Many studies have reported that symptoms of depression following an acute myocardial infarction (AMI) predict subsequent adverse cardiovascular outcomes. Reference van Melle, de Jonge, Spijkerman, Tussen, Ormel and van Veldhuisen1,Reference Sorensen, Friis-Hasche, Haghfelt and Bech2 Questions have been raised, however, about the validity of assessing symptoms of depression with self-report questionnaires in the context of acute or chronic medical illness. Reference Sorensen, Friis-Hasche, Haghfelt and Bech2–Reference Koenig, George, Peterson and Pieper5 Symptoms that commonly occur following an AMI, including fatigue or loss of energy, changes in sleep patterns and changes in appetite, may be misinterpreted by healthcare providers, researchers or patients as mood-related. The Beck Depression Inventory (BDI) and its revised version, the BDI–II, are the most commonly used self-report depressive symptom measures in post-AMI depression research. Reference Beck and Steer6–Reference Thombs, Bass, Ford, Stewart, Tsilidis and Patel8 Continuous scores on the BDI and BDI–II, as well as scores above a cut-off level, have been found to predict poor cardiovascular outcomes. Reference van Melle, de Jonge, Spijkerman, Tussen, Ormel and van Veldhuisen1,Reference Sorensen, Friis-Hasche, Haghfelt and Bech2 The authors of one systematic review of the association between post-AMI depression and mortality, however, argued that 50–75% of medical patients endorse some of the somatic items on the BDI and that patients with cardiovascular disease may score close to 10 points on the BDI (the standard cut-off for identifying possible depression) on somatic symptoms alone. Reference Sorensen, Friis-Hasche, Haghfelt and Bech2 Owing to the practical difficulty of determining whether non-specific somatic symptoms such as fatigue and appetite change are the result of depression or medical illness, alternative self-report measures such as the Hospital Anxiety and Depression Scale (HADS), Reference Zigmond and Snaith9 which exclude somatic symptoms, have been developed. The argument for using alternative measures that do not include somatic symptoms, however, is not based on empirical evidence from studies showing that assessment methods that include somatic symptoms, such as the BDI or BDI–II, are confounded by the presence of a medical illness. Reference Simon and Von Korff10
We investigated whether self-report of depressive symptom severity is confounded by the presence of an acute medical condition by examining BDI–II responses in three different groups: cardiac patients, psychiatric out-patients and undergraduate students. The objective of this study was to compare scores from post-AMI patients with those of the other two groups on items of the BDI–II that reflect somatic concerns common both following AMI and in depression, after matching on cognitive/affective symptom scores. Essentially, this was an empirical test of the hypothesis that scores on depression symptom measures that include somatic symptoms, such as the BDI and BDI–II, are exaggerated among medically ill patients owing to the misattribution of somatic symptoms to depression.
Method
Participants
Post-AMI cohort
The post-AMI cohort consisted of patients who were treated for acute myocardial infarction at one of five tertiary care hospitals or one of five community hospitals in Québec, Canada, between December 1996 and November 1998. Patients were eligible for enrolment in the study if they were admitted through the emergency department and not transferred from another hospital, survived at least 24 h after admission, read and understood French or English, and were medically capable of giving informed consent. Research nurses approached eligible patients for informed consent and enrolment within 2–3 days of admission to hospital. Participants completed self-administered questionnaires that included the BDI–II in English or French at the time of enrolment, and 477 patients completed all BDI–II items. This study was ancillary to a prospective cohort study of quality of life after AMI, Reference Pilote, Lauzon, Huynh, Dion, Roux and Racine11 which received ethical approval from the Montréal General Hospital ethics review board.
