Cu, as a redox protein cofactor or structural element, is required for both ruminal microbes and animal per se (1,Reference Spears2) . Studies in dairy cows reported that dietary Cu addition did not affect DM intake (DMI) and milk yield but changed milk fatty acid composition(Reference Engle, Fellner and Spears3,Reference Morales, Palmquist and Weiss4) . Studies in goats found that addition of 10, 20 and 30 mg/kg DM of Cu from copper sulphate (CS) increased ruminal concentrations of total volatile fatty acids (VFA) and acetate as well as apparent total tract digestibility of DM, organic matter (OM) and fibre(Reference Zhang, Wang and Zhu5,Reference Mondal and Biswas6) . Vázquez-Armijo et al.(Reference Vázquez-Armijo, Martínez-Tinajero and López7) observed that ruminal production of SCFA and gas increased with supplementation of 21·7 mg/kg DM of Cu from CS in vitro fermentation of goats. However, studies about the impacts of dietary Cu addition on ruminal fermentation and nutrient digestion could not be found in dairy cows. Moreover, other studies reported that ruminal pH, VFA molar proportion and DM degradability were unchanged with the addition of 10 or 20 mg/kg DM of Cu from CS in steers consuming a high-concentrate finishing diet(Reference Engle and Spears8) or with supplementation of 100 mg Cu/d from CS in goats(Reference Solaiman, Craig and Reddy9). The divergent results might be associated with different addition levels of Cu and dietary composition in these studies(1,Reference Spears2) . It was necessary to study the effects of dietary addition of Cu on ruminal fermentation, microbial population and enzyme activity in dairy cows.
The current widely used inorganic Cu supplement is CS, and the absorption rate of dietary Cu was only 1−5 % in adult dairy cows(1). Cu is absorbed in the small intestine, but Cu from the rumen-soluble CS could form insoluble complexes with S, Mo and Fe in the rumen, impairing the absorption rate of Cu(Reference Bremner, Humphries and Phillippo10,Reference Gould and Kendall11) . Wang et al.(Reference Wang, Li and Xin12) reported that replacing one-half of dietary CS with methionine hydroxy, Cu increased the milk yield and blood Cu concentration and tended to increase apparent total tract digestibility of neutral-detergent fibre (NDF) and acid-detergent fibre (ADF) in dairy cows. The release percentage of Cu from coated copper sulphate (CCS) in the rumen was determined using cannulated Holstein bulls in the present study and was 24 %. However, CS has a high solubility and would release 100 % of its Cu in the rumen. Therefore, it was hypothesised that cows receiving CCS supplementation might have higher milk yield and nutrient digestibility compared with cows receiving CS supplementation. Therefore, the present study was conducted to evaluate the effects of dietary CS and CCS supplementation on lactation performance, nutrient digestibility, ruminal microbial population and enzyme activity in dairy cows.
Materials and methods
Animal welfare
Cow protection and welfare, cow husbandry and experimental protocol were authorised by the Animal Care and Ethics Committee of Shanxi Agriculture University.
Preparation and properties of coated copper sulphate
Supplement of CCS was produced based on the procedure of Wang et al.(Reference Wang, Liu and Guo13). First, 312·5 g/kg of CuSO4.5H2O, 187·5 g/kg of silicon dioxide and 160 g/kg of calcium stearate were mixed together. Second, 340 g/kg of hydrogenated fat (ratio of C16:0:C18:0 = 2:1) was heated to 80°C and then mixed with the first mixed ingredients. Third, the mixture was pelleted to particles with a diameter of 1·00−1·25 cm in a rotating pelletiser (HJ-400-S; Chongqing Rongkai Machinery Manufacturing Co. Ltd.
The disappearance rate of CCS in the rumen was measured using four ruminally cannulated Holstein bulls (657 (sd 11·6) kg of body weight). Eight ruminal nylon bags (12 × 8 cm; pore 37 μm) for each bull and about 3 g CCS of each bag were incubated in the rumen for 24 h. The collected bags from the rumen were washed in cool water for 3 min and freeze-dried at a freezer dryer. DM and Cu concentration of each sample were measured. The CCS contained 80·2 g/kg of Cu, and the ruminal disappearance rate of CCS was 0·24.
