Household food insecurity (HFI) happens when people do not have, at all times, physical, social and economic access to sufficient, safe and nutritious food which meets their dietary needs and food preferences for an active and healthy life( 1 ). HFI is a global epidemic that can lead to hunger and malnutrition. The FAO estimates that 795 million people are undernourished globally( 2 ). In Brazil, only 62·5 % of households are considered food secure and 4·8 % are severely food insecure, a percentage that reaches 5·8 % in households with minors under the age of 18 years( Reference Brasil 3 ).
HFI is known to be associated with insufficient or inadequate food intake( Reference Addo, Marquis and Lartey 4 ) and this low intake of healthy foods and micronutrients is associated with worse health status( Reference Stuff, Casey and Szeto 5 ), both in developed and developing countries. Children are negatively affected by HFI( Reference Saha, Frongillo and Alam 6 , Reference Monteiro, Schmidt and Costa 7 ) as it has been associated with a higher prevalence of hospitalization, respiratory infection, fever, diarrhoea, and nutritional deficiencies such as Fe deficiency and other forms of malnutrition compared with children living in food-secure households( Reference Cook, Frank and Berkowitz 8 – Reference Ohemeng, Marquis and Lartey 10 ).
Food deprivation and poor dietary quality in childhood influence children’s growth, psycho-emotional, social and cognitive development, and overall health( Reference Perez-Escamilla and Vianna 11 , Reference Perez-Escamilla 12 ). According to the 2006 Brazilian Demographic and Health Survey (DHS), only 57 % of 12- to 18 month-old children ate fruits and vegetables and 25 % ate meat on a daily basis( Reference Brasil 3 ). In Brazil, 7 % of children suffer from chronic malnutrition (height-for-age Z-score (HAZ)<−2)( Reference Brasil 3 ); additionally, 1·6 % of deaths of children under 5 years old are caused by acute diarrhoea and 5·3 % by acute respiratory infection( Reference Brasil 13 ).
Although the influence of HFI on dietary intake has been investigated in the last few years in Brazil and elsewhere( Reference Morais, Dutra and Franceschini 14 – Reference Rosas, Harley and Fernald 16 ), the association between HFI and common childhood infectious diseases needs more investigation( Reference Ohemeng, Marquis and Lartey 10 ). Thus, our study aimed to identify the independent association of HFI with the risk of vitamin A deficiency and anaemia, morbidities such as cough and fever, and hospitalizations for diarrhoea and pneumonia in children under 5 years old.
Methods
We analysed data from the 2006 Brazilian DHS, which is a nationally representative cross-sectional survey with complex probability sampling that covered rural and urban areas of each of the five Brazilian geographic regions. This survey was approved by the Research Ethics Committee of the Sexually Transmitted Diseases/AIDS Reference and Training Center of the São Paulo State Department of Health.
The process of sample selection for the 2006 Brazilian DHS has been described in detail elsewhere( Reference Brasil 3 ). Briefly, participating households were selected in two stages, with the first being the selection of census tracts (primary sampling units) and the second the selection of households within each tract (secondary sampling units). A total of 14 617 households were eligible for the survey. The target population of the survey was women of reproductive age (15–49 years) and their children living in the selected households. The survey interviewed 15 468 women and collected data on 5461 children up to 59 months of age.
Our study focused on children under 5 years old. In our study, households were excluded if the target children had died before the interview, did not live with their mother (because HFI is assessed within households and the available HFI data refer to the mother’s household), had missing HFI or anthropometric data, or had implausible anthropometric data (nutritional status Z-scores <–4 or >+4). Our analytical sample included 4064 children living in 3375 households.
In the 2006 Brazilian DHS, three finger-prick blood samples were collected from a sub-sample of children under 5 years (n 3499) on to filter paper. Of the 4064 children selected for the present study, 3425 and 3361 had retinol and Hb levels available, respectively. Hb and retinol concentrations were measured by the cyanmethaemoglobin method and HPLC, respectively, using the dried-drop technique( Reference Brasil 3 ). Children with Hb levels below 110 g/l were considered anaemic( 17 ). Vitamin A deficiency was defined as vitamin A levels below 0·70 μmol/l( 18 ). Child anthropometric status was assessed using the indices HAZ, weight-for-age Z-score (WAZ) and weight-for-height Z-score (WHZ). Children were considered stunted or wasted when HAZ or WAZ was <−2 and overweight-for-height when WHZ was >+2( 19 ). The DHS also investigated household conditions, such as source of water consumption, quality of sanitation, location (urban or rural), Brazil’s geographical region (North, Northeast, Southeast, South, Middle-West) and maternal characteristics (age and educational level).
