Mammalian intra-uterine growth retardation (IUGR) is characterised by impaired organ development and embryo/fetus growth during pregnancy(Reference Rosenberg1, Reference Wu, Bazer and Wallace2). It is mainly caused by uteroplacental insufficiency, intra-uterine infection, genetic defects, congenital malformations, environmental insults or severe malnutrition(Reference Wu, Bazer and Wallace2, Reference Baserga, Bertolotto and Maclennan3). In humans, IUGR affects 5–10% of all newborn infants(Reference McMillen and Robinson4) and increases the risk of obesity, hypertension and type 2 diabetes mellitus in adulthood(Reference Valsamakis, Kanaka-Gantenbein and Malamitsi-Puchner5). In livestock production such as the pig-rearing industry, 15–20% of all neonates suffer from this pregnancy condition(Reference Wu, Bazer and Wallace2). Animals born with IUGR have increased neonatal morbidity and reduced metabolic efficiency, postnatal growth rate, meat quality and reproductive performance(Reference Wu, Bazer and Wallace2). In neonatal piglets, IUGR also severely impairs the development and function of the gastrointestinal tract(Reference Wang, Huo and Shi6–Reference Xu, Mellor and Birtles8), and this is the leading cause of malnutrition and delayed growth of IUGR piglets after birth(Reference Wang, Huo and Shi6).
l-Arginine (Arg) is an essential amino acid for neonatal piglets, particularly under stressful conditions(Reference Kim, McPherson and Wu9, Reference Wu, Jaeger and Bazer10). In sow-reared piglets, only < 40% of Arg requirement can be met through breast-feeding(Reference Wu, Davis and Flynn11, Reference Wu, Knabe and Kim12). It has been reported that dietary supplementation of Arg significantly promoted the growth of neonatal piglets(Reference Kim, McPherson and Wu9, Reference Yao, Yin and Chu13). In addition to postnatal growth, the embryonic development of animals is also affected by maternal Arg levels. Maternal Arg deficiency commonly leads to a higher incidence of preterm or IUGR in infants and newborn animals(Reference Wu, Jaeger and Bazer10, Reference Vosatka, Hassoun and Harvey-Wilkes14, Reference Richir, Siroen and van Elburg15). Supplementation of Arg during gestation increased the reproductive performance of gilts and the litter birth weight(Reference Mateo, Wu and Bazer16). Thus, Arg has great potential in preventing and treating IUGR, as well as in promoting postnatal growth and health.
The small intestine (SI) is the site where the majority of food digestion and nutrient absorption occurs(Reference Guilloteau, Zabielski and Hammon17). The development of the SI appears to be faster than many other organs in piglets within the first four postnatal weeks(Reference Zabielski, Godlewski and Guilloteau18). Previous studies have demonstrated that the ratio between the number of apoptotic and proliferating enterocytes can be used as the marker for intestinal mucosal maturation(Reference Zabielski, Godlewski and Guilloteau18). However, it is unclear whether the impaired SI development in IUGR neonates is due to increased apoptosis or decreased cell proliferation. Akt, a protein kinase also referred to as protein kinase B, plays a critical role in promoting cell survival by inhibiting apoptosis(Reference Franke, Kaplan and Cantley19, Reference Cardone, Roy and Stennicke20). The activation of Akt by insulin and many other growth factors(Reference Franke, Kaplan and Cantley19) can directly phosphorylate mammalian target of rapamycin (mTOR)(Reference Nave, Ouwens and Withers21). The phosphorylated mTOR activates its effectors, p70 S6 kinase (S6K1) and eukaryotic translation initiation factor 4E-binding protein 1, to initiate translation and stimulate protein synthesis and cell growth(Reference Dennis, Jaeschke and Saitoh22, Reference Murgas Torrazza, Suryawan and Gazzaneo23). It has been reported that Arg supplementation in the diet of neonatal piglets enhanced mTOR signalling in skeletal muscle(Reference Yao, Yin and Chu13). Similarly, in vitro studies have shown that Arg stimulates protein synthesis in intestinal epithelial cells by enhancing mTOR/S6K1 signalling(Reference Rhoads, Chen and Gookin24–Reference Ban, Shigemitsu and Yamatsuji26). In addition, dietary Arg supplementation enhanced intestinal growth and development in 21-d-old normal piglets(Reference Yao, Guan and Li27). However, it is still unknown whether Arg supplementation would have beneficial effects on the development of the SI in IUGR neonatal piglets. Therefore, we hypothesised that oral Arg supplementation would regulate SI growth and development in the neonatal IUGR piglets through enhancing the activation of Akt and mTOR and reducing the ratio between apoptosis and proliferation in the SI mucosa. Since the pig is also an ideal animal model for studying human nutritional and digestive disorders(Reference Guilloteau, Zabielski and Hammon17), results from the present study will also have direct relevance to the understanding of human nutrition.
Materials and methods
Milk replacer diets
Experimental diets used for the present study were prepared by supplementing with either 0·60% (w/w) Arg (Arg diet) or 1·23% (w/w) l-alanine (Control diet) in the basic milk replacer powder to be isoenergetic and isonitrogenous (Table 1). Basic milk replacer powder was formulated according to previous studies(Reference Yao, Yin and Chu13). The ingredients of the formulation were purchased from Tianke and Hejia Company; and Arg and l-alanine were provided by Ajinomoto (China) Company Limited. The dosage of supplemental Arg (0·60%) was chosen according to previous studies in 7-d-old piglets(Reference Kim, McPherson and Wu9, Reference Yao, Yin and Chu13). The level of Arg in the control milk replacer diet was 0·59% (w/w), as analysed according to the methods of the Association of Official Analytical Chemists(28). The diets were prepared by mixing 1 kg of milk replacer powder (DM 87.5%) with 4000 ml of water to a final 5030 ml of milk solution and were given to piglets by bottle feeding six times per 24 h(Reference Yao, Yin and Chu13, Reference Shulman29).