Holstein dairy cows, experimental design and diet
The study was carried out from October 2018 to December 2018 at a commercial dairy farm (Datong Sanxin DairyFarm). Fifty Holstein cows (2–4 of parity, 642 (sd 11·6) kg of body weight, 103 (sd 8·2) d in milk and 35·3 (sd 2·8) kg of daily milk yield), blocked by parity, daily milk yield and days in milk, were randomly assigned to one of the five treatments. Treatments were control (without Cu addition), CS addition (7·5 mg Cu from CS per kg DM) or CCS addition (5, 7·5 and 10 mg Cu from CCS per kg DM for low coated copper sulphate (LCCS), medium coated copper sulphate (MCCS) and high coated copper sulphate (HCCS), respectively). Supplementary CS or CCS was mixed into traditional premix and then into concentrate before the trial, respectively. The level of Cu in control, CS, LCCS, MCCS and HCCS groups was 8, 16, 14, 16 and 21 mg/kg DM, respectively. Experimental period was 111 d including 21 d of adaptation period and 90 d of sampling and data collection. Cows were raised in a naturally ventilated tie-stall barn, individually fed and were allowed to exercise for 2 h in an open dry lot before each milking. Cows were fed ad libitum a total mixed ration (Table 1). The total mixed ration was formulated according to the National Research Council(1) recommendation for a 650 kg cow producing 35 kg/d of milk containing 34 g/kg of milk fat and 32 g/kg of milk protein. Cows were milked three times/d at 06.00, 14.00 and 21.00 hours.
Measurements and collection of samples
Feed offered and orts were measured daily for each cow to estimate DMI. The orts and total mixed ration were sampled daily and stored at –20°C. Milk yield was recorded at each milking and was calculated by each cow. Milk was sampled every 10 d from each milking and preserved with 2-bromo-2-nitropropane-1, 3-diol before chemical analysis. At the end of the experiment, milk samples were pooled for each cow by day based on the milk yield at each milking.
From day 91 to day 108, each cow was dosed orally 5 g of chromic oxide (in gelatin capsule) twice daily (06.50 and 15.50 hours) for using as a digestion marker. Approximately 250 g of wet faeces was sampled from the rectum at 06.30, 12.30, 18.30 and 00.30 hours during days 99–108 and stored at –20°C. At the end of the experiment, samples of total mixed ration, orts and faeces were mixed by cow, respectively, dried at 55°C for 48 h and milled to pass a 1 mm sieve with a cutter mill (SF-320; Taizhou Chuangjian Machinery Co. Ltd) for chemical analyses. The DM flow (g/d) excreted in faeces was estimated by dividing daily Cr intake (mg) by faecal Cr content (mg Cr/g faeces)(Reference Ferret, Plaixats and Caja14). The flow of nutrient was evaluated by multiplying nutrient concentration in faecal DM by DM flow.
On days 109–110, ruminal fluid of each cow was sampled via oesophagus using a stomach tube at 06.00, 12.00, 18.00 and 24.00 hours. The initial 200 ml ruminal fluid was discarded to avoid saliva contamination, and the subsequent 200 ml was retained. Ruminal pH was immediately measured using an electric pH meter (HK-1309; Beijing Huakeyi Technology Co. Ltd). Samples of ruminal fluid were strained through four layers of cheesecloth. Filtrate used for the determination of VFA (5 ml) and ammonia-N (5 ml) was preserved by adding 1 ml of 250 g/l meta-phosphoric acid and 1 ml of 20 g/l (w/v) H2SO4, respectively, and frozen at –20°C. Filtrate used for the determination of enzymatic activity (20 ml) and ruminal microbe DNA (5 ml) was frozen at –80°C.
Blood samples of each cow were collected from the coccygeal vessels with disposable venous blood lancet (19 G, diameter 1·00 × 20 mm) into 5 ml vacuum tubes (diameter 13 × 100 mm; Jiangsu Xinkang Medical Equipment Co. Ltd) containing potassium oxalate and 4 g/100 ml sodium fluoride for glucose analysis, 10 ml serum separator tube (diameter 13 × 100 mm; Jiangsu Xinkang Medical Equipment Co. Ltd) containing coagulant (serum separating gel) for analysis of serum total protein, albumin, total cholesterol, TAG and ceruloplasmin and 10 ml heparin-impregnated tubes (diameter 13 × 100 mm; Jiangsu Xinkang Medical Equipment Co. Ltd) for analysis of superoxide dismutase (SOD) activity on day 111 after 3 h of the morning feeding. Samples were centrifuged at 2000 g and at 4°C for 15 min in order to separate serum and plasma and then frozen at –20°C.
On day 112, the liver was biopsied under local anaesthesia by blind percutaneous needle biopsy (14 G × 152 mm; Dispomed Witt oHG) as described by Gross et al.(Reference Gross, Dorland and Schwarz15). Liver biopsy samples were dried at 100°C for 48 h, weighed and then prepared for Cu analysis.
