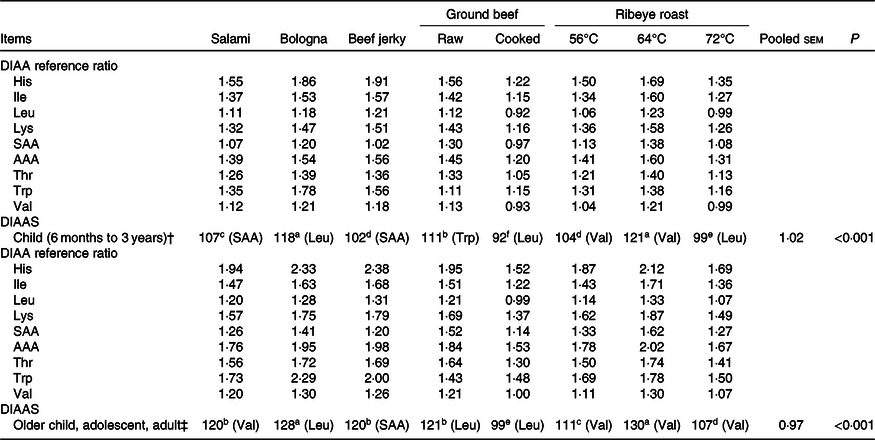
DIAA, digestible indispensable amino acid; SAA, sulphur amino acids (methionine + cysteine); AAA, aromatic amino acids (phenylalanine + tyrosine); AA, amino acid.
a,b,c,d,e,f Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* First-limiting AA is in parentheses.
† DIAAS were calculated using the recommended AA scoring pattern for a child (6 months to 3 years). The indispensable AA reference patterns are expressed as mg AA/g protein: histidine, 20; isoleucine, 32; leucine, 66; lysine, 57; sulphur AA, 27; aromatic AA, 52; threonine, 31; tryptophan, 8·5; valine, 40(1).
‡ DIAAS were calculated using the recommended AA scoring pattern for older child, adolescent and adult. The indispensable AA reference patterns are expressed as mg AA/g protein: histidine, 16; isoleucine, 30; leucine, 61; lysine, 48; sulphur AA, 23; aromatic AA, 41; threonine, 25; tryptophan, 6·6; valine, 40(1).
For children older than 3 years, adolescents and adults, ribeye roast heated to 64°C and bologna had the greatest (P < 0·05) value for DIAAS and cooked ground beef had the least (P < 0·05) DIAAS. Raw ground beef, salami and beef jerky were not different, but these proteins had DIAAS that were greater (P < 0·05) than ribeye roast heated to 56°C, which had a greater (P < 0·05) DIAAS than the 72°C ribeye roast.
For DIAAS calculated for both children from 6 months to 3 years and children older than 3 years, adolescents and adults, the first-limiting AA in the three types of ribeye roast products was valine, with the exception that leucine was the first-limiting AA for DIAAS in ribeye roast heated to 72°C. The first-limiting AA in raw and cooked ground beef and in bologna was leucine for both children from 6 months to 3 years and children older than 3 years, adolescents and adults, with the exception that tryptophan was the first-limiting AA in raw ground beef for children from 6 months to 3 years. The first-limiting AA for children from 6 months to 3 years in beef jerky and salami were the sulphur-containing AA. The sulphur-containing AA were also the first-limiting AA in beef jerky for children older than 3 years, adolescents and adults, whereas valine was the first-limiting AA in salami.
Discussion
Global undernutrition is estimated to affect 815 million people, or 11 % of the global population(20). For children, stunting is considered the best available measurement of chronic malnutrition and globally about one in four children under the age of 5 years are affected(20,Reference Semba, Shardell and Ashour21) . Stunting before the age of 2 years is associated with increased morbidity and mortality and impaired cognitive and motor development(Reference Black, Victora and Walker22). Stunting also has the potential to decrease economic development of entire nations, as indicated by the high prevalence of stunting in developing countries(20).