Table 1 Composition and nutrient content of diets (87·5% DM basis)
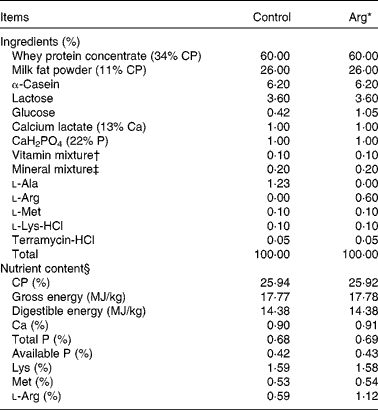
Arg, l-arginine; CP, crude protein.
* In the Arg diets, 1·23% l-alanine was replaced by 0·60% Arg (expressed on the basis of milk replacer powder). The two diets were isonitrogenous and isoenergetic.
† Vitamin mixture provided per mg/kg powder diet: retinyl acetate, 0·76; cholecalciterol, 0·055; all-rac-α-tocopheryl acetate, 16; menadione sodium bisulphate, 0·50; cyanocobalamin, 0·02; riboflavin, 4·0; niacin, 20; pantothenic acid, 12; choline chloride, 600; colic acid, 0·30; thiamine, 1·5; pyridoxine, 2·0; biotin, 0·08.
‡ Mineral mixture provided per mg/kg powder diet: Zn (as [C5H11NO2S]2Zn), 100; Mn (as [C2H4N2O2]2Mn), 5·0; Fe (as [C5H4O2N]2Fe), 100; Cu (as [C2H5NO2]2Cn), 10; I (as KI), 0·2; Se (as Na2SeO3), 0·3.
§ All nutrient contents, except digestible energy, were analysed values.
Animals and treatment
All the procedures were approved by the Institutional Animal Care and Use Committee of Nanjing Agricultural University. A gestating diet formulated to meet National Research Council(30) nutrient requirements was fed ad libitum to the pregnant sows with a similar birth order (3rd or 4th) during the entire period of pregnancy, as was drinking water. At the time of parturition (day 114 (sd 1) of gestation), newborn piglets (Duroc × (Landrace × Yorkshire)) from six sows with the same litter size (ten piglets/litter) were selected for this study. In each litter, one normal piglet with body weight (BW) of 1·57 (sd 0·13) kg and two IUGR littermates with BW of 0·90 (sd 0·12) kg were chosen according to previous studies(Reference Valsamakis, Kanaka-Gantenbein and Malamitsi-Puchner5, Reference Wang, Huo and Shi6, Reference Zhong, Wang and Zhang31). All piglets were weaned at 7 d of age and were fed with liquid diets at 75 ml/kg BW per meal every 4 h by bottle feeding between 7 and 14 d of age(Reference Shulman29). In each litter, one of the IUGR piglets (IUGR control, n 6) and normal littermate (Normal control, n 6) received the control diet, and the other IUGR littermate received the Arg diet (IUGR+Arg, n 6). The Arg intake by Normal and IUGR piglets fed the control diet was 0·418 g/kg BW per d, which was similar to the piglets fed by sows. All piglets were housed individually in plastic floored pens (1·5 m × 0·5 m) at an ambient temperature of 33°C in an environmentally controlled room and had free access to water.
Tissue sampling
From each group, four piglets with nearly equal BW, at 14 d of age, were selected for tissue collection. The piglets were killed by intramuscular injection of sodium pentobarbital (50 mg/kg BW) at 2 h after the last meal. The entire SI starting from the pyloric sphincter to the ileocecal valve was removed from the abdominal cavity and divided into four segments, including the duodenum, proximal and distal jejunum, and ileum(Reference Wang, Chen and Li32). Each intestinal segment was immediately flushed with ice-cold physiological saline to remove luminal contents, and weighed after careful removal of the mesenteric attachments. The length of the entire SI was measured by a metric ruler. Sections of approximately 1 cm in length were carefully collected from the mid of each segment, and fixed in 4% (w/v) paraformaldehyde in 100 mm-PBS, pH 7·4 for 24 h for histological and immunohistochemical analyses. Jejunal mucosa was scraped from the rest of the tissue using a glass microscope slide(Reference Wang, Huo and Shi6, Reference Zhong, Wang and Zhang31), and was immediately frozen in liquid N2 for protein and hormone analyses.
Measurement of growth factors in small intestine mucosa
The insulin and insulin growth factor 1 (IGF-1) levels in the jejunal mucosa were determined using commercial ELISA kits (USCN Life Science Corporation) following the manufacturer's protocols. The supernatant of the mucosal homogenate was prepared according to a previous study in our laboratory(Reference Zhong, Wang and Zhang31). The concentration of protein was measured using Lowry's method(Reference Martin, Wallace and Sigalet33). Values of insulin and IGF-1 were expressed as per g wet tissue and per mg protein basis.
Analysis of amino acids in small intestine mucosa
Norleucine was added to 100 mg of jejunal mucosa, and homogenised in a mixture of 10 ml trifluoroacetic acid and 100 ml methanol, followed by centrifugation at 3500 g for 5 min at 4°C(Reference Bidlingmeyer, Cohen and Tarvin34). The concentrations of amino acids were analysed by reverse-phase HPLC (HP1100; Agilent) following a method described previously(Reference Wu and Knabe35).
Morphological analysis
SI sections (5 μm) were prepared from paraffin-embedded samples and stained with haematoxylin and eosin. Villus height, villus width and crypt depth of twenty well-oriented villi per section were measured using a Nikon ECLIPSE 80i light microscope with a computer-assisted morphometric system (Nikon Corporation).