Chemical analyses
Analytical DM content of oven-dried samples was determined by drying at 135°C for 3 h (Association of Official Analytical Chemists (AOAC), method 930.15)(16). OM content was calculated as the difference between DM and ash content, with ash determined by combustion at 550°C for 5 h. Ether extract was determined according to AOAC (method 930.39)(16). NDF was determined using the methods described by Van Soest et al.(Reference Van Soest, Robertson and Lewis17) with heat-stable α-amylase and sodium sulphite used in the NDF procedure, whereas ADF was according to AOAC (method 973.18)(16). The content of N was determined by the Kjeldahl method (AOAC, method 976.05)(16). Milk samples were determined for fat, true protein and lactose using infrared spectrophotometry (Foss 120 Milko-Scan; Foss Electric) according to AOAC (method 972.16) procedures(16). Oven-dried samples of liver (100°C for 48 h), diets and faeces for mineral analysis were prepared using a microwave digestion (Model MDS-81D; CEM Corp.) procedure as described by Gengelbach et al.(Reference Gengelbach, Ward and Spears18). Cr content of faecal samples was measured by atomic absorption spectrophotometry (TAS-990, Beijing Purkinje General instrument Co. Ltd). The contents of Ca, P, S, Cu, Fe, Zn, Mn and Mo in diets and liver Cu were determined by inductively coupled plasma optical emission spectrophotometer (Optima 7300 DV; Perkin Elmer) according to the procedure described by Webb et al.(Reference Webb, Bartos and Boles19). Ruminal VFA was measured by GC (GC-8890B; Liaoning Dongke Analytical Instrument Co. Ltd) with 2-ethylbutyric acid as the internal standard. Ruminal ammonia-N content was determined by a colorimetric spectrophotometer (P8; Nanjing Meipuda Instrument Co. Ltd) based on the method of AOAC(16). Microbial enzymatic activities (carboxymethyl cellulase (CMC), xylanase, cellobiase, pectinase, protease and α-amylase) were determined according to the method of Agarwal et al.(Reference Agarwal, Kamra and Chaudhary20). Serum total protein, glucose, albumin, total cholesterol and TAG were measured by the Infinite 200 PRO Microplate reader (Tecan Austria GmbH) using the double-antibody sandwich method. All double-antibody sandwich ELISA kits for serum total protein, glucose, albumin, total cholesterol and TAG were specific kits for bovine and purchased from Shanghai Du Ma Biology Science & Technology Co. Ltd. Commercial colorimetric kits were used for the determination of SOD (WST-1 method; Nanjing Jiancheng Biological Engineering Institute) and ceruloplasmin (p-phenylenediamine oxidation method; Nanjing Jiancheng Biological Engineering Institute).
Extraction of DNA and real-time PCR
Microbial DNA was extracted from 1·5 ml ruminal fluid with the repeated bead beating plus column method described by Yu & Morrison(Reference Yu and Morrison21). Briefly, the method employs two rounds of bead beating in the presence of NaCl and SDS, followed by sequential ammonium acetate and isopropanal precipitations. The precipitated nucleic acids were then treated with RNase A and proteinase K, and the DNA was purified using columns from the QIAGEN DNA Mini stool Kit (QIAGEN). The extracted DNA was kept frozen at –20°C for PCR. The targeted populations were total bacteria, total anaerobic fungi, total protozoa, Ruminococcus albus, Ruminococcus flavefaciens, Butyrivibrio fibrisolvens, Fibrobacter succinogenes, Ruminobacter amylophilus and Prevotella ruminicola. All primer set sequences are shown in Table 2. The conventional PCR was used to generate sample-derived DNA standards for each real-time PCR assay. Ten sample-derived standards were prepared from the treatment pool set of microbial DNA. Then, the PCR product was purified using a QIA quick PCR purification kit (QIAGEN, Inc.) and quantified using a spectrophotometer. Ten-fold serial dilution was made in Tris-EDTA prior to real-time PCR using serial 10-fold gradient dilutions from 101 to 108 DNA copies(Reference Kongmun, Wanapat and Pakdee22). The conditions of the real-time PCR assays of target genes were following cycle conditions: one cycle of 95°C for 1 min for initial denaturation, forty cycles of 95°C for 15 s, primer annealing at 60°C for 30 s and product elongation at 95°C for 15 s, 60°C for 60 s and 95°C for 15 s with fluorescence detection at the end of each denaturation and extension step. Amplicon specificity was performed via dissociation curve analysis of PCR end products by increasing the temperature at a rate of 1°C every 30 s from 60 to 95°C. Real-time PCR amplification and detection were performed in a StepOne Plus™ real-time quantitative PCR system (Applied Biosystems, Life Technologies). Biotools QuantiMix EASY SYG KIT (B&M Labs, S.A.) was used for the real-time PCR amplification. Quantitative test samples were assayed in triplicate in a 20 μl reaction mixture contained 10 μl SYBR Green Premix Ex Taq- (2×) (TaKaRa Biotechnology), 0·8 μl PCR forward primer (10 μmol/l), 0·8 μl PCR reverse primer (10 μmol/l), 0·4 μl ROX reference dye (50×), 6·0 μl dH2O and 2 μl of the template DNA. The final copy number of targeted populations was calculated by the equation: (M × C × V2)/(S × V1), where M is the quantitative mean of the copy number, C is the DNA concentration of each sample, V2 is the dilution volume of isolated DNA, S is the DNA amount (ng) used in analysis and V1 is the volume (ml) of the sample used to DNA extraction.