Sub-Saharan Africa and Southern Asia have rates of stunting that are among the highest in the world(20). Diets in these regions are largely composed of cereal grains, such as sorghum, wheat, rice or maize, which are poor sources of IAA, especially lysine(Reference Shaheen, Islam and Munmun5,Reference Cervantes-Pahm, Liu and Stein19,20,Reference Abelilla, Liu and Stein23) . There is a strong association between low dietary intakes of high-quality proteins and stunting(Reference Semba, Shardell and Ashour21,Reference Ghosh, Suri and Uauy24) . Consequently, complementing cereal-based diets with higher-quality proteins may overcome IAA deficiencies. The excellent quality of dairy proteins was demonstrated by Rutherfurd et al. (Reference Rutherfurd, Fanning and Miller3) and Mathai et al. (Reference Mathai, Liu and Stein4), and high DIAAS for beef cooked to an internal temperature of 71°C was reported by Hodgkinson et al. (Reference Hodgkinson, Montoya and Scholten7). Meat can be prepared by various techniques and to different degrees of doneness, but to our knowledge, there have been no studies conducted to determine DIAAS for meat that have undergone extensive processing (i.e. drying, curing or fermenting), nor have the effects of varying degrees of doneness on DIAAS been reported.
In the present experiment, concentrations of CP and AA in all meat products were generally within the range of published values(Reference Bodwell, Anderson and Bechtel25,26) , with the exception that, to our knowledge, there are no published values for concentrations of AA in beef jerky. The physicochemical characterisation of the beef jerky used in this experiment was in accordance with the United States Department of Agriculture and Food Safety and Inspection Services compliance guidelines for meat and poultry jerky(27). When compared with similar dried beef products, the concentration of AA was slightly greater in beef jerky, which is probably due to variations in formulation(26). For all meat products used in this experiment, the AA with the lowest concentration were tryptophan, methionine and histidine, in that order, regardless of the cooking method, which is in agreement with the reported data(26).
The concentration of CP in all treatment diets, except the N-free diet, was formulated to 16 % to provide the pig with quantities of AA according to current requirements(14), which are greater than the FAO recommended CP concentration of 10 %(1). However, when calculating SID of AA, values are corrected for basal endogenous losses of AA, which are independent of the CP concentrations of the treatment diets(Reference Stein, Sève and Fuller2). Subsequently, DIAAS values are calculated based on SID of AA, and therefore, DIAAS values are also independent of the CP of the diets. A few AA had SID values above 100, which is an artifact of using the N-free diet to determine basal endogenous losses of AA(Reference Stein, Sève and Fuller2).
The protein quality of raw ground beef clearly decreased after cooking. The meat was fully browned using a Groen model IT-40 steam jacketed rendering kettle with bottom draw-off (Groen Division/Dover Corporation). Steam was circulated between the kettle and the outer wall, or jacket, and did not come into contact with the meat. Cooking of the ground beef was constantly monitored by a technician, and the ground beef was continuously turned with a spatula to prevent burning or over-browning. The United States Department of Agriculture has set the safe minimum internal temperature for ground meats at 71·1°C(28). In this experiment, the ground beef was considered fully cooked when the batch was fully browned with no remaining pink/red meat and the temperature was recorded above 72°C in three locations. Cooking meat to 70°C may enhance protein hydrolysis by proteolytic enzymes due to the progressive effect heat has on protein denaturation(Reference Gatellier, Kondjoyan and Portanguen29–Reference Bax, Buffière and Hafnaoui31). However, cooking at 100°C may lead to protein oxidation and the formation of carbonyl groups that may interact with free amino groups of non-oxidised AA leading to modification of AA, especially lysine, histidine and arginine(Reference Santé-Lhoutellier, Astruc and Marinova10,Reference Gatellier, Kondjoyan and Portanguen29) . Cooking is important to inactivate pathogenic micro-organisms and is used to enhance the flavour of the meat(Reference Santé-Lhoutellier, Astruc and Marinova10), but in the present experiment, analysed concentrations of lysine and histidine decreased in raw ground beef post-cooking, which indicates that heating to temperatures <100°C may negatively affect AA bioavailability. The decrease in digestibility and protein quality of cooked ground beef compared with raw ground beef further indicates that heat damage occurred even though the temperature was closely monitored. It therefore appears that heat damage of ground beef may occur well before the cooking temperature reaches 100°C.