Apoptosis and proliferation
To evaluate cell proliferation in the intestinal crypt, Ki67, a marker for proliferative activity was determined using the streptavidin–perosidase method(Reference Danilenko, Ring and Tarpley36). Briefly, SI sections (distal jejunum segment, 5 μm thick) were deparaffinised in xylene, dehydrated with alcohol and rehydrated in PBS. Endogenous peroxidase activity was blocked by incubating sections with 3% H2O2 in methanol for 15 min. Then, normal goat serum (Sigma) was added to block non-specific interactions of the tissue with the primary antibody used. The tissue sections were then incubated with a rabbit polyclonal antibody for human Ki67 (1:100; Dako) for 2 h, and for 1 h with secondary antibody goat anti-rabbit (1:200; Dako) conjugated to alkaline phosphatase at room temperature in a humid chamber. Apoptosis was evaluated in parallel sections by terminal deoxynucleotidyl transferase-mediated nick end labelling analysis using an apoptosis assay kit (Beyotime Institute of Biotechnology) according to the manufacturer's instructions(Reference Miyazawa, Aso and Kanaya37). After visualisation with diaminobenzidine, cell nuclei were counterstained with haematoxylin in the tissue sections.
The number of positive cells was counted from ten random microscope fields per section at 100 × magnification by a Nikon morphometric system (Nikon Corporation). Apoptosis index (AI) and proliferation index (PI) were defined as the ratio of single-stranded DNA-positive nuclei or Ki67-positive nuclei to total nuclei multiplied by 100, respectively. The ratio of AI:PI was calculated by dividing the AI by the PI(Reference Hong, Turner and Carroll38).
Caspase-3 activity assay
The mucosal caspase-3 activity in jejunum was determined by the colorimetric assay using a commercial kit (Beyotime Institute of Biotechnology) based on the ability of caspase-3 to change acetyl-Asp-Glu-Val-Asp p-nitroanilide into a yellow formosan product p-nitroanilides(Reference Yin, Tang and Deng39). The relative activity of caspase-3 was normalised to total proteins, and expressed as fold change relative to Normal control piglets(Reference Yin, Tang and Deng39).
Protein immunoblot analysis
Jejunal mucosa stored in liquid N2 was ground into powder, followed by centrifugation(Reference Yao, Yin and Chu13). Protein concentration in the supernatant was determined using Bradford's method(Reference Bradford40). Equal amounts of protein (50 μg per lane) were subjected to SDS-PAGE (Mini-PROTEAN II electrophoresis system; Bio-Rad Laboratory)(Reference Laemmli41), and transferred on to an activated polyvinylidene difluoride membrane. For immunostaining, membranes were incubated with appropriate primary antibodies generated in rabbit (Cell Signaling Technology), and then incubated with horseradish peroxidase-conjugated secondary antibodies (ZDR-5306, Goat anti-rabbit; Zhongshan Goldenbridge) at a dilution of 1:5000. Blots were developed using an enhanced chemiluminescence kit (ECL), and visualised on Kodak-X-Omat film in a DuPont Lighting plus intensifying screen. The intensity of the protein bands was quantified by a computerised densitometry method in Adobe Photoshop software (Adobe). The polyclonal antibodies for phospho-Akt (Ser473) (no. 4058, 1:1000), total Akt (no. 9272, 1:1000), phospho-mTOR (Ser2448) (no. 2971, 1:1000), total mTOR (no. 2983, 1:1000), phospho-S6K1 (Thr389) (no. 9234, 1:1000), total S6K1 (no. 2708, 1:1000) were used in this study. The ratio of phosphorylated form to total protein was presented next to the blots.
Statistical analysis
Normal control, IUGR control and IUGR+Arg were paired within litters, and this set was a randomised block design, where blocks were litters. Hence, comparisons between any two groups were made using the paired t test(Reference Dauncey, Burton and Tivey42). For the BW data, there were two repeated measures. Modelling of repeated records was done using the MIXED procedure. Effects were compared using the CONTRAST and LSMEANS statement in the repeated MIXED analysis. The diarrhoea rate was analysed by the χ2 test. Pearson correlation coefficients were determined (CORR procedure) between the Arg supplementation levels and all the parameters measured in IUGR piglets. All statistical analyses were performed using SAS (SAS Institute, Inc.). Probability values < 0·05 were considered as significant difference. Data are presented as means with their standard errors.
Results
Milk intake and growth performance
In the present study, repeated analysis showed that, group, day, and group and day interaction had significant effect (P < 0·05) on BW in piglets from 7 to 14 d of age (Table 2). The BW of IUGR piglets was lower (P < 0·05) than that of Normal piglets. IUGR piglets treated by Arg had a higher (P < 0·05) BW compared with IUGR control piglets. However, it was still lower (P < 0·05) than that of Normal piglets. These were consistent with the results of BW at 14 d of age. A positive correlation was established between 14 d BW of IUGR piglets and Arg-supplemented levels (r 0·70, P = 0·017). Piglets in the IUGR group showed decreased daily weight gain, and daily DM intake (P < 0·05) in comparison to the Normal control group (Table 3). Arg treatment increased (P < 0·05) the daily weight gain and daily DM intake by 47·3 and 21·4%, respectively, and decreased the diarrhoea rate by 61·5% compared with IUGR piglets fed the control diet (P < 0·05). For daily weight gain and daily DM intake, positive correlations with Arg supplementation in IUGR piglets from 7 to 14 d of age were found (P < 0·05), and an inverse correlation between diarrhoea rate and Arg supplementation in IUGR piglets was observed (P < 0·05).
Table 2 Body weight (BW) in Normal and intra-uterine growth retarded (IUGR) piglets* from 7 to 14 d of age, reared on a bottle-feeding system (Mean values with their standard errors, n 6)

Arg, l-arginine; LS, least square.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* A piglet was defined as IUGR when its birth weight was 2 sd below the mean BW of the total population; when its birth weight was within 1 sd of the mean BW, it was defined as Normal.