Table 1. Ingredient and chemical composition of the basal diet
(Mean values and standard deviations)

ME, metabolic energy.
* Contained per kg premix: 20 000 mg Fe, 8000 mg Mn, 7500 mg Zn, 1·20 mg iodine, 60 mg Se, 20 mg Co, 1640 mg vitamin A, 600 mg vitamin D and 20 mg vitamin E.
† ME was estimated based on National Research Council(1) recommendations.
Table 2. PCR primers for real-time PCR assay
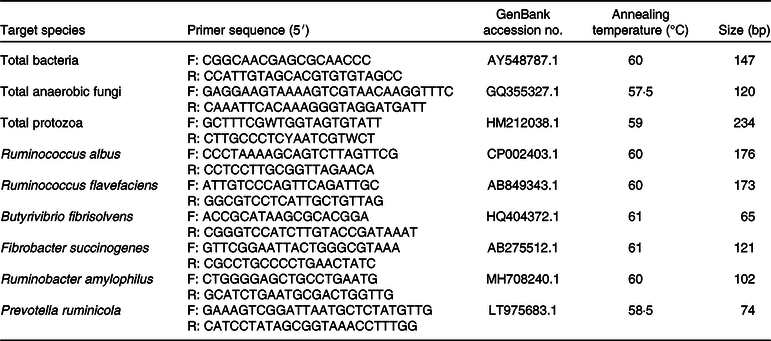
F, forward; R, reverse.
Calculations and statistical analysis
Feed efficiency (FE) was calculated as daily milk yield divided by DMI for each cow. Data for DMI, lactation performance and FE were analysed using the MIXED procedure of SAS (2002; Proc Mixed)(23), and the compound symmetry was the covariance structure that fitted the model best. A randomised block experiment design with repeated measurements was adopted according to the following model:
where Y ijkl is the dependent variable, μ is the overall mean, B i is the random effects of the ith block, C j is the fixed effects of treatments, T k is the fixed effect of time, (TC) jk is the time × treatment interaction, R ijkl is the random effects of the lth cow and ϵ ijkl is the residual error. Because the effects of time on variable and the time × treatment interaction for overall variable were not significant, the results are not listed in Table 3. Other data for nutrient digestibility, rumen fermentation parameters, enzyme activity, microbial flora and blood indicators were analysed with the same statistical model as suggested above except that the time and the interaction between time and treatment were omitted. Contrasts were carried out to examine the effect of control v. CS + MCCS, MCCS v. CS and linear and quadratic effects of CCS. Significant difference was suggested at P < 0·05.
Table 3. Effects of copper sulphate (CS) and coated copper sulphate (CCS) addition on DM intake (DMI), lactation performance and feed efficiency (FE) in lactating dairy cows
(Mean values with their standard errors; n 10)
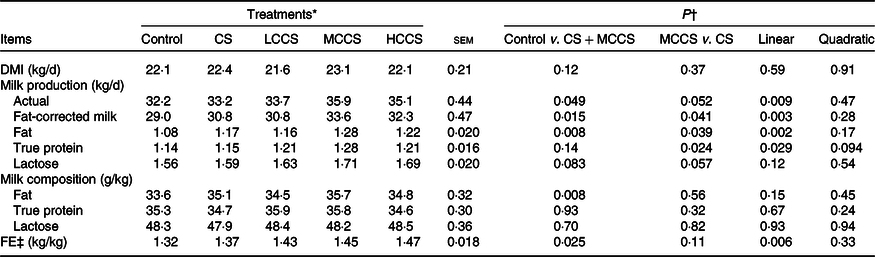
LCCS, low coated copper sulphate; MCCS, medium coated copper sulphate; HCCS, high coated copper sulphate.
* Control = without Cu addition; CS = 7·5 mg Cu/kg DM from CS; LCCS, MCCS and HCCS with 5, 7·5 and 10 mg Cu/kg DM from CCS, respectively.
† Linear and quadratic: orthogonal polynomials for response due to CCS (0, 5, 7·5 and 10 mg Cu/kg DM).
‡ FE = fat-corrected milk/DMI.