The observation that there was no difference in the SID of IAA between ribeye roast cooked to internal temperatures of 56 and 72°C may be attributed to the use of finite temperature targets in combination with thermometers to obtain the desired degree of doneness. In addition, ribeye roasts were cooked as whole-muscle, intact roasts, which reduced the protein exposure to O2 and light. The lower SID of most IAA in ribeye roast heated to 64°C compared with the ribeye roast heated to 56°C may be a result of the reduced amount of fat in the product because increased fat increases SID of AA due to reduced rate of passage(Reference Li and Sauer32,Reference Cervantes-Pahm and Stein33) . The reason the ribeye roast heated to 64°C had the greatest DIAAS compared with all other meat products, despite lower SID values, is that this product had a higher concentration of IAA compared with other meat products included in the present experiment, which is probably also a consequence of the lower concentration of fat. Overall, cooking the ribeye roasts to different degrees of doneness had little impact on AA digestibility or protein quality.
Salami and bologna are cured, smoked sausages and in this experiment, both were fully cooked to the United States Department of Agriculture recommended safe internal temperature of 71·1°C(11,28) . Salami and bologna differ in that salami was subjected to a fermentation process to lower the pH and to improve shelf-life. Curing with sodium chloride and sodium nitrite is another method of meat preservation; however, with the advancement of modern preservation methods in developed countries, curing is more commonly used to obtain a reddish-pink colour and induce a particular flavour in the end product(Reference Heinz and Hautzinger34). In developing countries, curing as a means of preserving meat is important in ensuring microbial stability and is essential in improving food distribution to malnourished populations(Reference Weaver, Dwyer and Fulgoni35). Addition of ingredients that prevent oxidation during processing (i.e. nitrite and sodium chloride) may also aid in maintaining the structure of protein prior to digestion(Reference Van Hecke, Vossen and Vanden Bussche36). Beef jerky, another highly processed, cured meat product, is nutrient dense and shelf-stable due to removal of the majority of the moisture by drying(27). For a product to be labelled jerky, it must be dried to a moisture:protein ratio of <0·75:1 and have a water activity of <0·85, and prior to dehydration, a temperature of 71·1°C must be reached, which enables the jerky to be kept at room temperature for up to 12 months(27). Salami, bologna and beef jerky were all classified as ‘excellent’-quality proteins with similar DIAAS to raw ground beef, further confirming that the processing techniques used to produce salami, bologna and jerky do not negatively affect protein quality.
The high digestibility of the meat proteins in this experiment may partially be attributed to grinding. Grinding of whole-muscle proteins (i.e. intact ribeye roast) prior to consumption may have an impact similar to chewing, which increases the surface area of the protein during ingestion leading to more rapid digestion and absorption of AA(Reference Rémond, Machebeuf and Yven37,Reference Pennings, Groen and van Dijk38) . In contrast, grinding meat prior to cooking increases the surface area exposed to O2 and light(Reference Soladoye, Juárez and Aalhus39), and in combination with higher cooking temperatures may have had a more negative impact on the DIAAS for cooked ground beef than for the ribeye roasts. For salami and bologna, the meat was ground prior to cooking, but these products were also encased and heated from the outside, which may have resulted in more uniform cooking.