Psychiatry out-patient comparison sample
The psychiatry out-patient sample consisted of adults (≥18 years old) seeking treatment for mental health problems at an outpatient psychiatric clinic in New Jersey, USA. These participants completed the BDI–II as part of a standardised intake assessment prior to being interviewed and diagnosed by experienced psychiatrists with a clinical interview using DSM–IV criteria. 12 The sample was drawn from participants in four studies that investigated the use of the BDI–II in people receiving psychiatric out-patient treatment. Reference Steer, Clark, Beck and Ranieri13–Reference Ball and Steer16 Data collection was conducted with the approval of the institutional review board of the University of Medicine and Dentistry of New Jersey, School of Osteopathic Medicine. Patient data used in this study were drawn from participants matched as closely as possible in terms of age, gender and race/ethnicity (White or Black and minority ethnicity) to the 477 patients in the post-AMI cohort. Reference Thombs, Ziegelstein, Beck and Pilote17 If more than one individual from the psychiatry out-patient group was found to match with a post-AMI patient, a computer-generated random number selected the participant to be included. When there was no exact match, the closest possible match was sought by selecting the psychiatric out-patient nearest in age. Following initial matching, the BDI–II scores in the psychiatry out-patient group (n = 477) were substantially higher than in the post-AMI group (n = 477); a subset of the initially matched patients was therefore extracted based on exact matches on BDI–II cognitive/affective symptom scores. To do this, for each possible cognitive/affective score, all post-AMI or psychiatry patients were included from the group with fewer patients with that score. Then, the same number of patients from the other group with that score was included, selected using computer-generated random numbers. For example, if 10 people from the psychiatry out-patients group and 15 people from the post-AMI group had a cognitive/affective score of 6, all 10 from the first group were selected along with 10 of the 15 patients in the post-AMI group, based on random number selection.
Student comparison sample
The undergraduate student sample consisted of 996 psychology students from the University of Calgary who completed all items on the BDI–II. Reference Dozois, Dobson and Ahnberg18 Students were approached during class time and asked to participate in the study. Informed consent was provided, and data collection was approved by the institutional review board at the University of Calgary. Undergraduate students were matched with patients in the post-AMI group for both gender and cognitive/affective symptom total scores, since a much greater proportion of the student sample was female (66.9%) compared with the post-AMI group (17.4%). For females and males separately, at each cognitive/affective symptom score level, all post-AMI or psychiatry patients were included from the smaller group, and matches from the other group were based on computer-generated random numbers.
Measures
Symptoms of depression were assessed using the 21-item BDI–II. Reference Beck, Steer and Brown7 Items consist of four statements scored 0–3, with higher scores indicating increasing symptom severity. Respondents are instructed to describe the way they have been feeling during the past 2 weeks. There is extensive evidence of the validity and reliability of the BDI–II in both psychiatric and non-psychiatric populations. Reference Dozois, Covin, Segal and Hilsenroth19,Reference Joiner, Walker, Pettit, Perez and Cukrowicz20 Studies have reported several different factor structures for the BDI–II. Reference Ward21,Reference Osman, Barrios, Gutierrez, Williams and Bailey22 In our study, in order to test whether somatic symptoms might be overreported by the post-AMI in-patients, a total score was computed for BDI–II items that potentially overlap with somatic symptoms common after an AMI. Based on a review of existing factor models and item content, scores on BDI–II items 15–21 (loss of energy, sleep problems, irritability, appetite problems, concentration, fatigue, loss of interest in sex) were summed to calculate somatic symptom scores. Items 1–14 (sadness, pessimism, past failure, loss of pleasure, guilty feelings, punishment feelings, self-dislike, self-criticalness, suicidal ideation, crying, agitation, loss of interest, indecisiveness, worthlessness) were summed to calculate cognitive/affective scores.
Statistical analysis
Differences between the three study cohorts were assessed on categorical variables using chi-squared tests and on continuous variables with two-tailed t-tests. No adjustment was made for multiple comparisons since the statistical tests were done for illustrative purposes rather than hypothesis testing. To test whether somatic scores on the BDI–II differed between the groups overall and at different levels of cognitive/affective scores, we used two-tailed t-tests. In addition, we reported the effect size statistic Hedges's g, which represents a standardised difference between two means. Reference Hedges23 In a post hoc analysis, score distributions on each of the seven somatic items for the study groups were compared using the Mann–Whitney U-test. Hochberg's sequential method was used to maintain a family-wise type I error rate of α < 0.05 for item comparisons between the post-AMI and psychiatry out-patient groups and between the post-AMI group and the students.