Results
Feed intake, lactation performance and feed efficiency
Addition of 7·5 mg/kg DM of Cu did not affect DMI but increased (P < 0·05) the yields of milk and milk fat as well as FE in dairy cows (Table 3). When comparing Cu source at equal addition level (7·5 mg Cu/kg DM), DMI, actual milk yield, milk lactose yield, milk composition content and FE were similar for cows receiving CS and MCCS addition, but yields of fat-corrected milk, milk fat and milk protein were higher (P < 0·05) for MCCS addition than for CS addition. Increasing dietary CCS addition, DMI, milk lactose yield and milk composition content were unaltered, but yields of actual milk, fat-corrected milk, milk fat and milk protein as well as FE linearly increased (P < 0·05).
Apparent nutrient digestibility and rumen fermentation
Compared with control, cows receiving 7·5 mg/kg DM of Cu addition had higher (P < 0·05) apparent total tract digestibility of DM, OM, crude protein, NDF and ADF (Table 4). Dietary 7·5 mg/kg DM of Cu addition decreased (P < 0·05) ruminal pH, propionate molar proportion and ammonia-N concentration; increased (P < 0·05) total VFA concentration, molar proportions of butyrate, valerate and isovalerate and the ratio of acetate:propionate; but did not affect molar proportions of acetate and isobutyrate. When comparing Cu source at equal inclusion level (7·5 mg Cu/kg DM), apparent total tract digestibility of DM, OM and NDF and ruminal total VFA concentration were higher (P < 0·05) for MCCS addition than for CS addition. Increasing dietary CCS addition, apparent total tract digestibility of DM, OM, NDF and ADF, ruminal total VFA concentration, butyrate molar proportion and acetate:propionate ratio increased linearly (P < 0·05), whereas propionate molar proportion and ammonia-N concentration decreased linearly (P < 0·05). Apparent total tract digestibility of crude protein, ruminal pH and molar proportions of acetate, valerate, isobutyrate and isovalerate were not affected by CCS.
Table 4. Effects of copper sulphate (CS) and coated copper sulphate (CCS) addition on nutrient digestibility and ruminal fermentation in lactating dairy cows
(Mean values with their standard errors; n 10)
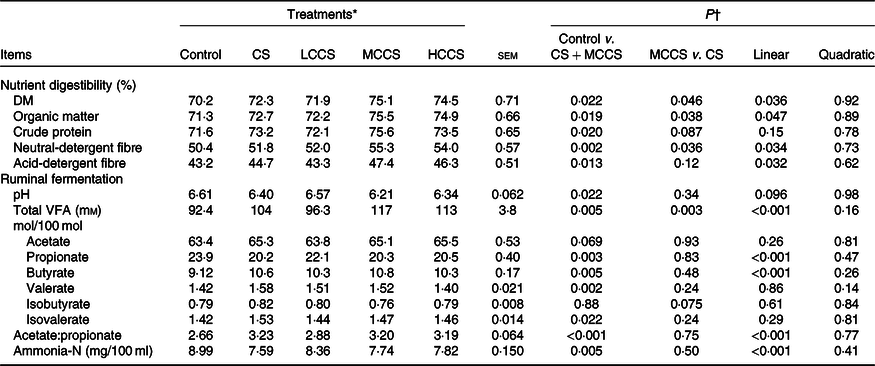
LCCS, low coated copper sulphate; MCCS, medium coated copper sulphate; HCCS, high coated copper sulphate; VFA, volatile fatty acids.
* Control = without Cu addition; CS = 7·5 mg Cu/kg DM from CS; LCCS, MCCS and HCCS with 5, 7·5 and 10 mg Cu/kg DM from CCS, respectively.
† Linear and quadratic: orthogonal polynomials for response due to CCS (0, 5, 7·5 and 10 mg Cu/kg DM).
Ruminal microbial enzyme activity and microbiota
Addition of 7·5 mg/kg DM of Cu increased (P < 0·05) activities of CMC, cellobiase, xylanase and pectinase and populations of total bacteria, protozoa, R. albus, R. flavefaciens, F. succinogenes and B. fibrisolvens but decreased (P < 0·05) α-amylase activity and populations of P. ruminicola and Rb. amylophilus (Table 5). When comparing Cu source at equal inclusion level (7·5 mg Cu/kg DM), activities of CMC, cellobiase, pectinase and α-amylase and populations of R. albus, R. flavefaciens and F. succinogenes were higher (P < 0·05) for cows receiving MCCS addition compared with cows receiving CS addition. Activities of xylanase and protease and populations of total bacteria, fungi, protozoa, B. fibrisolvens, P. ruminicola and Rb. amylophilus were not affected by dietary Cu sources. Increasing addition of CCS, activities of CMC, cellobiase, xylanase and pectinase and populations of total bacteria, protozoa, R. albus, R. flavefaciens, F. succinogenes and B. fibrisolvens increased linearly (P < 0·05), activity of α-amylase and populations of P. ruminicola and Rb. amylophilus decreased linearly (P < 0·05), but protease activity and total fungi population were unchanged.
