Introduction
The extreme conditions to which sperm are subjected during the cryopreservation process result in several changes that diminish their fertilizing capacity (Waberski et al., Reference Waberski, Riesenbeck, Schulze, Weitze and Johnson2019). To reduce this damage to sperm cells, various cryoprotective agents are used in freezing–thawing protocols. Trehalose (α-d-glucopyranosyl α-d-glucopyranoside) is a disaccharide that is used as a nonpermeable cryoprotectant because it does not permeate the plasma membrane and exerts its effects at the extracellular level (Yeste, Reference Yeste2016). It acts by forming complexes with the biomacromolecules of the plasma membrane through hydrogen bonds, replacing water molecules, decreasing phospholipid-phase transition temperatures, preventing protein denaturalization, promoting an increase in the viscosity of the medium, and reducing the effects of lipid oxidation (Crowe, Reference Crowe2002; Oku et al., Reference Oku, Watanabe, Kubota, Fukuda, Kurimoto, Tsujisaka, Komori, Inoue and Sakurai2003; Sampedro and Uribe, Reference Sampedro and Uribe2004).
While it has been reported that trehalose maintains boar sperm quality upon thawing (Athurupana et al., Reference Athurupana, Takahashi, Ioki and Funahashi2015; Gutiérrez-Pérez et al., Reference Gutiérrez-Pérez, Juárez-Mosqueda, Carvajal and Ortega2009), trehalose must be present on both sides of the lipid bilayer to exercise its cryoprotective function by neutralizing reactive oxygen species (ROS) (da Costa Morato Nery et al., Reference da Costa Morato Nery, da Silva, Mariani, Fernandes, Pereira, Panek and Eleutherio2008; Herdeiro et al., Reference Herdeiro, Pereira, Panek and Eleutherio2006). Although the above hypothesis has now been confirmed in several types of cells (Eroglu et al., Reference Eroglu, Russo, Bieganski, Fowler, Cheley, Bayley and Toner2000; Holovati et al., Reference Holovati, Gyongyossy-Issa and Acker2009; Motta et al., Reference Motta, Paraguassú-Braga, Bouzas and Porto2014; Stefanic et al., Reference Stefanic, Ward, Tawfik, Seemann, Baulin, Guo, Fleury and Drouet2017; Uchida et al., Reference Uchida, Furukawa, Kikawada, Yamazaki and Gohara2017), it has still not been confirmed for sperm.
Trehalose exercises its antioxidant function through interactions that are carried out via hydrogen bonds between disaccharides (α,α-1,1) and the double bonds of unsaturated fatty acids (Oku et al., Reference Oku, Watanabe, Kubota, Fukuda, Kurimoto, Tsujisaka, Komori, Inoue and Sakurai2003). Docosahexaenoic acid (22:6n-3) is the most plentiful fatty acid in the boar-sperm membrane. As this fatty acid is particularly sensitive to lipid peroxidation during freezing (Cerolini et al., Reference Cerolini, Maldjian, Surai and Noble2000), the use of cryoprotectants is recommended to minimize damage at that level.
Several methods of facilitating the intracellular incorporation of trehalose have been described, and one example is liposomes. These vesicles were quickly identified as being an efficient means of releasing molecules due to their biocompatibility and low toxicity levels (Akbarzadeh et al., Reference Akbarzadeh, Rezaei-Sadabady, Davaran, Joo, Zarghami, Hanifehpour, Samiei, Kouhi and Nejati-Koshki2013; Toh and Chiu, Reference Toh and Chiu2013). Research into erythrocytes (Holovati et al., Reference Holovati, Gyongyossy-Issa and Acker2009) and stem cells obtained from umbilical cord blood (Motta et al., Reference Motta, Paraguassú-Braga, Bouzas and Porto2014) has shown that liposomes are an efficient means of facilitating the permeabilization of trehalose.
Holovati et al. (Reference Holovati, Gyongyossy-Issa and Acker2009) asserted that the extent to which trehalose exerts cryoprotective effects depends on its concentration. This research stresses the importance of determining the specific cryoprotective concentration for each type of cell. One of the techniques that has been described for analyzing trehalose is high-performance liquid chromatography (HPLC), which can be used in conjunction with high-sensitivity evaporative light-scattering detection (ELSD) (Allgeier et al., Reference Allgeier, Nussbaum and Risley2003; Dvořáčková et al., Reference Dvořáčková, Šnóblová and Hrdlička2014). ELSD detection represents an alternative to the inconveniences inherent in chemical derivatization as trehalose is an analyte that lacks chromophores (Allgeier et al., Reference Allgeier, Nussbaum and Risley2003).