Results
Prior to matching, the mean BDI–II scores were 9.2 (s.d. = 7.9) for the 477 post-AMI patients, 22.6 (s.d. = 12.2) for the 477 psychiatry out-patients and 9.1 (s.d. = 7.6) for the 996 college students. The percentage with very low scores on the BDI–II (total score 0–3) was 24.1% (n = 115) among post-AMI patients, 3.8% among psychiatry out-patients (n = 18) and 25.2% (n = 251) among the students. Among the two patient groups there were 209 exact matches based on cognitive/affective scores. Of the student group, 366 were successfully matched with post-AMI patients. The post-AMI patients were on average 3.4 years older than the psychiatry out-patient comparison group (P = 0.006), but gender and ethnicity were similar owing to the matching protocol (Table 1). In the psychiatry out-patients matched group, primary DSM–IV Axis I diagnoses included major depressive disorder (n = 81; 38.8%), dysthymia (n = 10; 4.8%), depression not otherwise specified (n = 9; 4.3%), alcohol dependence (n =3;1.4%),anxiety disorders (n = 52; 24.9%) and adjustment disorders (n = 54; 25.8%). Compared with the post-AMI patients, the matched students were on average 39.5 years younger (P<0.001). The number of women in the post-AMI and student groups were the same owing to the matching protocol, and ethnicity was similar. Clinical characteristics of the matched post-AMI patients are provided in online Table DS1.
Table 1 Demographic characteristics of the matched samples

| Post-AMI patients (n = 209) | Psychiatric out-patients (n = 209) | P | Post-AMI patients (n = 366) | College students (n = 366) | P | |
|---|---|---|---|---|---|---|
| Age, years: mean (s.d.) | 58.9 (12.2) | 55.5 (12.9) | 0.006 | 60.4 (12.6) | 20.9 (4.1) | < 0.001 |
| Female, n (%) | 39 (18.7) | 35 (16.7) | 0.608 | 82 (22.4) | 82 (22.4) | 1.000 |
| White, n (%) | 196 (93.8) | 201 (96.2) | 0.263 | 348 (95.1) | 345 (94.3) | 0.622 |
Cognitive/affective and somatic symptoms
Post-AMI patients v. psychiatry out-patients
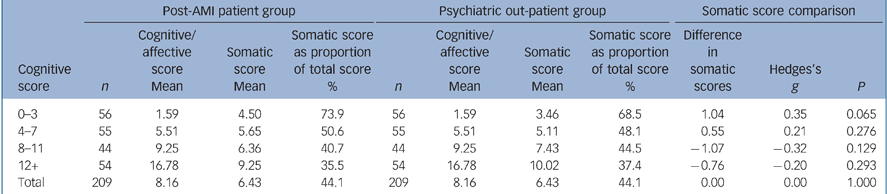
Overall, somatic scores accounted for 44.1% of the total BDI–II scores for both the 209 post-AMI patients and the 209 matched psychiatry out-patients, with no difference between the groups. However, there was variation across the range of cognitive/affective scores. The post-AMI group had relatively higher somatic symptom scores at lower levels of cognitive/affective symptoms, whereas those in the psychiatry out-patient group reported relatively higher somatic scores at higher levels of cognitive/affective symptoms (Table 2). None of these differences was statistically significant, however, and, as shown by the Hedges's g effect size statistic, the differences were generally small based on Cohen's operational definitions (small 0.2, medium 0.5, large 0.8). Reference Cohen24 The only item with a statistically significant difference between the two patient groups was item 19 (concentration), for which participants in the psychiatric out-patient group scored higher than those in the post-AMI group (mean score 0.86 v. 0.67). Item comparisons are shown in online Table DS2.
Table 2 Comparison of cognitive and somatic scores for the myocardial infarction and psychiatric out-patient groups
| Post-AMI patient group | Psychiatric out-patient group | Somatic score comparison | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cognitive score | n | Cognitive/affective score Mean | Somatic score Mean | Somatic score as proportion of total score % | n | Cognitive/affective score Mean | Somatic score Mean | Somatic score as proportion of total score % | Difference in somatic scores | Hedges's g | P |
| 0-3 | 56 | 1.59 | 4.50 | 73.9 | 56 | 1.59 | 3.46 | 68.5 | 1.04 | 0.35 | 0.065 |
| 4-7 | 55 | 5.51 | 5.65 | 50.6 | 55 | 5.51 | 5.11 | 48.1 | 0.55 | 0.21 | 0.276 |
| 8-11 | 44 | 9.25 | 6.36 | 40.7 | 44 | 9.25 | 7.43 | 44.5 | -1.07 | -0.32 | 0.129 |
| 12+ | 54 | 16.78 | 9.25 | 35.5 | 54 | 16.78 | 10.02 | 37.4 | -0.76 | -0.20 | 0.293 |
| Total | 209 | 8.16 | 6.43 | 44.1 | 209 | 8.16 | 6.43 | 44.1 | 0.00 | 0.00 | 1.000 |
In both groups, somatic symptoms accounted for the highest proportion of total BDI–II scores among patients with low cognitive/affective scores.