Table 5. Effects of copper sulphate (CS) and coated copper sulphate (CCS) addition on microbial enzyme activity and ruminal microflora in lactating dairy cows
(Mean values with their standard errors; n 10)
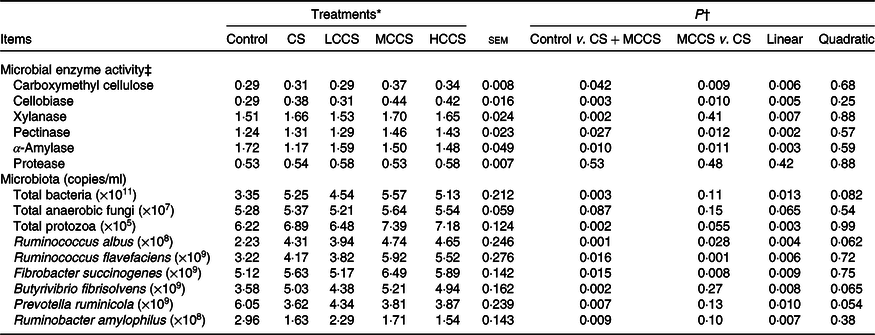
LCCS, low coated copper sulphate; MCCS, medium coated copper sulphate; HCCS, high coated copper sulphate.
* Control = without Cu addition; CS = 7·5 mg Cu/kg DM from CS; LCCS, MCCS and HCCS with 5, 7·5 and 10 mg Cu/kg DM from CCS, respectively.
† Linear and quadratic: orthogonal polynomials for response due to CCS (0, 5, 7·5 and 10 mg Cu/kg DM).
‡ Units of enzyme activity are carboxymethyl cellulase (μmol glucose/min per ml), cellobiase (μmol glucose/min per ml), xylanase (μmol xylose/min per ml), pectinase (μmol d-galactouronic acid/min per ml), α-amylase (μmol maltose/min per ml) and protease (μg hydrolysed protein/min per ml).
Blood metabolites and liver copper content
Dietary addition of 7·5 mg/kg DM of Cu increased (P < 0·05) serum concentrations of albumin, TAG and Cu as well as activities of SOD and ceruloplasmin and decreased (P < 0·05) total cholesterol concentration but did not affect concentrations of glucose and total protein (Table 6). When comparing Cu source at equal inclusion level (7·5 mg Cu/kg DM), lower (P < 0·05) serum total cholesterol concentration was observed for MCCS than for CS. Increasing addition of CCS, serum concentrations of glucose, total protein and TAG were unchanged, concentrations of albumin and Cu as well as activities of SOD and ceruloplasmin increased linearly (P < 0·05), but total cholesterol concentration decreased linearly (P < 0·05). Hepatic Cu concentration was increased (P < 0·05) by dietary Cu addition and was higher (P < 0·05) for MCCS than for CS. Increasing addition of CCS, a linear (P < 0·05) response was observed for hepatic Cu concentration.
Table 6. Effects of copper sulphate (CS) and coated copper sulphate (CCS) addition on blood metabolites and liver copper concentrations in lactating dairy cows
(Mean values with their standard errors; n 10)

LCCS, low coated copper sulphate; MCCS, medium coated copper sulphate; HCCS, high coated copper sulphate.
* Control = without Cu addition; CS = 7·5 mg Cu/kg DM from CS; LCCS, MCCS and HCCS with 5, 7·5 and 10 mg Cu/kg DM from CCS, respectively.
† Linear and quadratic: orthogonal polynomials for response due to CCS (0, 5, 7·5 and 10 mg Cu/kg DM).
Discussion
The level of Cu in the control group (8·51 mg/kg DM) was below the requirement (15·7 mg/kg DM) of the National Research Council(1) and might be insufficient to support the optimum performance of dairy cows. Therefore, an increase in milk yield was observed with the addition of 7·5 mg/kg DM of Cu. The increment in milk yield, nutrient digestibility and ruminal total VFA concentration as well as microbial cellulolytic enzyme activity and bacteria population indicated that dietary addition of Cu was required for ruminal cellulolytic bacteria and animal per se (1,Reference Spears2) . The design of current study was to investigate the effects of dietary Cu source and CCS level on milk performance and ruminal fermentation in dairy cows. In addition, dietary added fat due to the coating of CCS was 0·02, 0·03 and 0·04 g/kg DM for LCCS, MCCS and HCCS, respectively and had little impact on the total fat percentage of the overall diets. The influence of dietary added fat due to the coating of CCS would not be discussed below.