Therefore, this study used HPLC in conjunction with ELSD to determine the intracellular presence of trehalose in boar sperm cryopreserved with liposomes after conducting a preliminary analysis of its effects on the integrity, progressive motility, and rheological behaviour of sperm upon thawing. To the best of our knowledge, there have been no reports of trehalose use at the intracellular level in sperm cells.
Materials and methods
The experimental procedures in this work that involved animals were approved by the Internal Committee for the Care and Use of Animals of the Autonomous National University of Mexico (Spanish acronym: UNAM).
The reagents used to prepare liposomes and for cryopreserving sperm, namely, soybean lecithin, cholesterol, and trehalose, were acquired from Sigma–Aldrich (St. Louis, MO, USA). Glycerol and phosphate-buffered saline solution (PBS) were obtained from JT Baker (Phillipsburg, NJ, USA). Dichloromethane (analytical grade), acetonitrile, and methanol (HPLC grade) were acquired from JT Baker, whereas trichloroacetic acid was obtained from Mallinckrodt AR (Paris, KY, USA).
Preparation and characterization of the liposomes
The liposomes were formulated using lecithin (3 mM), cholesterol (2 mM) at a 6:4 molar ratio, trehalose (300 mM), and glycerol (3% v/v) in a PBS (e.g. NaH2PO4·H2O, NaCl and NaOH; 0.1. M, pH 7.45). The liposomes were prepared using a heating method with the adaptations proposed by Linares-Alba et al. (Reference Linares-Alba, Gómez-Guajardo, Fonzar, Brooks, García-Sánchez and Bernad-Bernad2016). After heating the PBS-diluted cholesterol to 83°C while stirring at 750 rpm for 15 min, the remaining constituents of the formula were added, the final volume was adjusted with PBS, and N2 was applied. After heating the mixture to 75°C with constant stirring (750 rpm, 35 min), it was kept at room temperature for 60 min. The mixture was extruded and subsequently sterilized under aseptic conditions using a 0.45-µm Millex PVDF Millipore syringe filter (Billerica, MA, USA) in the first case and a 0.22-µm Millex PVDF Millipore syringe filter in the second case. The liposomes were refrigerated until used.
The liposome properties that were evaluated were particle size, polydispersity index (PDI), zeta potential (ζ), morphology, and entrapment efficiency (EE%).
Particle size and PDI: The analysis was carried out via dynamic light scattering (DLS) using a Zetasizer Nano-ZS analyzer ZEN 3600 (Malvern Instruments Ltd, Malvern, UK.) at a temperature of 25°C and a detection angle of 173°. Each dispersion was analyzed three times, and the average of the three results was reported.
Zeta potential (ζ): The zeta potential was determined via electrophoretic light scattering (ELS) using the aforementioned Zetasizer Nano-ZS ZEN 3600, each dispersion was analyzed three times, and the average of the three results was reported.
Morphology: The appearance of the liposomes was studied using transmission electron microscopy (TEM) and a JEOL JEM 1200EX II transmission electron microscope (JEOL, Tokyo, Japan) with ×10,000 magnification and using uranyl acetate at 2% as a negative stain.
Entrapment efficiency (EE%): The entrapment efficiency of the liposomes was determined by determining the amount of trehalose via HPLC with an Infinity LC 1260 system (Agilent Technologies, Santa Clara, CA, USA) adapted to work with a 1290 Infinity evaporative light-scattering detector (Agilent Technologies) and an 8-µm (300 × 7.7 mm) Hiplex H Agilent PL1170–6830 column. The mobile phase consisted of HPLC-grade water filtered through a 0.22-µm membrane. The injection volume was 1 µl, while a flow rate of 0.6 ml/min and temperature of 65°C were used. Before carrying out the chromatographic analysis of the liposomes, a calibration curve was plotted using aliquots with known trehalose concentrations (e.g. 0.1 to 10 mg/ml), and each aliquot was analyzed three times.
To measure the trehalose amounts in the liposome suspensions, it was necessary to centrifuge the latter at 14,000 g for 40 min to separate the liposomes in the samples using Amicon® Ultra 0.5 ml, 10K Centrifugal Filters (Millipore Co, Billerica, MA, USA). The liposomes were recovered by placing the same filter face-downward in a clean tube and centrifuging the sample again at 14,000 g for 10 min. Dichloromethane (1:1 v/v) was used to lyse the liposomes, and the sample was vortexed for 2 min before finally obtaining the aqueous phase for analysis.