Food security status was measured using the fourteen-item Brazilian Household Food Insecurity Measurement Scale (Escala Brasileira de Insegurança Alimentar, EBIA)( Reference Brasil 20 ). Based on the HFI additive score, obtained from the number of affirmative answers to EBIA questions, as recommended households were classified into: food secure (score=0), mildly food insecure (score=1 to 5), moderately food insecure (score=6 to 9) and severely food insecure (score=10 to 14).
Child morbidity data were reported by the children’s mothers to capture the occurrence of cough and fever in the last two weeks before the survey and hospitalizations for diarrhoea and pneumonia during the year prior to the survey.
Statistical analyses were performed with the statistical software package IBM SPSS Statistics Version 20·0 using the Complex Samples add-on module that computes estimates considering the complex sampling survey design. Chi-square bivariate and logistic regression analyses were conducted. Adjusted odds ratios and their corresponding 95 % confidence intervals were estimated by adjusting for child anthropometric status (except for the stunting, wasting and overweight models), source of water consumption in the household, quality of household sanitation, maternal education level, maternal age and household location (rural/urban). Analyses were also adjusted for Brazilian regions to take regional socio-economic and food insecurity contextual factors into account( Reference Gubert, Benício and Santos 21 ). Based on the pattern of bivariate associations, multivariate analyses were conducted using two HFI categories (severe HFI v. food secure/mild and moderate HFI).
Results
Of the children under 5 years old assessed in the present study, 6·7 % lived in households experiencing severe HFI, 18·8 % had vitamin A deficiency and 22·0 % had anaemia. With regard to common childhood morbidities, 36·4 % of the children were reported to have experienced cough and 23·7 % fever during the two weeks preceding the survey. Additionally, 2·6 % of them were hospitalized for diarrhoea or pneumonia in the year prior to the survey. Among the children suffering from malnutrition, 6·8 % were stunted and 6·9 % had a high WHZ, indicating overweight-for-height (Table 1).
Table 1 Characteristics of households and children under 5 years of age whose 15–49-year-old mothers were interviewed in the 2006 Brazilian Demographic and Health Survey. Brazil, 2006

* Vitamin A deficiency defined as retinol<0·70 µmol/l (n 3425).
† Anaemia defined as Hb <110 g/d (n 3361).
‡ In the last two weeks prior to the survey.
§ In the last year prior to the survey.
|| Height-for-age Z-score<−2 (n 4064).
¶ Weight-for-age Z-score<−2 (n 4064).
** Weight-for-height Z-score>+2 (n 4064).
The prevalence of cough was significantly higher in children living in severely food-insecure households (Table 2). A similar result was also found for hospitalization for diarrhoea. The difference in the prevalence of vitamin A and anaemia between the categories of food insecurity and security was quite small and not statistically significant. A dose–response association was found between level of HFI severity and stunting. By contrast, high WHZ was more prevalent among children experiencing severe food insecurity compared with their food-secure counterparts.
Table 2 Prevalence of morbidities and nutritional deficits, according to food security status, among 4064 children under 5 years of age whose 15–49-year-old mothers were interviewed in the 2006 Brazilian Demographic and Health Survey. Brazil, 2006
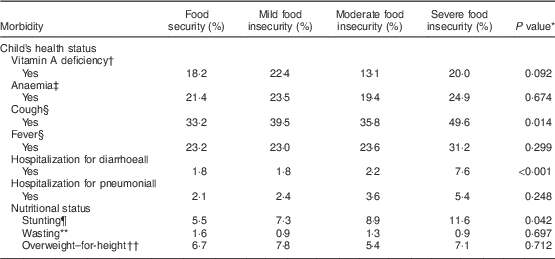
* Using the χ 2 test.