When comparing Cu source at equal inclusion level (7·5 mg Cu/kg DM), higher yields of fat-corrected milk, milk fat and milk protein were observed for MCCS addition and were likely related to the higher digestibility of DM, OM and NDF for cows receiving MCCS addition compared with cows receiving CS addition. The observed higher nutrient digestibility was consistent with the higher ruminal total VFA concentration and should be associated with the greater activities of CMC, cellobiase, pectinase and α-amylase and populations of R. albus, R. flavefaciens and F. succinogenes for MCCS addition compared with CS supplementation. The results suggested that the responses of ruminal microbes and nutrients digestion to dietary Cu supplementation were in a dose-dependent manner, and replacing CS with CCS was more favourable for ruminal cellulolytic bacteria growth. Similarly, Zhang et al.(Reference Zhang, Wang and Zhu5) reported that ruminal total VFA concentration and NDF digestibility increased quadratically with increasing the level of Cu supplementation in goats. Wang et al.(Reference Wang, Li and Xin12) found that replacing one-half of dietary CS with methionine hydroxy Cu did not affect DMI but tended to increase milk yield and fibre digestibility in dairy cows receiving dietary 12 mg/kg DM of Cu addition. Other studies found that DMI and yields of milk and milk components of dairy cows were unchanged with the addition of 10 or 40 mg/kg DM of Cu as CS in diets containing 8·9 mg/kg DM of Cu(Reference Engle, Fellner and Spears3) or with the addition of 20 mg/kg DM of Cu as CS in diets containing 8·0 mg/kg DM of Cu(Reference Morales, Palmquist and Weiss4). The unchanged DMI reported in these studies(Reference Engle, Fellner and Spears3,Reference Morales, Palmquist and Weiss4,Reference Wang, Li and Xin12) was in agreement with the current findings that DMI of cows was not affected by dietary Cu source or the supplementing level of CCS. That liver Cu concentration was greater for cows in MCCS group indicated that more Cu might be absorbed with MCCS addition compared with CS addition.
That ruminal total VFA concentration increased with increasing CCS supplementation indicated that nutrients degradation might be elevated by CCS addition. Similarly, Vázquez-Armijo et al.(Reference Vázquez-Armijo, Martínez-Tinajero and López7) reported that ruminal SCFA production, gas production and DM degradability increased with the addition of 21·7 mg/kg DM of Cu as CS in vitro. Zhang et al.(Reference Zhang, Wang and Zhu5) observed that apparent total tract digestibility of NDF and ruminal concentrations of total VFA and acetate increased with supplementation of 10 or 20 mg/kg DM of Cu as CS in diets containing 7·46 mg/kg DM of Cu in goats. The increase in ruminal concentrations of acetate (58·6, 61·4, 76·2 and 74·0 mm or control, LCCS, MCCS and HCCS, respectively) and butyrate (8·4, 9·9, 12·6 and 11·6 mm for control, LCCS, MCCS and HCCS, respectively) indicated that ruminal acetate and butyrate production increased(Reference Noziere, Glasser and Sauvant24), supporting the observed increase in milk fat yield. Ruminal acetate and butyrate are precursors of milk fat synthesis(Reference Mansson25). Maxin et al.(Reference Maxin, Glasser and Hurtaud26) reported that ruminal addition of acetate increased milk fat content of dairy cows. Sheng et al.(Reference Sheng, Yan and Qi27) observed that acetate and β-hydroxybutyrate addition stimulated the expression of genes related to milk fat and protein synthesis in bovine mammary epithelial cells in vitro. The observed increase in milk protein production was consistent with the increase in blood albumin concentration with increasing CCS supplementation.