The entrapment efficiency was calculated based on the formula shown below. The total trehalose values were obtained in the chromatographic analysis of the lysed liposomes, without having them undergo a separation protocol with the Amicon® filter:
Freezing–thawing method
Fifteen ejaculates were obtained from four mature boars that were provided by the Center for Education, Research and Extension in Swine Production (Spanish acronym: CEIEPP) of UNAM and were evaluated and diluted (1:1 v/v) in a commercial extender (Androstar Plus, Minitube, Tiefenbach, Germany). Only those ejaculates with progressive motilities greater than 85% and less than 20% abnormalities were accepted.
Two freezing media, an experimental medium and control medium, were evaluated. The experimental medium was composed of 10% liposomes, 10% egg yolk, 300 mM trehalose, and 3% glycerol. For the control medium, a tested extender containing 20% egg yolk and no liposomes was used (Gutiérrez-Pérez et al., Reference Gutiérrez-Pérez, Juárez-Mosqueda, Carvajal and Ortega2009). The freezing medium used during the freezing process were split into two parts, namely, Part A without glycerol and Part B with glycerol.
Sperm samples with concentrations adjusted to 600 × 106 cells/ml were centrifuged for 10 min at 800 g. Supernatants were removed, and Part A of the freezing medium was added to the pellets. The samples were kept at room temperature for 60 min, then at 16°C for 60 min, and finally at 4°C for 120 min. Part B of the freezing medium was gradually added to these samples over a period of 30 min.
The samples were placed in 0.5-ml straws, sealed with polyvinyl alcohol, exposed to liquid nitrogen vapour (−130 to −150°C) for 20 min, and finally conserved in liquid nitrogen (−196°C). At 15 days after being frozen, they were thawed in a water bath at 36°C for 30 s, and the contents of the straws were diluted with a commercial extender (Androstar Plus) (1:4 v/v).
Post-thaw sperm assessment
The viability and progressive motility of the sperm were studied by analyzing the contents of three straws per control and experimental treatment 10 min after thawing.
Cell viability: The percentages of living cells upon thawing were determined by eosin–nigrosin staining (Gutiérrez-Pérez et al., Reference Gutiérrez-Pérez, Juárez-Mosqueda, Carvajal and Ortega2009). After diluting the semen sample with the stain (1:8 v/v) and then incubating it at 37°C for 5 min, smears were taken and left to dry at room temperature. The evaluation consisted of distinguishing the live (unstained) cells from the dead (stained) cells with a ×100 optical microscope by examining at least 200 cells.
Progressive motility: Motility was ascertained by placing a drop of the sample on a pretempered slide (37°C) with a coverslip and observing the sample with an optical microscope (×10 and ×40).
Ultrastructure of the sperm: TEM was used to study the ultrastructure of the cryopreserved sperm in the presence of trehalose-containing liposomes, and the samples were fixed with 3% glutaraldehyde. After being postfixed with osmium tetroxide (OSO4) at 1%, the samples were dehydrated with ethanol at concentrations of 30% to 100%, with acetonitrile being used between the dehydration of the samples and their embedding in EPON resin. The samples were contrasted using uranyl acetate at 2% and lead citrate at 2% and were examined with a JEOL JEM 1200EX II transmission electron microscope.
Rheological analysis
Characterization of the rheological properties was conducted by studying the ejaculates from two different boars to ensure that the test was replicable. The semen samples were analyzed under the different cryopreservation protocol conditions (e.g. fresh, diluted with commercial extender, diluted with freezing medium, thawed, and thawed–diluted in a commercial extender) using a stress-controlled DHR3® Discovery Hybrid Rheometer with a concentric-cylinder geometry (TA Instruments, New Castle, Delaware, USA). The steady simple shear flow tests were carried out over a range 1–100 s−1, whereas the linear oscillatory shear flow tests used to estimate the viscoelastic properties (storage modulus, G′ and loss modulus, G′′) were carried out under small-amplitude oscillatory flow (γ = 30%) at a constant temperature of 37°C (circulating water bath, Cole-Parmer Polystat, and Peltier AR-G2) with an observation window of 0.1–300 rad/s. Experimental data were obtained and analyzed directly using TA Rheology Advantage Data Analysis v.5.7.0 (TA Instrument Ltd, Crawley, UK) software.