† Piglets fed the control diet containing 1·23% l-alanine (isonitrogenous control).
‡ Piglets fed the Arg diet supplemented with 0·60% Arg.
Table 3 Growth performance and diarrhoea rate in Normal and intra-uterine growth retarded (IUGR) piglets* from 7 to 14 d of age, reared on a bottle-feeding system (Mean values with their standard errors, n 6)

Arg, l-arginine; ADG, daily weight gain; DMI, daily DM intake.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* A piglet was defined as IUGR when its birth weight was 2 sd below the mean body weight (BW) of the total population; when its birth weight was within 1 sd of the mean BW, it was defined as Normal.
† Piglets fed the control diet containing 1·23% l-alanine (isonitrogenous control).
‡ Piglets fed the Arg diet supplemented with 0·60% Arg.
§ Pearson correlation coefficients were determined in IUGR piglets supplemented with 0 and 0.60% Arg.
Small intestine weight, length and mucosa weight
IUGR decreased (P < 0·05) the entire SI weight:length ratio by 29·4% compared with Normal piglets at 14 d of age. The entire SI length ( − 12·1%, P = 0·403), the SI weight ( − 35·0%, P = 0·065), the mucosal weight ( − 23·8%, P = 0·266), the SI weight:BW ratio ( − 17·1%, P = 0·063) and the mucosal weight:length ratio ( − 17·2%, P = 0·201) also showed a trend to be lower in IUGR piglets (Table 4). Dietary Arg increased (P < 0·05) the SI weight by 73·2% and SI weight:length ratio by 62·4% in IUGR piglets, respectively, and did not differ (P = 0·071 and 0·155, respectively) from those of Normal piglets. Interestingly, the SI weight:BW ratio, mucosal weight and mucosal weight:length ratio of SI in IUGR piglets treated by Arg were increased (P < 0·05) by 43·3, 87·9 and 76·7% compared with IUGR piglets, and by 18.8, 43.2 and 16.4% compared with Normal piglets, respectively. The mucosal weight:BW ratio in IUGR piglets fed with the Arg diet was also elevated compared with both IUGR piglets (P = 0·058) and Normal piglets (P < 0·05) fed the control diet. In this study, as expected, all SI growth parameters except length measured were positively correlated (P < 0·05) with Arg supplementation in IUGR piglets. These results suggest that dietary supplementation of Arg improves the growth of SI mucosa in IUGR piglets.
Table 4 Length and weight of the entire small intestine (SI), SI mucosal weight in Normal and intra-uterine growth retarded (IUGR) piglets* at 14 d of age, reared on a bottle-feeding system (Mean values with their standard errors, n 4)

Arg, l-arginine; BW, body weight; L, length.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* A piglet was defined as IUGR when its birth weight was 2 sd below the mean BW of the total population; when its birth weight was within 1 sd of the mean BW, it was defined as Normal.
† Piglets fed the control diet containing 1·23% l-alanine (isonitrogenous control).
‡ Piglets fed the Arg diet supplemented with 0·60% Arg.
§ Pearson correlation coefficients were determined in IUGR piglets supplemented with 0 and 0.60% Arg.
Intestinal morphology
Measurements of villus height, crypt depth, villus height:villus width ratio, and villus height:crypt depth ratio did not differ (P>0·05) in the duodenum of piglets among all groups (Table 5). However, the villus width in both IUGR and IUGR+Arg piglets was lower (P < 0·05) than that of Normal piglets. Both proximal and distal jejunal villus height, villus height:villus width ratio and villus height:crypt depth ratio were increased (P < 0·05) by Arg supplementation in IUGR piglets, and had no difference (P>0·05) compared with Normal piglets. No significant difference (P>0·05) was observed in crypt depth and villus width of both the proximal and distal jejunum. In the ileum, the decreased (P < 0·05) villus height in IUGR piglets was recovered (P < 0·05) by Arg supplementation. However, the ileal crypt depth, villus width, villus height:villus width ratio and villus height:crypt depth ratio were not affected (P>0·05) by Arg supplementation in IUGR piglets. Moreover, Arg supplementation in IUGR piglets was positively correlated (P < 0·05) with villus height and villus width of the distal jejunum and ileum, proximal jejunal villus width and distal jejunal villus height:crypt depth ratio, respectively.
Table 5 Morphometric measurements of the duodenum, proximal jejunum, distal jejunum and ileum in Normal and intra-uterine growth retarded (IUGR) piglets* at 14 d of age, reared on a bottle-feeding system (Mean values with their standard errors, n 4)

Arg, l-arginine; HWR, villus height:villus width ratio; VCR, villus height:crypt depth ratio.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* A piglet was defined as IUGR when its birth weight was 2 sd below the mean body weight (BW) of the total population; when its birth weight was within 1 sd of the mean BW, it was defined as Normal.
† Piglets fed the control diet containing 1·23% l-alanine (isonitrogenous control).
‡ Piglets fed the Arg diet supplemented with 0·60% Arg.
§ Pearson correlation coefficients were determined in IUGR piglets supplemented with 0 and 0.60% Arg.