Post-AMI patients v. students
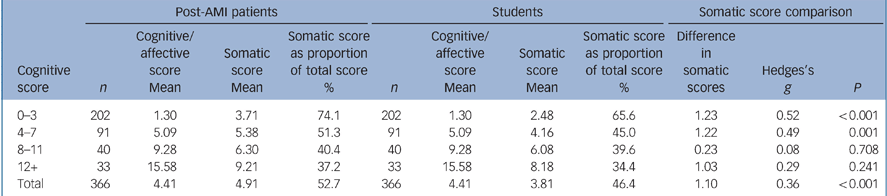
Table 3 shows cognitive/affective and somatic scores for the post-AMI patient group and the student group. Overall, somatic symptom scores accounted for 52.7% of the total BDI–II score for the 366 post-AMI patients compared with 46.4% in the matched student sample. Post-AMI patients scored on average 1.1 points higher on somatic scores than the students matched on cognitive/affective scores (P<0.001), and this was generally consistent across the range of cognitive/affective scores, with the exception of the 8–11 range where there was no difference between the groups. The higher somatic scores among post-AMI patients were due to significantly higher scores on items 15 (loss of energy), 20 (tiredness or fatigue) and 21 (loss of interest in sex), as shown in online Table DS2. The proportion of total scores accounted for by somatic items in the student cohort across the range of cognitive/affective scores was similar to the pattern in the two patient groups.
Table 3 Comparison of cognitive and somatic scores for the myocardial infarction and college student groups
| Post-AMI patients | Students | Somatic score comparison | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cognitive score | n | Cognitive/affective score Mean | Somatic score Mean | Somatic score as proportion of total score % | n | Cognitive/affective score Mean | Somatic score Mean | Somatic score as proportion of total score % | Difference in somatic scores | Hedges's g | P |
| 0-3 | 202 | 1.30 | 3.71 | 74.1 | 202 | 1.30 | 2.48 | 65.6 | 1.23 | 0.52 | < 0.001 |
| 4-7 | 91 | 5.09 | 5.38 | 51.3 | 91 | 5.09 | 4.16 | 45.0 | 1.22 | 0.49 | 0.001 |
| 8-11 | 40 | 9.28 | 6.30 | 40.4 | 40 | 9.28 | 6.08 | 39.6 | 0.23 | 0.08 | 0.708 |
| 12+ | 33 | 15.58 | 9.21 | 37.2 | 33 | 15.58 | 8.18 | 34.4 | 1.03 | 0.29 | 0.241 |
| Total | 366 | 4.41 | 4.91 | 52.7 | 366 | 4.41 | 3.81 | 46.4 | 1.10 | 0.36 | < 0.001 |
Discussion
Clinical lore, which has been echoed in the research literature, Reference Sorensen, Friis-Hasche, Haghfelt and Bech2–Reference Koenig, George, Peterson and Pieper5 suggests that assessment of depression symptoms with self-report tools that include somatic symptoms (such as the BDI or BDI–II) may inflate symptom severity scores substantially among patients with acute or chronic medical disease, including post-AMI patients. Reference Koenig, George, Peterson and Pieper5,Reference Irvine, Basinski, Baker, Jandciu, Paquette and Cairns25,Reference de Jonge, Ormel, van den Brink, van Melle, Spijkerman and Kuijper26 The results of this study showed that post-AMI patients did not have higher somatic symptom scores than psychiatry out-patients matched on age, gender and cognitive/affective scores, and reported, on average, somatic symptom scores only one point higher than undergraduate students matched on gender and cognitive/affective scores. Furthermore, the large proportion of post-AMI patients who scored below 4 on the BDI–II appears to be inconsistent with previous speculation of substantial upward bias of BDI and BDI–II scores in post-AMI patients compared with people who are not medically ill. Reference Koenig, George, Peterson and Pieper5,Reference Irvine, Basinski, Baker, Jandciu, Paquette and Cairns25,Reference de Jonge, Ormel, van den Brink, van Melle, Spijkerman and Kuijper26
Given the degree of physical burden associated with an acute coronary event, it may seem surprising that post-AMI patients did not have substantially higher somatic symptom scores than psychiatry out-patients or college students after matching for cognitive/affective symptom scores. One explanation for this finding may relate to the overt nature of symptom assessment, which has been shown to influence responses to self-report questionnaires. Reference Hunt, Auriemma and Cashaw27,Reference Tourangeau, Rips and Rasinski28 Indeed, it is well established that the content of items presented early in a questionnaire or survey may influence