Chromatographic analysis
The analysis was carried out by means of HPLC using an Agilent Technologies HPLC Infinity Quaternary 1260 system that was adapted to work with a PL-ELS 1000 ELSD detector (Polymer Laboratories, Amherst, MA, USA) and a 250 × 4.6 mm Unison UK-Amino column (Imtakt, Kyoto, Japan).
Chromatographic conditions: 70% acetonitrile and 30% HPLC-grade water were filtered through a 0.22-µm membrane and used in the mobile phase. The injection volume was 0.5 µl of sample at a flow rate of 0.7 ml/min and temperature of 60°C.
Calibration curve: The calibration curve was constructed using aliquots with known trehalose concentrations (e.g. 5–25 mg/ml) that were dissolved in ultrapure water.
Sample preparation: Samples of four thawed ejaculates were centrifuged for 10 min at 800 g, and the supernatants were removed. Sperm lysis was carried out on the pellets obtained to evaluate the effectiveness of the two protocols. The first protocol (P1) consisted of reconstituting the pellets with 450 µl of ultrapure water and vortexing for 2 min. The samples were then ultrasonicated in VCX 500 ultrasonic processors (Sonics & Materials Inc., Danbury, CT, USA) for 30 min at 30% amplitude. Next, 50 µl of trichloroacetic acid was added to the samples, which were centrifuged at 14,000 g for 15 min. Finally, the supernatants were recovered, filtered through a 0.45-µm nylon syringe filter and analyzed via HPLC.
The second cell lysis protocol (P2) was based on the findings reported by Kralikova et al., (Reference Kralikova, Crha, Huser, Melounova, Zakova, Matejovicova and Ventruba2017) but with some changes. Cell pellets were reconstituted with 450 µl of 80% methanol and incubated for 60 min at room temperature. Five freezing processes were carried out using liquid nitrogen and ultrasound thawing at 36°C, after which 50 µl of trichloroacetic acid was added. The samples were then centrifuged again for 15 min at 14,000 g. Finally, after the supernatants had been recovered and filtered using a 0.45-µm Millex®-HN nylon filter, chromatographic analysis was carried out.
Statistical analysis
The regression coefficients for the calibration curves, the results of the variance analysis, and mean differences (as determined by Tukey’s test) were calculated using Version 20 of the IBM SPSS statistical package. The distribution of sperm evaluation data was normal.
Results
Characterization of the liposomes
The results of the liposome characterization are shown in Table 1. DLS analysis revealed the presence of particles with an average size of 357 nm. The PDI of the liposomes was 0.4, which indicated that the sample was moderately homogeneous with respect to the vesicle sizes. The electrostatic stability of the dispersion was evaluated based on the ζ value (e.g. −18.13 mV) and was considered moderate. The surface load of the vesicles that were obtained was negative.
Table 1. Physical characterization of liposomes

The values presented are the means ± standard error (SE) of the three measurements that were taken.
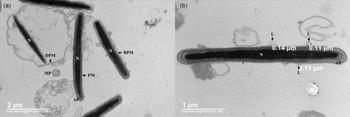
The TEM images confirmed the formation of liposomes (Figure 1) and showed spherical electron-lucid vesicles that were surrounded by electron-dense edges. The average diameter of the liposomes that were observed measured ∼325 nm. This was consistent with the DLS data.

Figure 1. (a–c) TEM micrographs of trehalose-containing liposomes.
The chromatographic analysis provided a determination coefficient (R2) of 0.993, obtained from the calibration curve. This indicated a strong correlation between the concentrations used in the standards and the areas below the peaks. A total trehalose concentration of 94.6 ± 0.5 mg/ml was observed in the liposome-dispersion samples, while the trehalose concentration found in the vesicles was 69.4 ± 3.8 mg/ml, with a 73% entrapment efficiency. A trehalose retention time of 8.383 min was observed in the chromatogram.
Post-thaw sperm assessment
The resulting semen quality that was observed upon thawing is presented in Table 2, and shows that the levels of viability and progressive motility of the thawed semen did not differ significantly from the results that were obtained for the freezing medium that were used (P > 0.05). Although no significant differences were found for the studied variables, the largest percentage of viable spermatozoa (28%) was found in the ejaculates that were cryopreserved in the presence of trehalose-containing liposomes whereas, in contrast, a smaller percentage of spermatozoa with progressive movement was found in the experimental group.
Table 2. Effects of liposomes on cryopreserved sperm
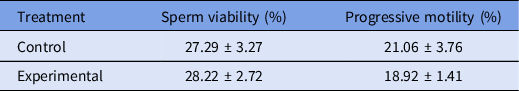
The values presented are the means ± SE. n = 15.