Apoptosis and proliferation
In the present study, the apoptotic cells were located in the upper region of the villus, while the proliferating cells were distributed around the lower part of the intestinal crypt in 14-d-old piglets (data not shown). These were consistent with previous studies(Reference Danilenko, Ring and Tarpley36, Reference Miyazawa, Aso and Kanaya37). IUGR showed a trend to decrease (P = 0·059) the PI in the distal jejunal mucosa, increased (P < 0·05) the AI by 32·7%, and the ratio of AI to PI by 44·1% compared with Normal piglets, respectively (Table 6). Dietary supplementation with Arg decreased (P < 0·05) AI by 43·0%, the ratio of AI:PI by 45·3% in IUGR piglets, and did not differ compared to Normal piglets (P = 0·077 and 0·118, respectively). Arg supplementation in IUGR piglets was inversely correlated (P < 0·05) with both AI and the ratio of AI:PI. The relative activity of caspase-3 in the distal jejunal mucosa was also decreased (P < 0·05) by Arg supplementation in IUGR piglets. PI in Arg-treated IUGR piglets did not differ from IUGR piglets and Normal piglets (P = 0·309 and 0·268, respectively).
Table 6 Apoptosis and proliferation of small intestine mucosa in Normal and intra-uterine growth retarded (IUGR) piglets * at 14 d of age, reared on a bottle-feeding system (Mean values with their standard errors, n 4)

Arg, l-arginine; AI, apoptosis index; PI, proliferation index.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* A piglet was defined as IUGR when its birth weight was 2 sd below the mean body weight (BW) of the total population; when its birth weight was within 1 sd of the mean BW, it was defined as Normal.
† Piglets fed the control diet containing 1·23% l-alanine (isonitrogenous control).
‡ Piglets fed the Arg diet supplemented with 0·60% Arg.
§ Pearson correlation coefficients were determined in IUGR piglets supplemented with 0 and 0.60% Arg.
Small intestine mucosal concentration of amino acids
The concentrations of amino acids in SI mucosa of piglets at 14 d of age, including histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophane, valine, aspartate, glutamate, glutamine, alanine, asparagines, cystine, glycine, serine and tyrosine did not differ (P>0·05) among the three groups (data not shown), and no correlations (P>0·05) with Arg supplementation in IUGR piglets were found. However, IUGR piglets revealed decreased (P < 0·05) concentrations of Arg, citrulline (Cit), ornithine (Orn) and proline (Pro) in SI mucosa compared with Normal piglets (Table 7). Dietary Arg supplementation in IUGR piglets increased (P < 0·05) the concentrations of Arg, Cit and Pro by 10·5, 21·6 and 9·6%, respectively. The concentrations of these amino acids in IUGR+Arg piglets were similar to those of Normal piglets (Arg, P = 0·090; Cit, P = 0·852 and Pro, P = 0·605). Mucosal concentrations of Arg, Cit and Pro were positively correlated (P < 0·05) with Arg supplementation in IUGR piglets, respectively.
Table 7 Amino acid concentrations of small intestine mucosa in Normal and intra-uterine growth retarded (IUGR) piglets* at 14 d of age, reared on a bottle-feeding system (Mean values with their standard errors, n 4)
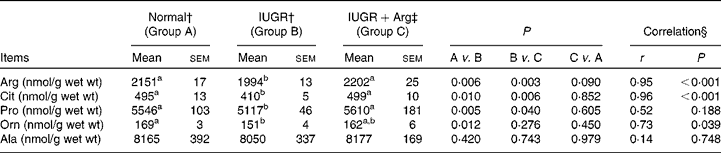
Arg, l-arginine; Cit, citrulline; Orn, ornithine; Pro, proline; Ala, alanine.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* A piglet was defined as IUGR when its birth weight was 2 sd below the mean body weight (BW) of the total population; when its birth weight was within 1 sd of the mean BW, it was defined as Normal.
† Piglets fed the control diet containing 1·23% Ala (isonitrogenous control).
‡ Piglets fed the Arg diet supplemented with 0·60% Arg.
§ Pearson correlation coefficients were determined in IUGR piglets supplemented with 0 and 0.60% Arg.
Insulin and insulin growth factor 1 level in small intestine mucosa
In comparison with Normal piglets, IUGR decreased (P < 0·05) the levels of insulin and IGF-1 by 23·2 and 24·2% in terms of per g wet mucosa and by 19·8 and 20·6% when normalised to the total protein level in mucosa (Table 8). In Arg-treated IUGR piglets, the levels of insulin in terms of per g wet mucosa and per g mucosal protein were increased (P < 0·05) by 27·0 and 24·4% compared with IUGR control piglets. Similarly, the IGF-1 levels showed an increased trend (P = 0·173 and 0·222, respectively). For insulin levels, the correlations with Arg supplementation in IUGR piglets were positive (P < 0·05), but no correlations (P>0·05) between Arg supplementation and IGF-1 levels were observed. Moreover, the levels of insulin and IGF-1 in IUGR+Arg piglets were similar (P>0·05) with Normal piglets fed the control diet at 14 d of age.
Table 8 Protein concentration, insulin and insulin growth factor 1 (IGF-1) level in small intestine mucosa of Normal and intra-uterine growth retarded (IUGR) piglets* at 14 d of age, reared on a bottle-feeding system (Mean values with their standard errors, n 4)
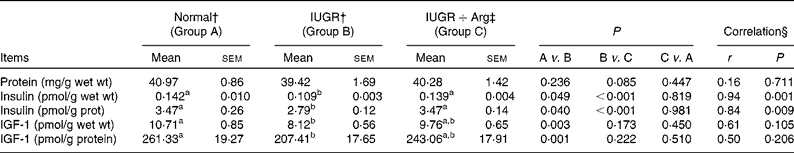
Arg, l-arginine.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* A piglet was defined as IUGR when its birth weight was 2 sd below the mean body weight (BW) of the total population; when its birth weight was within 1 sd of the mean BW, it was defined as Normal.
† Piglets fed the control diet containing 1·23% l-alanine (isonitrogenous control).
‡ Piglets fed the Arg diet supplemented with 0·60% Arg.
§ Pearson correlation coefficients were determined in IUGR piglets supplemented with 0 and 0.60% Arg.