† Vitamin A deficiency defined as retinol<0·70 µmol/l (n 3425).
‡ Anaemia defined as Hb <110 g/d (n 3361).
§ In the last two weeks prior to the survey.
|| In the last year prior to the survey.
¶ Height-for-age Z-score<−2.
** Weight-for-age Z-score<−2.
†† Weight-for-height Z-score>+2.
The odds for cough, fever, and hospitalizations for diarrhoea and pneumonia were significantly higher in children living in severely food-insecure households (Table 3). For example, these children had 4·28 and 2·31 greater odds of being hospitalized for diarrhoea and pneumonia, respectively, compared with those living in food-secure households. After adjusting for confounders, strong and significant associations remained between severe HFI and the prevalence of cough (adjusted OR=1·67) and hospitalization for diarrhoea (adjusted OR=2·93).
Table 3 Unadjusted and adjusted odds ratios for morbidities and nutritional deficits, according to food security status, among 4064 children under 5 years of age whose 15–49-year-old mothers were interviewed in the 2006 Brazilian Demographic and Health Survey. Brazil, 2006

* Adjusted by type of water for consumption, presence of adequate sanitation, maternal education level, maternal age, household location (urban or rural area), Brazilian geographic region and child’s nutritional status.
† Adjusted by type of water for consumption, presence of adequate sanitation, maternal education level, maternal age, household location (urban or rural area) and Brazilian geographic region.
Discussion
Bivariate results of the present study show that, in Brazil, HFI is associated with stunting and the occurrence of common childhood illnesses. This is consistent with what has been reported in other countries( Reference Ohemeng, Marquis and Lartey 10 , Reference Ghattas 15 , Reference Garrett and Ruel 22 ). Our findings on HFI and malnutrition in the context of the nutrition transition are in agreement with previous studies focusing on both child undernutrition( Reference Monteiro, Schmidt and Costa 7 , Reference Hackett, Melgar-Quiñone and Álvarez 23 , Reference Saaka and Osman 24 ) and overweight( Reference Casey, Szeto and Lensing 25 – Reference Franklin, Jones and Love 28 ). In our study, bivariate analyses showed that severely food-insecure children had a higher prevalence of stunting and overweight compared with food-secure children. This is indeed what would be expected in the context of the nutrition transition in an upper middle-income country( Reference Kac and Pérez-Escamilla 29 ). A study conducted in Colombia revealed that food-insecure children were three times more likely to be underweight, but not stunted, than food-secure children( Reference Isanaka, Mora-Plazas and Lopez-Arana 30 ). However, other studies have identified a dose–response relationship between HFI severity and the risk of child stunting( Reference Hackett, Melgar-Quiñone and Álvarez 23 , Reference Saaka and Osman 24 , Reference Shamah-Levy, Mundo-Rosas and Rivera-Dommarco 31 ). In our study we found a similar prevalence of overweight between children living in food-secure, mildly and moderately food-insecure households. The higher prevalence of overweight in severely food-insecure children (7·1 %) in our study may be partially explained by poor dietary quality resulting from the hyperenergetic diet to which these children are exposed. Individuals living in food-insecure households have a lower intake of animal protein( Reference Isanaka, Mora-Plazas and Lopez-Arana 30 ) and higher intakes of snack foods( Reference Kaiser, Melgar-Quinonez and Townsend 32 , Reference Perez-Escamilla, Segall-Correa and Kurdian Maranha 33 ) and high-energy-density foods, because of the cost of these products( Reference Drewnowski and Specter 34 , Reference Drewnowski, Monsivais and Maillot 35 ).
There have also been previous reports on the association between HFI and child overweight and obesity( Reference Sharkey, Nalty and Johnson 36 ); however, we could not confirm this association in our study. In agreement with previous studies( Reference Girard, Self and McAuliffe 37 , Reference Rodrigues and Roncada 38 ) our results did not confirm an association of HFI with anaemia and vitamin A deficiency. Micronutrient deficiencies, as well as excess weight, are related to poor dietary quality( Reference Cook, Frank and Berkowitz 8 , Reference Isanaka, Mora-Plazas and Lopez-Arana 30 ) and reflect the effect of HFI on children’s health in the context of poverty, low education, poor housing conditions and other vulnerabilities( Reference Skalicky, Meyers and Adams 9 , Reference Park, Kersey and Geppert 39 ). In the case of Brazil as well as in other countries deeply immersed in the nutrition transition, it is possible that all social classes experience similar prevalence of micronutrient deficiencies as a result of well-established food fortification and supplementation programmes.