Using DIAAS cut-off values, protein quality can be described as ‘excellent’, if DIAAS is >100 and ‘good’, if DIAAS is between 75 and 99(1). Based on these cut-off values, all meat products used in this experiment, except cooked ground beef, are ‘excellent’-quality proteins if consumed by children older than 3 years, adolescents and adults. The ‘good’ quality of cooked ground beef is attributable to the inadequate amount of leucine to meet the requirement of children older than 3 years, adolescents and adults. For children from 6 months to 3 years, ribeye roast heated to 72°C and cooked ground beef are ‘good’- quality proteins due to the limiting amount of leucine, but all other proteins are ‘excellent’ quality.
Hodgkinson et al. (Reference Hodgkinson, Montoya and Scholten7) reported DIAAS for beef from 80 to 99 as evaluated in the growing pig. Raw beef and roasted beef were reported with a DIAAS of 97 and 91, respectively(Reference Hodgkinson, Montoya and Scholten7). Thus, the DIAAS for raw ground beef and the three ribeye roast products calculated in the present experiment are greater than the scores reported by Hodgkinson et al. (Reference Hodgkinson, Montoya and Scholten7). The beef prepared by Hodgkinson et al. (Reference Hodgkinson, Montoya and Scholten7) was trimmed of all adipose and connective tissue, whereas the meat in the present experiment had 12 to 24 % analysed fat, with the exception of cooked ground beef and beef jerky. In addition, in the experiment by Hodgkinson et al. (Reference Hodgkinson, Montoya and Scholten7), ileal digesta were collected by anaesthetising and then euthanising each pig and dissecting the terminal ileum from the body. In contrast, in the present experiment, ileal digesta were collected for 9 h on two consecutive days, which may have resulted in a more representative sampling of digesta. The DIAAS reference pattern was not stated by Hodgkinson et al. (Reference Hodgkinson, Montoya and Scholten7), which may explain DIAAS being <100, as younger age groups have higher requirements for AA.
Rutherfurd et al. (Reference Rutherfurd, Fanning and Miller3) reported DIAAS for whey and milk proteins determined in the growing rat ranging from 97 to 118, and Mathai et al. (Reference Mathai, Liu and Stein4) reported DIAAS for whey and milk proteins determined in the growing pig ranging from 123 to 141. The DIAAS for the meat proteins evaluated in the present experiment ranged from 99 to 130, and according to the FAO(1), DIAAS >100 indicate the potential of that protein to complement lower-quality proteins. Specifically, meat proteins may complement cereal grains because of the high concentration of lysine in meat, whereas cereal grains are limiting in lysine(Reference Rutherfurd, Fanning and Miller3,Reference Cervantes-Pahm, Liu and Stein19,Reference Abelilla, Liu and Stein23) . In addition, most of the meat products included in this experiment had a low concentration of leucine, but leucine is available in high concentration in maize and sorghum(Reference Cervantes-Pahm, Liu and Stein19), which indicates that a combination of cereal grains and meat will result in a high-quality protein source.
In conclusion, data from this experiment indicate that meat products are generally high-quality proteins and are comparable in quality with dairy proteins. Fermenting, curing, drying or cooking meat to minimally safe internal temperatures does not negatively affect SID of AA or DIAAS. However, cooking meat that is ground or cooking meat with direct heat, for example, on the stovetop, may reduce protein quality, especially if overcooking occurs. The use of thermometers to monitor the internal temperature may aid in reducing the risk of overcooking and, therefore, contribute to maintaining the high quality of meat protein.
Acknowledgements
Funding for this research was provided by the National Cattlemen’s Beef Association, Centennial, CO, USA.
The contributions of the authors were as follows: H. M. B. conducted the animal work and laboratory work, analysed the data and wrote the majority of the manuscript. J. K. M. assisted with the animal work and data analysis. E. P. B. secured the funding and contributed to interpretation of data. H. H. S. was the principle investigator. He prepared the experiment proposal, secured approval from appropriate animal welfare regulatory bodies, designed the experiment, oversaw the development of the experiment and wrote the final version of the manuscript.
The authors declare no conflicts of interest.