Phosphorylation levels of Akt, mammalian target of rapamycin and p70 S6 kinase
Results of Western blots showed that IUGR decreased (P < 0·05) the phosphorylation of Akt on Ser473 by 34·1%, the phosphorylation of mTOR on Ser2448 by 37·5% and the phosphorylation of S6K1 on Thr389 by 27·7% in jejunal mucosa compared with Normal piglets at 14 d of age (Fig. 1). Dietary supplementation with 0·60% Arg increased (P < 0·05) the phosphorylation of Akt by 47·7% and the phosphorylation of mTOR by 61·9% in IUGR piglets, and restored them to the levels in Normal BW piglets (P = 0·673 and 0·899, respectively). As expected, the phosphorylation levels of Akt and mTOR were positively correlated with Arg supplementation in IUGR piglets (r 0·93, P < 0·05 and r 0·90, P < 0·05, respectively). In contrast, the phosphorylation of S6K1 was not affected (P = 0·116) by Arg supplementation in IUGR piglets. No correlation between phosphorylation of S6K1 and Arg supplementation in IUGR piglets was observed (r 0·70, P = 0·052).

Fig. 1 Phosphorylation state of (A) Akt, (B) mammalian target of rapamycin (mTOR) and (C) p70 S6 kinase (S6K1) in the jejunal mucosa of Normal and IUGR piglets at 14 d of age. A piglet was defined as intra-uterine growth-retarded (IUGR) when its birth weight was 2 sd below the mean body weight (BW) of the total population; when its birth weight was within 1 sd of the mean BW, it was defined as Normal. Normal and IUGR piglets fed the control diet containing 1·23% l-alanine (isonitrogenous control). IUGR+l-arginine (Arg) piglets fed the Arg diet supplemented by 0·60% Arg between day 7 and day 14 after birth. At 14 d of age, 2 h after feeding, the jejunal mucosa was obtained from piglets. Akt phosphorylation on Ser473, mTOR phosphorylation on Ser2448 and S6K1 phosphorylation on Thr389 were measured by Western blot analysis using antibodies that recognise these proteins only when that residue was phosphorylated. Western blots are shown above each graph. Values for phosphorylation of each protein were normalised for total protein. Values are means, with their standard errors represented by vertical bars (n 4). a,b Mean values with unlike letters were significantly different (P < 0·05).
Discussion
IUGR is a prevalent pathological phenomenon both in animals and in humans(Reference Wu, Bazer and Wallace2, Reference Wang, Huo and Shi6). The neonatal pig has been well established as an ideal model system for investigating the development of the gastrointestinal tract in human neonates(Reference Wang, Huo and Shi6, Reference Zabielski, Godlewski and Guilloteau18, Reference Murgas Torrazza, Suryawan and Gazzaneo23). Studies in the pig model have shown that IUGR could negatively affect birth weight, neonatal survivability, gastrointestinal tract development and postnatal growth(Reference Wu, Bazer and Wallace2, Reference Wang, Huo and Shi6). In both children and young pigs, Arg appears to be one of the most important amino acids for tissue growth(Reference Wu, Knabe and Kim12, Reference Wu, Bazer and Davis43) and SI development(Reference Rhoads, Liu and Niu25). Sufficient Arg provision effectively improved the growth and protein synthesis in piglets at 14 d of age(Reference Kim, McPherson and Wu9, Reference Yao, Yin and Chu13). It also enhanced the migration of intestinal epithelial cells by phosphorylating S6K1 in vitro (Reference Rhoads, Liu and Niu25). To our knowledge, this is the first study to determine the effects of dietary Arg supplementation on the growth performance and SI development through Akt and mTOR signals using IUGR piglets. However, the number of observations in the present study is limited (n 4).
Effect of l-arginine supplementation on body weight, growth and morphology of small intestine
IUGR negatively affects birth weight and postnatal growth of animals(Reference Wu, Bazer and Wallace2). This is consistent with our findings that IUGR decreased the growth performance of piglets from 7 to 14 d of age. The difference in daily DM intake we found is due to the change of BW and daily weight gain of piglets, because we fed them according to the BW, which was similar to the previous study(Reference Yao, Yin and Chu13). Although the reduced growth and development of SI has been reported in newborn IUGR piglets(Reference Wang, Huo and Shi6–Reference Xu, Mellor and Birtles8, Reference Zhong, Wang and Zhang31), few studies have investigated the effects of IUGR on the postnatal SI development. At 14 d of age, we found that IUGR decreased the SI weight per unit length as well as the ileal villus height. These results indicated that the negative effect of IUGR on pig gut development persists up to 14 d of age at least. This persistent effect may be one of the reasons for the long-term effect of IUGR on growth and development.
As an essential amino acid for suckling piglets, Arg deficiency is a major factor limiting maximum growth of milk-fed piglets(Reference Wu, Knabe and Kim12). The deficiency is even more prominent in IUGR piglets(Reference Wu, Jaeger and Bazer10). Consequently, dietary supplementation with Arg(Reference Kim, McPherson and Wu9, Reference Yao, Yin and Chu13) or activation of endogenous Arg synthesis(Reference Frank, Escobar and Nguyen44) is effective to increase Arg availability and growth performance in milk-fed piglets. In the present study, dietary supplementation with 0·60% Arg increased the growth traits of milk-fed IUGR piglets. This agrees with previous studies on milk-fed normal young pigs in the same period(Reference Kim, McPherson and Wu9, Reference Yao, Yin and Chu13), and is similar to the results found in 21-d-old weaned piglets(Reference Yao, Guan and Li27).