Our bivariate analyses showed that severely food-insecure children were more likely to have experienced cough, fever, diarrhoea and pneumonia. This was expected, as higher morbidity rates have been observed previously among food-insecure children in other countries( Reference Ohemeng, Marquis and Lartey 10 , Reference Cook, Frank and Levenson 40 , Reference Cook, Frank and Casey 41 ). Specifically, children living in food-insecure households are more susceptible to diarrhoea, respiratory infection and chronic diseases( Reference Skalicky, Meyers and Adams 9 , Reference Hackett, Melgar-Quiñone and Álvarez 23 , Reference Nord 42 , Reference Reis 43 ). Child nutritional status has a close association with the occurrence of morbidities( Reference Addo, Marquis and Lartey 4 ), as well as with income conditions and household characteristics such as location in the rural area and quality of sanitation( Reference Black, Allen and Bhutta 44 ). These factors were also closely associated with diarrhoea in previous studies( Reference Black, Allen and Bhutta 44 ). However, our findings clearly indicate that severe food insecurity remained strongly associated with the occurrence of hospitalization for diarrhoea after controlling for these key confounders. This confirms the previous conclusion that experience-based HFI scales such as EBIA indeed capture health risk information that goes well above and beyond what traditional socio-economic and demographic variables do( Reference Pérez-Escamilla 45 ). In this context, it is worth emphasizing the importance of food insecurity (especially in its more severe form) as a strong risk factor for the occurrence of common childhood illnesses such as cough and diarrhoea among Brazilian children. Our study did not find any associations of morbidities, anthropometric status or micronutrient deficiencies with mild or moderate food insecurity. This was unexpected because dietary quality has been previously reported to be negatively affected by mild and moderate HFI( Reference Saha, Frongillo and Alam 6 , Reference Ghattas 15 , Reference Rosas, Harley and Fernald 16 ). This finding may be explained by strong improvements in the social determinants of health across social classes over the past decades( Reference Monteiro, Benicio and Konno 46 ).
One limitation of our study is that we were able to adjust only for the factors likely to be involved in the association between childhood morbidity and HFI that were available from the 2006 DHS. For example, we did not have data on specific health literacy of the mother or health-care access issues affecting the child. A second limitation is that all data, including HFI and morbidity information, were reported by the mothers. However, previous research shows that EBIA has strong internal and external validity( Reference Segall-Corrêa and Marin-León 47 , Reference Segall-Corrêa, Marin-León and Melgar-Quiñonez 48 ) and that caregivers’ report of child morbidity is reliable( Reference Rousham, Northrop-Clewes and Lunn 49 ). Further prospective studies are needed to better understand the actual role of HFI on the prevalence of childhood morbidity and to allow for a thorough investigation on the matter, including other socio-economic, health and food intake data.
Conclusion
In conclusion, severe HFI is associated with a higher risk of cough and hospitalization due to diarrhoea among Brazilian children under 5 years of age, illustrating the importance of linking better the food systems and the health-care systems in Brazil.
Acknowledgements
Financial support: This work was supported by funding from the National Council of Technological and Scientific Development (CNPq). CNPq had no role in the design, analysis or writing of this article. Conflict of interest: M.B.G., A.M.S., G.A.B. and R.P.-E. declare no conflicts of interest. Authorship: M.B.G. and R.P.-E. designed the research; M.B.G. and A.M.S. performed statistical analyses; R.P.-E. and G.A.B. supplied technical assistance and advice; M.B.G., A.M.S., G.A.B. and R.P.-E. wrote the paper and M.B.G. had primary responsibility for the final content. All authors read and approved the final manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the Research Ethics Committee of the Sexually Transmitted Diseases/AIDS Reference and Training Center of the São Paulo State Department of Health.