The TEM images (Figure 2) showed cryopreserved spermatozoa from the experimental medium that remained structurally intact (Figure 2a), along with cells with changes commonly associated with the freezing–thawing process. Additionally, vesicles that, due to their appearance, were identified as liposomes interacted with the plasma membrane of the cryopreserved spermatozoa (Figure 2b).
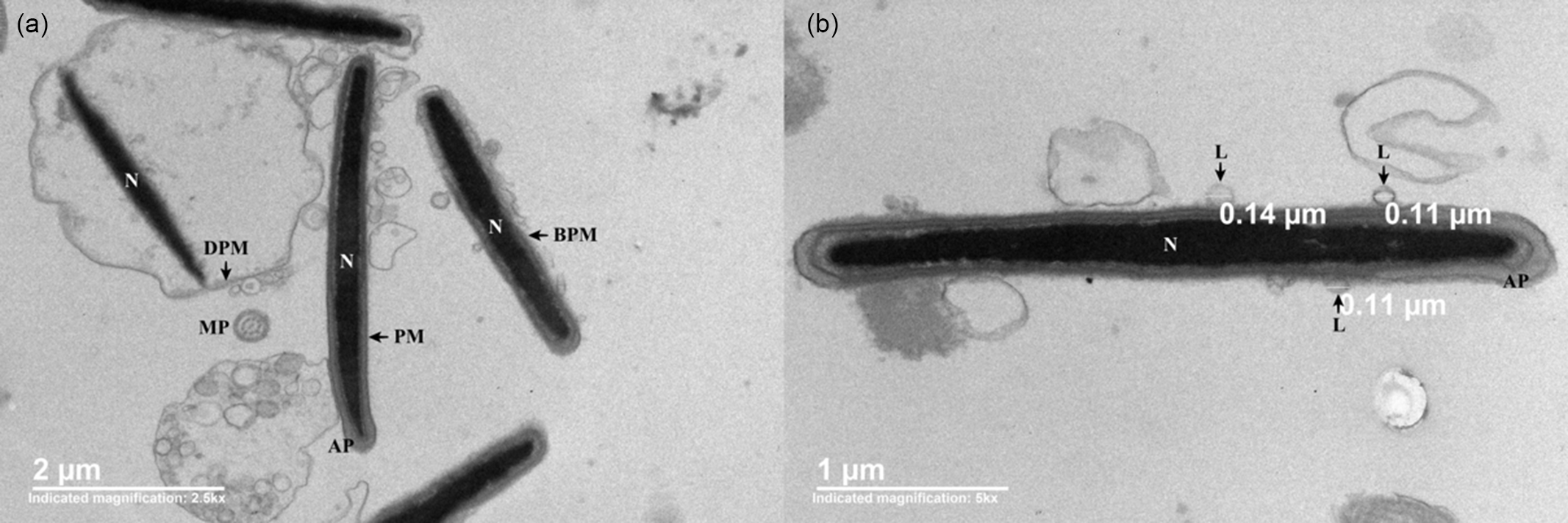
Figure 2. TEM micrographs of different segments of boar sperm cells cryopreserved in the presence of trehalose-containing liposomes. (a) Sperm cells with intact (PM), distended (DPM) and broken plasma membranes (BPM). (b) Cross-section of the sperm head showing the presence of liposomes (L) close to the plasma membrane of spermatozoa. AP, acrosomal protuberance; MP, main piece; N, nucleus.
Rheological analysis
The results obtained from the simple shear viscosity tests (Figure 3a) showed that the boar semen examined was a non-Newtonian shear-thinning fluid, that is, the viscosity decreases with respect to shear rate (shear-thinning behaviour, n < 1). Furthermore, no variations were observed in the sample viscosities before and after cryopreservation. When diluted with a commercial extender (1:4 v/v), the sample viscosities were one order of magnitude less while retaining their non-Newtonian shear-thinning properties (n < 1). Conversely, the linear viscoelastic oscillatory test (Figure 3b) showed a significant viscoelastic response, with elastic behaviour prevailing over viscous behaviour (G′ > G′′) in the fresh boar semen, whereas in the samples that were diluted with a commercial extender or experimental freezing medium, G′′ (viscous modulus) prevailed over G′ (elastic modulus), so the ejaculate changed its behaviour from a viscoelastic solid material (G′ > G′′) to a viscoelastic liquid material (G′′ > G′). The freezing–thawing process changed the microstructure of the samples (G′). This phenomenon was more evident over short times or high frequencies, as shown in Figure 4. The Han plot allows us to visualize and compare the sample microstructures before and after cryopreservation. The solid black line represents the equimodulus line (where G′ = G′′), which shows the transition from viscous (G′ < G′′) to elastic (G′ > G′′) behaviour, so that the systems above the diagonal line (fresh semen) exhibit predominantly elastic solid behaviour, while the diluted samples below the diagonal line mainly exhibit the behaviour of a viscoelastic liquid (G′′ > G′). It should be noted that, in the samples of diluted semen, the data relating to the experimental freezing medium lie closer to the diagonal line, indicating a predominant viscoelastic liquid behaviour and therefore pointing to more interactions among the components of the system. The rheological results of frozen–thawed boar semen suggested typical pseudosolid-like behaviour. The presence of this pseudosolid-like behaviour needs to be confirmed with other experimental evidence; however, it is consistent and could explain the microscopic behaviour and mechanical properties observed.