The SI is a major site for Arg metabolism and synthesis(Reference Wu45) and plays a critical role in maintaining Arg homeostasis in neonates(Reference Wu, Knabe and Kim12). On the other hand, as an essential precursor for intestinal synthesis of glutathione, NO, polyamines, amino acids (Cit and Pro)(Reference Wu45) and protein synthesis(Reference Rhoads, Chen and Gookin24, Reference Corl, Odle and Niu46), Arg plays an important role in intestinal functionality(Reference Rhoads and Wu47). Recent research(Reference Yao, Guan and Li27) has shown that dietary supplementation with 1 % Arg enhanced intestinal growth in 21-d-old weaned piglets. We also showed that supplementation of 0·60% Arg improved villus development and SI morphology in neonatal IUGR piglets. These effects were more evident in the jejunum, which was the primary site of nutrient absorption and amino acid metabolism in the SI(Reference Wu, Knabe and Kim12).
Effect of l-arginine supplementation on the concentrations of amino acids in small intestine mucosa
In neonatal mammals, SI enterocytes, the main place of endogenous Arg synthesis(Reference Kim and Wu48), play a critical role in the homeostasis of Arg(Reference Wu, Davis and Flynn11). Arg is synthesised from glutamine, and Pro via the SI in humans and pigs(Reference Wu, Bazer and Davis43). In suckling piglets, most of the Cit synthesised in enterocytes is converted locally into Arg(Reference Wu, Davis and Flynn11, Reference Wu, Bazer and Davis43). Arg degradation in the SI produces NO, polyamines, Pro, creatine and agmatine, with each having enormous biological importance(Reference Wu, Knabe and Kim12, Reference Wu, Bazer and Davis43). In the present study, we found that IUGR decreased the concentration of Arg, and its metabolism referring to amino acids (including Cit, Orn and Pro). This is similar to the previous finding in skeletal muscle of fetal pigs as a result of maternal malnutrition(Reference Wu, Bazer and Datta49) and several other observations in the SI of humans and pigs(Reference Rosenberg1, Reference Wang, Huo and Shi6, Reference Xu, Mellor and Birtles8, Reference Zhong, Wang and Zhang31).
Dietary supplementation or infusion with Arg elevated the plasma Arg concentrations in young piglets(Reference Kim, McPherson and Wu9, Reference Yao, Yin and Chu13) and humans(Reference Nagasaka, Yorifuji and Murayama50). The plasma concentrations of Cit and Orn were also increased(Reference Kim, McPherson and Wu9, Reference Yao, Yin and Chu13). Similarly, we found that the concentrations of Arg, Cit and Pro were increased in SI mucosa of IUGR piglets fed diets supplemented with Arg. However, as one of the major products of Arg catabolism in the mitochondria of enterocytes, the level of Orn was not significantly changed by Arg treatment. This may be due to the negligible arginase activity(Reference Wu, Davis and Flynn11). The metabolism of Arg in SI is oriented to anabolism rather than catabolism in the enterocytes of neonatal and suckling piglets, which exhibits a net production of Arg in the enterocytes of suckling piglets(Reference Blachier, M'Rabet-Touil and Posho51). The reduced Cit, a precursor of Arg(Reference Wu, Davis and Flynn11), has been shown as a biomarker of intestinal failure in infants and adults(Reference Crenn, Messing and Cynober52). Therefore, the increased Cit in intestinal mucosa of IUGR piglets by Arg supplementation suggests the improvement of the SI, which is further confirmed by our morphological observations in this study.
Effect of l-arginine supplementation on apoptosis and proliferation of enterocytes in small intestine
Previous studies showed that within the SI, IUGR enhanced apoptosis in rats(Reference Baserga, Bertolotto and Maclennan3) and reduced cell proliferation in newborn rabbits(Reference Cellini, Xu and Buchmiller53). In the present study, IUGR increased the AI and reduced the PI of enterocytes in piglets. This indicated that IUGR might affect intestinal growth and morphology through enhancing apoptosis and reducing proliferation in suckling piglets(Reference Baserga, Bertolotto and Maclennan3, Reference Zabielski, Godlewski and Guilloteau18). The enhanced apoptosis of enterocytes may create transient leaking gaps on the intestinal epithelium, which provide bacteria with easy access to the lamina propria and often induce inflammatory responses and cause diarrhoea. This has been reported in necrotising enterocolitis(Reference Upperman, Potoka and Grishin54).
Arg plays an important role in improving intestinal absorption and gut recovery after injury(Reference Liu, Huang and Hou55). We also found that a diet supplemented with Arg decreased apoptosis and the ratio of AI:PI in SI mucosa of IUGR piglets, suggesting that Arg protects the enterocytes probably via regulating apoptosis in the SI. Previous studies(Reference Rhoads, Chen and Gookin24, Reference Corl, Odle and Niu46, Reference Tan, Yin and Kong56) have demonstrated that Arg stimulates ex vivo intestinal cell apoptosis, proliferation and protein synthesis, while enhancing mTOR activity. Caspase-3, a protease activated by Bcl-2 and Bax protein, plays a key role in the apoptosis of enterocytes(Reference Hyoh, Ishizaka and Horii57). Its activity in the SI was elevated by IUGR in rats(Reference Baserga, Bertolotto and Maclennan3). We found that, consistent with the results of apoptosis, Arg supplementation significantly reduced the activity of caspase-3 in the SI of IUGR piglets. NO is synthesised from Arg by NO synthase in almost all mammalian cells. In IUGR infants, the production of NO was reduced due to the low availability of plasma Arg, which may be involved in the pathophysiology of necrotising enterocolitis(Reference Richir, Siroen and van Elburg15). A certain amount of NO is required for maintaining cellular functions in physiological conditions(Reference Wu and Meininger58). However, an excess production of NO or its toxic metabolite, ONOO−, may promote mucosal injury and gut barrier failure, possibly through the induction of enterocyte apoptosis by the activation of caspases and accumulation of p53(Reference Upperman, Potoka and Grishin54). In this study, mucosal caspase-3 activity and enterocyte apoptosis were reduced in the IUGR piglets treated by Arg, which indicated that the Arg-dependent production of NO was not too much impaired in SI mucosa.