responses on subsequent items. Reference Bowling29,Reference Schwarz30 Respondents to self-report questionnaires use the same tacit assumptions that guide everyday conversation and attempt to make their input relevant to the ongoing conversation or, in the case of questionnaires, to the purposes of the research. Reference Schwarz30 The seven BDI–II items with potential somatic overlap between depression and an AMI are found near the end of the 21-item questionnaire (items 15–21) and follow items on self-dislike, sadness, guilt and suicidal ideation, among other questions likely to be recognised by patients as related to depression. Thus, responses to the somatic items may be perceived implicitly as queries about depression rather than literally as questions about physical health status. Given this, post-AMI in-patients who are tired or not eating well, for instance, may still not endorse these somatic symptoms on the BDI–II, since they recognise this as a ‘depression questionnaire’. Some of these patients may either attribute these symptoms to their acute cardiac event and the hospitalisation itself or refrain from endorsing these symptoms so that they do not appear depressed.
To the best of our knowledge, no previous study has reported the degree to which patients with relatively low scores on the BDI–II report predominantly somatic symptoms compared with patients with higher overall score levels. It is possible that patients with lower-grade symptoms might experience their distress more somatically, although this has not been documented and would appear to contradict current understanding of depression. Reference Joiner, Walker, Pettit, Perez and Cukrowicz20 It would appear more likely that BDI–II scores reflect some degree of common somatic experiences, such as fatigue, that many people report regardless of their medical status, and that the relative effect of this on overall BDI–II scores is more prominent among respondents with very low cognitive/affective scores. This interpretation is consistent with previous reports using factor analytic methods that found that 6–10% of the explained variance in BDI–II scores is due to variance from somatic symptoms that is orthogonal or unrelated to a general depression factor in samples of post-AMI patients, substance-dependent men and psychiatry out-patients. Reference Thombs, Ziegelstein, Beck and Pilote17,Reference Ward21,Reference Thombs, Ziegelstein, Parakh, Stewart, Abbey and Grace31 It is also consistent with evidence from 2481 patients enrolled in the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) trial that both somatic and cognitive symptoms of depression on the BDI were associated with medical comorbidity, Reference Watkins, Schneiderman, Blumenthal, Sheps, Catellier and Taylor32 but that the association of somatic symptoms with medical comorbidity was much more robust than for cognitive symptoms. Similarly, a number of studies have found that somatic symptoms of depression as measured by the BDI are more robustly related to cardiac prognosis than cognitive symptoms. Reference Irvine, Basinski, Baker, Jandciu, Paquette and Cairns25,Reference de Jonge, Ormel, van den Brink, van Melle, Spijkerman and Kuijper26,Reference Linke, Rutledge, Johnson, Vaccarino, Bittner and Cornell33–Reference de Jonge, Mangano and Whooley35
One limitation of this study is that the three cohorts were not drawn from the same setting. An additional limitation is that information on medical comorbidities was not available for the psychiatry out-patient or student groups. One might expect in particular that some of the participants in the psychiatry outpatient group, given the mean age of the analysed sample, would have some medical comorbidity. This would be less of an issue in the student sample. Thus, the general consistency of the main finding, that post-AMI patients do not report substantially more somatic symptoms than either psychiatry out-patients or college students, provides confidence in the results.