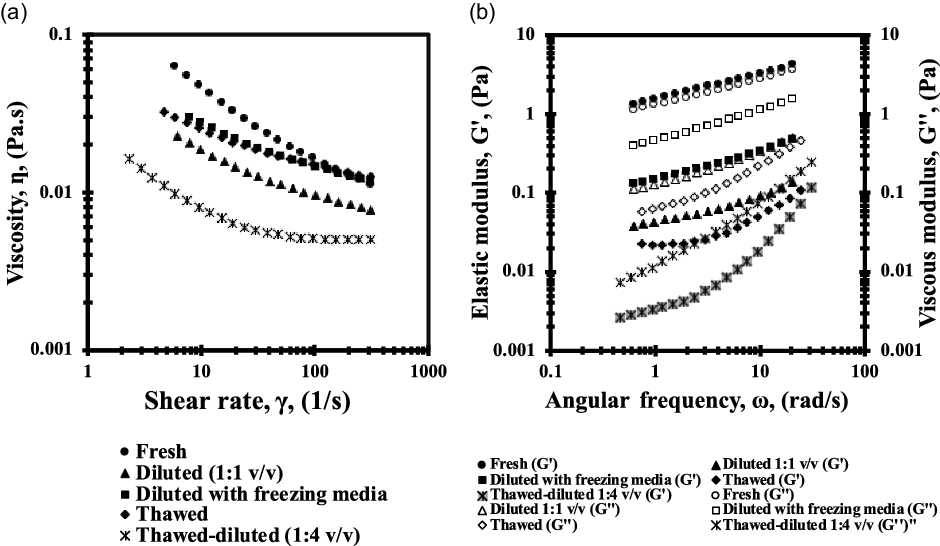
Figure 3. Rheological characterization of boar semen before and after cryopreservation. (a) Viscosity curves (viscosity vs. shear rate, γ) and (b) oscillatory shear curves (filled symbols are G′: elastic module and empty symbols are G′′: viscous module).

Figure 4. Han plot showing the elastic module (G′) versus viscous module (G′′) of boar semen samples before and after cryopreservation.
Chromatographic analysis
The calibration curve results showed a linear relationship between the trehalose concentrations (5–25 mM) and the areas below the peaks that were detected by the device, with a determination coefficient (R 2) of 0.999. Table 3 shows the trehalose concentrations that were detected in cryopreserved boar sperm with respect to the freezing medium used and the sample-preparation protocol. The trehalose concentrations that were observed in the cryopreserved sperm in the experimental group were higher in P2 (2.8 ± 0.4 mg/ml), although no significant differences were observed between the two sample-preparation protocols (P > 0.05).
Table 3. Mean trehalose (mg/ml) concentrations in frozen-–thawed semen

The values presented are the means ± SE. n = 4.
Although the freezing medium formula did not include trehalose-containing liposomes, a trehalose concentration level similar to that observed in the experimental group was also observed in the cryopreserved sperm pertaining to the control group for both sample-preparation protocols (P > 0.05).
Discussion
HPLC-ELSD was used in this study to detect trehalose at the intracellular level in cryopreserved boar sperm. The obtained results revealed the presence of trehalose inside the spermatozoa that had been frozen and subsequently thawed, and an effective analytical method for measuring sugars in cryopreserved cell samples is proposed.