Effect of l-arginine supplementation on the levels of insulin and phosphorylated Akt
Akt plays a critical role in promoting cell survival by inhibiting apoptosis(Reference Franke, Kaplan and Cantley19, Reference Cardone, Roy and Stennicke20). Akt can be activated by insulin and various growth factors(Reference Franke, Kaplan and Cantley19) through phosphorylation sites at Ser473 and Thr308 (Reference Jacinto, Facchinetti and Liu59). In the present study, the levels of insulin and the phosphorylated Akt were decreased in the SI mucosa of 14-d-old IUGR piglets, suggesting that the insulin–Akt pathway is affected by IUGR.
Arg is a potent stimulator of insulin secretion(Reference Cochard, Guilhermet and Bonneau60). Dietary supplementation with Arg elevated the plasma insulin level in artificially reared Normal piglets at 14 d of age(Reference Kim, McPherson and Wu9, Reference Yao, Yin and Chu13). We also observed increased Arg concentration in the SI mucosa of IUGR piglets. However, blood glucose is a well-known factor contributing to insulin production(Reference Park, Yang and Shinde61). In the present study, no difference was observed in the concentration of serum glucose (data not shown). In addition, only minimal metabolism of ingested glucose occurs in SI mucosa(Reference Bjorkman, Crump and Phillips62). Consequently, the elevated mucosal insulin level in IUGR piglets was probably due to the increased dietary Arg supplementation. The elevated insulin level by Arg may enhance the activation of Akt(Reference Franke, Kaplan and Cantley19), which inhibits intestinal cell DNA fragmentation and reduces cell apoptosis(Reference Rhoads and Wu47). We found that Arg increased the concentrations of insulin and phosphorylated Akt and consequently improved both the development and morphology of the SI in IUGR piglets, indicating an essential role for Arg in insulin–Akt-mediated cytoprotection.
Effect of l-arginine supplementation on the phosphorylation of mammalian target of rapamycin and p70 S6 kinase in small intestine mucosa
mTOR, a Ser/Thr protein kinase, controls protein synthesis, cell survival and proliferation(Reference Jacinto, Facchinetti and Liu59, Reference Drakos, Rassidakis and Medeiros63). In the present study, IUGR decreased the mTOR phosphorylation compared with the Normal piglets in SI mucosa. mTOR is phosphorylated at Ser2448 via the PI3 kinase–Akt pathway(Reference Nave, Ouwens and Withers21). Meanwhile, mTOR is a sensor for amino acids to balance the availability of nutrients and cell growth(Reference Dennis, Jaeschke and Saitoh22). In our study, decreased Akt activity and amino acid concentrations were observed in SI mucosa of IUGR piglets. This indicates the dual effects on inhibition of mTOR activity. Inhibition of mTOR inhibits the protein synthesis due to inhibition of its effector S6K1 and activation of 4E-binding protein 1, an inhibitor of translation(Reference Inoki, Li and Zhu64). S6K1 is a mitogen-activated Ser/Thr protein kinase that is required for cell growth and G1 cell cycle progression(Reference Pullen and Thomas65). We found a decreased S6K1 phosphorylation, which correlated with the reduced enterocyte proliferation in SI mucosa of IUGR piglets.
Recent studies(Reference Rhoads, Chen and Gookin24, Reference Rhoads, Liu and Niu25) have shown that Arg stimulates intestinal cell migration, and ex vivo intestinal protein synthesis by enhancing the mTOR signalling activity and activating S6K1 in rat intestinal epithelial cells. We also found that dietary Arg increased the level of phosphorylated mTOR in SI mucosa of IUGR piglets at 14 d of age, which is similar to the finding in skeletal muscle of Normal piglets(Reference Yao, Yin and Chu13). mTOR activity can be stimulated by Akt-dependent(Reference Nave, Ouwens and Withers21) and/or Akt-independent pathway (amino acids signal)(Reference O'Connor, Bush and Suryawan66). In the present study, the levels of insulin, Arg metabolism referring to amino acids (including Cit, Orn and Pro), as well as phosphorylated Akt and mTOR in SI mucosa were all increased in IUGR piglets treated by Arg. These results indicate that the insulin–Akt pathway and amino acids signal may play important roles in regulating mTOR by Arg in SI mucosa. This regulation may be specific to SI mucosa, since it was not observed in the skeletal muscle of piglets(Reference O'Connor, Bush and Suryawan66).
In conclusion, IUGR decreased growth and development of the SI, induced enterocyte apoptosis and reduced Akt and mTOR activities of SI mucosa in neonatal piglets. Arg supplementation improved SI development and reduced the apoptosis of enterocytes in IUGR piglets through enhancing Akt and mTOR signalling. Our findings provide important implications for treating IUGR piglets after birth by stimulating the intestinal development with dietary supplementation with Arg. However, further studies are needed to confirm the effects of Arg on the pathway regulating mTOR and its effectors in SI mucosa, as well as to identify the effects of long-term supplementation of Arg on growth and intestinal development in IUGR pigs or humans.
Acknowledgements
The present study was jointly supported by the National Natural Science Foundation of China (30771569 and 30972116) and the Youth Sci-Tech Innovation Fund, NAU (KJ08014). The authors thank W. Xu, Q. Dong and J. Wang for their assistance in sample collection and the raising of piglets. The contributions of each author to the study were as follows: L. Z., G. Z. and Z. L. participated in the experiments together with Y. W., who also performed the data analysis and wrote the manuscript. T. W. and W. L. designed and supervised the study, and revised the manuscript together with H. A. The authors had no conflicts of interest in the research.