It may be tempting, based on the results of this study, to conclude that depressive symptoms among post-AMI patients are not influenced by the general somatic profile of acutely ill patients or that depressive symptoms in medically ill patients are no different from the symptoms of depression in non-medically ill populations. Such conclusions are beyond the scope of this study, which only established that the proportion of somatic symptoms reported by post-AMI patients does not exceed that reported by psychiatry out-patients or undergraduate students. The nature, quality and causal pathways of post-AMI depression were not addressed in this study. Indeed, a number of biological mechanisms linking depression and cardiovascular disease have been identified, including increased platelet activation, reduced heart rate variability, heightened inflammatory response and endothelial dysfunction. Reference Frasure-Smith and Lesperance36 With these factors in mind, it is likely that key aspects of depression may differ in patients with cardiovascular disease compared with non-medically ill patients, even though results from our study suggest that depression may be experienced – or at least reported – similarly. Likewise, it is important that the results of this study should not be used to infer the degree to which the BDI–II would be useful as a screening tool for major depressive disorder. The study addressed the degree to which continuous scores on the BDI–II, reflecting depression symptom severity, may be influenced by bias owing to somatic symptom overlap following an AMI. It did not address the degree to which the BDI–II accurately identifies cases of major depressive disorder, which is an altogether different question. Indeed, a recent systematic review demonstrated potential pitfalls in using the BDI, BDI–II and other self-report questionnaires as screening tools for major depressive disorder in cardiovascular care settings. Reference Thombs, de Jonge, Coyne, Whooley, Frasure-Smith and Mitchell37
Future research is needed to improve our understanding of how patients make sense of, and respond to, self-report questionnaires such as the BDI and BDI–II. For instance, a study that randomly assigned post-AMI or other medically ill patients to receive either the standard BDI–II questionnaire or a version of the BDI–II in which somatic symptom items were administered prior to the cognitive/affective items would provide valuable information about how patients respond to somatic symptom items embedded in self-report measures of depressive symptoms. Such a study would ideally include a qualitative component that would enable patients to explain factors that they considered in formulating item responses. There is increasing interest in the nature of depression following an AMI, Reference Irvine, Basinski, Baker, Jandciu, Paquette and Cairns25,Reference de Jonge, Ormel, van den Brink, van Melle, Spijkerman and Kuijper26,Reference Linke, Rutledge, Johnson, Vaccarino, Bittner and Cornell33–Reference de Jonge, Mangano and Whooley35 and a better understanding of the nature of self-report symptom scores would inform this research.
In summary, the results of this study challenge clinical lore suggesting that the assessment of depressive symptom severity with standard self-report instruments, such as the BDI or BDI–II, will be substantially biased in medically ill patients compared with non-medically ill patients owing to the misattribution of somatic symptoms from medical conditions to depression. This study found that post-AMI patients did not have higher somatic symptom scores than psychiatry outpatients who were matched on cognitive/affective scores. Compared with undergraduate students, somatic symptom scores for post-AMI patients were only approximately one point higher. Across groups, however, the preponderance of somatic symptoms at low score levels suggests that BDI–II scores may include a small amount of somatic symptom variance not necessarily related to depression in medically ill and non-medically ill respondents.
Funding
B.D.T. is supported by a New Investigator Award from the Canadian Institutes of Health Research (CIHR) and an Établissement de Jeunes Chercheurs award from the Fonds de la Recherche en Santé Québec. R.C.Z. is supported by grants from the National Institutes of Health (R21NS048593), the National Center For Complementary and Alternative Medicine (R24AT004641) and the Miller Family Scholar Program of the Johns Hopkins Center for Innovative Medicine. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Complementary and Alternative Medicine or the National Institutes of Health. The Depression and Myocardial Infarction (DepreMI) study was funded by a grant from the Netherlands Organisation for Scientific Research to Ormel (Zon MW, Grant no. 904-57-100). P.d.J. is supported by a Vidi grant from the Dutch Medical Research Council (016-086-397). The Toronto cohort study was conducted with funds from the Heart and Stroke Foundation of Ontario and the Samuel Lunenfeld Foundation of Toronto, Ontario, awarded to D.E.S., S.E.A. and S.L.G. is supported by a New Investigator Award from the CIHR. Collection of data for the Québec cohort was supported by a grant from the Fonds de la Recherche en Santé de Québec (961305-104) awarded to L.P., who is a research scholar funded by the CIHR and a William Dawson Professor of Medicine at McGill University. S.F. is a CIHR Strategic Training Fellow in Pain: Molecules to Community and is supported by a Canada Graduate Scholarship – Masters Award from the CIHR.
Acknowledgements
The authors are grateful to Dr Robert A. Steer for allowing us to use data from the psychiatry out-patient sample.






eLetters
No eLetters have been published for this article.