Two distinct trehalose-extraction protocols were used, and no significant differences were found between them. In the first protocol, sonication was used to break down the sperm-cell membranes whereas, in the second protocol, methanol was used along with repeated freezing–thawing cycles. Among the findings reported when using HPLC-ELSD to quantify intracellular trehalose levels, the detection of trehalose in the control-group samples should be stressed. Intracellular incorporation of trehalose during freezing has been reported in platelets (Gläfke et al., Reference Gläfke, Akhoondi, Oldenhof, Sieme and Wolkers2012) and fibroblasts (Zhang et al., Reference Zhang, Oldenhof, Sieme and Wolkers2016). It has been suggested that the lipid phase transition that occurs during freezing makes the plasma membrane more permeable, facilitating the permeation of trehalose (Stewart and He, Reference Stewart and He2019). However, the part played by liposomes in the incorporation of trehalose inside the cryopreserved sperm was found to be minimal, a finding that concurs with that of Holovati et al., (Reference Holovati, Gyongyossy-Issa and Acker2009), who reported low concentrations of intracellular trehalose when using liposomes to cryopreserve human erythrocytes. Notwithstanding the above, the adverse effects of liposomes are minimal compared with those of other methods used to facilitate the intracellular release of trehalose.
In this work, liposomes were prepared using a heating technique, which facilitates the production of high-entrapment efficiency liposomes (Linares-Alba et al., Reference Linares-Alba, Gómez-Guajardo, Fonzar, Brooks, García-Sánchez and Bernad-Bernad2016). Subsequently, those features that have a significant effect on the in vivo performance of liposomes were studied (Saadeldin et al., Reference Saadeldin, Khalil, Alharbi and Lee2020). The results of the characterization confirmed that there was dispersion of spherical liposomes measuring 357 nm, moderately polydisperse (Danaei et al., Reference Danaei, Dehghankhold, Ataei, Hasanzadeh Davarani, Javanmard, Dokhani, Khorasani and Mozafari2018), relatively stable (Bhattacharjee, Reference Bhattacharjee2016), and with a 73% trehalose-entrapment efficiency. The average vesicle size that was achieved via filtration is worth noting, and it is worth mentioning that the presence of trehalose fosters the formation of larger liposomes. Roy et al. (Reference Roy, Dutta, Kundu, Banik and Sarkar2016) reported that trehalose is able to form hydrogen bonds with the phospholipids of liposomes made from dimyristoylphosphatidylcholine (DMPC), increasing the size of the vesicles as a result of this interaction. Similar to the liposome size, the moderate levels of polydispersion that were observed in the system are due to the less effective interactions between trehalose molecules and phospholipids, therefore resulting in a lower percentage of vesicles with smaller sizes.
Regarding the influence of liposome size on the interactions between liposomes and cells, it has been shown that the use of small liposomes (<200 nm) favours interactions between the vesicles and plasma membranes of the target cells, providing more efficient cryoprotection of cells (Kheirolomoom et al., Reference Kheirolomoom, Satpathy, Török, Banerjee, Bali, Novaes, Little, Manning, Dwyre, Tablin, Crowe and Tsvetkova2005; Papahadjopoulos et al., Reference Papahadjopoulos, Poste and Schaeffer1973). We also measured the zeta potential to determine the stability of our dispersion, with at least ±30 mV being necessary for the formulation to be deemed stable. According to the zeta potential values, our dispersion was classified as relatively stable, indicating that the electrostatic repulsive force between the vesicles is not sufficient to prevent their agglomeration (Bhattacharjee, Reference Bhattacharjee2016). Nevertheless, studies on exosomes have shown that trehalose can maintain sufficiently high repulsion forces to keep the vesicles separate from each other (Bosch et al., Reference Bosch, de Beaurepaire, Allard, Mosser, Heichette, Chrétien, Jegou and Bach2016).
Concerning the effects of intracellular trehalose on cryopreserved boar semen, the percentages of viable cells and progressive motility upon thawing showed no significant changes despite the presence of trehalose-containing liposomes in the freezing medium. We suggest that the aforementioned results might be influenced by the incubation conditions used in this study (Holovati et al., Reference Holovati, Gyongyossy-Issa and Acker2008; Röpke et al., Reference Röpke, Oldenhof, Leiding, Sieme, Bollwein and Wolkers2011; Stewart et al., Reference Stewart, Arminan and He2020) and by the soybean lecithin concentrations in the liposome dispersion (Zhang et al., Reference Zhang, Hu, Li, Jiang and Zhang2009). There have been no reports to date of liposomes made with soybean lecithin being used in boar semen. This opens the door for further research that contributes to the development of boar-sperm freezing techniques, which are becoming an increasingly relevant method for maintaining biosecurity.