The increase in apparent total tract digestibility of NDF and ADF was consistent with the increment in ruminal acetate: propionate ratio which indicated that the fermentation mode was changed to more acetate formation by CCS addition. The results were likely related to the increase in activities of CMC, xylanase, cellobiase and pectinase and populations of total protozoa, R. flavefaciens, R. albus, F. succinogenes and B. fibrisolvens. Ruminal Cu is utilised by micro-organisms as redox protein cofactor or structural element(Reference Ridge, Zhang and Gladyshev28). In addition, Cu2+ from the rumen-soluble CS could act as a bridge between the negatively charged feed and microbes(Reference Lopez-Guisa and Satter29). Addition of CCS provided 1·2, 1·8 and 2·4 mg/kg DM of Cu in the rumen for LCCS, MCCS and HCCS, respectively. However, digestibility of NDF and ADF, ruminal microbial population and enzyme activity were similar for MCCS and HCCS addition, indicating that higher level of Cu in the rumen might not be necessary. Similarly, Hernández-Sánchez et al.(Reference Hernández-Sánchez, Cervantes-Gómez and Ramírez-Bribiesca30) found that ruminal total bacteria population and VFA concentration were unchanged with increasing addition of Cu (as CS) from 20, 40 to 60 mg/kg DM and were decreased by addition of 80 and 100 mg/kg DM of Cu as CS in vitro. The decrease in ruminal propionate percentage was consistent with the lower activity of α-amylase and populations of P. ruminicola and Rb. amylophilus, indicating that increasing CCS addition might have negative effect on amylolytic bacteria growth. Studies indicated that a decreased propionate molar percentage could decrease the production of hepatic glucose, causing milk lactose yield and blood glucose concentration decrease in dairy cows(Reference Huhtanen, Miettinen and Ylinen31,Reference Miettinen and Huhtanen32) . However, milk lactose yield and blood glucose concentration were unaltered with CCS addition, and this was probably associated with the unchanged ruminal propionate concentration (22·1, 21·2, 23·8 and 23·2 mm for control, LCCS, MCCS and HCCS, respectively). Dietary CCS addition did not affect ruminal protease activity but increased cellulolytic bacteria and protozoa population. The increase in protozoa population would contribute to an accumulation of ammonia-N, since protozoa engulf bacteria and do not use ammonia-N(Reference Bach, Calsamiglia and Stern33,Reference Dijkstra34) . However, a reduction in ruminal ammonia-N concentration was observed with CCS addition. The result might be associated with the increase in total bacteria population and total VFA concentration, causing more ammonia-N to be converted to microbial protein. Since about 80 % of the cell N of cellulolytic bacteria is derived from ammonia-N, the fermentation of carbohydrate provides energy and carbon skeletons for microbial protein synthesis(Reference Bach, Calsamiglia and Stern33).
The decrease in serum total cholesterol concentration with increasing CCS addition was also observed by Engle & Spears(Reference Engle and Spears8), in which serum cholesterol concentration reduced with the addition of 10 or 20 mg/kg DM of Cu as CS in steers. Engle & Spears(Reference Engle and Spears8) explained that addition of Cu reduced the activity of 3-hydroxy-3-methylglutaryl CoA reductase, which is a rate-limiting enzyme in cholesterol synthesis, thereby decreasing cholesterol synthesis. However, Engle et al.(Reference Engle, Fellner and Spears3) reported an increased serum cholesterol concentration with 10 or 40 mg/kg DM of Cu as CS addition in dairy cows. The inconsistent results might be related to the difference in genetic and sex of animals(Reference Engle, Fellner and Spears3) as well as the level of Cu supplementation in these studies. The increase in concentrations of Cu in serum and liver as well as serum activities of SOD and ceruloplasmin indicated that the absorption of Cu was elevated with the addition of CCS. Hepatic Cu concentration could be used to reflect Cu status of cows, with 89−357 mg/kg DM in the normal range(Reference Kendall, Holmes-Pavord and Bone35). Concentrations of hepatic Cu for cows in control, LCCS, MCCS and HCCS groups were 369, 390, 387 and 431 mg/kg DM, respectively. The accumulation of Cu in the liver due to CCS addition did not appear to cause any detrimental effects in these cows, but any increase of Cu above the normal range results in Cu loading and an increased risk of Cu toxicity(Reference Kendall, Holmes-Pavord and Bone35).
Conclusion
CCS could be used as a Cu supplement in dairy cows. Addition of 7·5 mg/kg DM of Cu in diets containing 8·51 mg Cu/kg DM, higher milk yield and nutrient digestibility were observed for cows receiving CCS compared with cows receiving CS. Milk performance, nutrient digestion and ruminal total VFA concentration responded positively and linearly to the increased addition of CCS. Dietary Cu addition might be necessary for ruminal cellulolytic bacteria growth and fibre digestion, but this required further study to verify.
Acknowledgements
The authors thank the staff of Shanxi Agriculture University dairy calves unit for care of the animals. All authors read and approved the manuscript.
The present work was supported by a grant from Key Research and Development project of Shanxi Province (201803D221025-2), Key Research and Development project of Shanxi Province (201903D211012) and Animal Husbandry ‘1331 project’ Key Discipline Construction programme of Shanxi Province.
C. W. and Q. L. designed the experiment. L. H., G. W. Z., H. S. D., Z. Z. W., J. Z. and Y. L. Z. conducted the experiment. G. G., W. J. H., S. L. Z. and C. X. P. collected and analysed data. C. W. and Q. L. wrote the manuscript.
The authors declare that there are no conflicts of interest exist.