Moreover, He et al. (Reference He, Bailey and Buhr2001) reported the use of liposomes made from a variety of phospholipids to cryopreserve boar sperm. These results are consistent with those presented here regarding the effects of liposomes on the viability and progressive motility of thawed boar sperm. The authors also described the use of liposomes combined with egg yolk to create a freezing medium.
A study of the rheological properties of boar semen was carried out to analyze sperm–medium and sperm–sperm interactions before and after the cryopreservation process, as well as the effect of trehalose-containing liposomes on the viscoelasticity of the samples. The results from simple shear tests showed higher viscosity levels in semen samples that were reconstituted in the experimental freezing medium. The higher viscosity levels were mainly due to the presence of cryoprotectants and remained unchanged even after freezing–thawing (Sampedro and Uribe, Reference Sampedro and Uribe2004; Morris et al., Reference Morris, Goodrich, Acton and Fonseca2006; Rodriguez-Martinez and Wallgren, Reference Rodriguez-Martinez and Wallgren2011). Dissolving cryopreserved sperm in a commercial extender reduces its viscosity, which is a phenomenon that is relevant in view of the protective effects of conserving these cells in a viscous medium. Several investigations have stated that sperm-cell dilution in a viscous medium prevents cellular sedimentation and favours a reduction in sperm metabolic demand (Nagy et al., Reference Nagy, Sinkovics and Kovács2002; López-Gatius et al., Reference López-Gatius, Sances, Sancho, Yániz, Santolaria, Gutiérrez, Núñez, Núñez and Soler2005; Corcini et al., Reference Corcini, Moreira, Pigozzo, Varela, Torres and Lucia2011). Furthermore, studies on boar sperm have shown that increased viscosity of the medium enhances sperm membrane stability and their ability to move (Coy et al., Reference Coy, Gadea, Rath and Hunter2009; Gil et al., Reference Gil, Barón, Guerrero, García-Marín and Gil2014; González-Abreu et al., Reference González-Abreu, García-Martínez, Fernández-Espín, Romar and Gadea2017)
With regard to the oscillatory shear flow tests, we observed that the viscoelastic properties of semen samples were affected by the freezing–thawing process. This is related to the diminished interactions among the components of the freezing medium and sperm with a damaged plasma membrane (Barnes, Reference Barnes2000; Medina-Torres et al., Reference Medina-Torres, Brito-de la Fuente, Torrestiana-Sanchez and Katthain2000). Additionally, a change in the slope and a slight plateau were observed in the flow curves of thawed semen at low frequencies (or long times), typical of a pseudosolid-like behaviour, which is associated with the strong interaction between the sample components (Medina-Torres et al., Reference Medina-Torres, Núñez-Ramírez, Calderas, González-Laredo, Minjares-Fuentes, Valadez-García, Bernad-Bernad and Manero2019). Interestingly, Tung et al. (Reference Tung, Lin, Harvey, Fiore, Ardon, Wu and Suarez2017) reported that the elasticity of a medium induced collective swimming patterns of bovine sperm, facilitating sperm migration and therefore the success of fertilization. To our knowledge, there have been no reported studies of the viscoelastic behaviour of cryopreserved boar semen; therefore, the above findings may serve as a basis for designing quantitative tests to determine the quality of this semen.
It is worth mentioning that we also carried out a rheological analysis of those samples that were reconstituted in the control freezing medium (results not shown) and determined that their viscosities were similar to those of the experimental group due to the presence of cryoprotectants in the freezing medium. Nevertheless, a slight effect of trehalose-containing liposomes on the viscoelastic properties of the samples (G’) was observed.
In conclusion, in this study, we measured the amounts of intracellular trehalose in cryopreserved boar-sperm lysates by means of HPLC-ELSD and developed and characterized a freezing medium made from trehalose-containing liposomes that was effective in maintaining the viability and motility of thawed sperm. The rheological response of the samples during cryopreservation shows that they are mechanically stable for flow and that their rheological properties change when they are diluted in a freezing medium that contains trehalose and after thawing.
Further research should be carried out using soybean lecithin and trehalose in the boar-sperm freezing–thawing process to ascertain what concentrations are needed to achieve optimal cryoprotection.
Acknowledgements
The authors wish to thank the National Council of Science and Technology (Spanish acronym: CONACYT) for scholarship number 593892 assigned to Claudia Denisse Mendoza-Viveros. The authors also wish to thank Miguel Ángel Valadez-García, Carolina Flores Ávila (FQ-UNAM), Rodolfo Paredes Días (IFC-UNAM), Fred Rogers and Laboratorio Avi-mex, SA de CV for their collaboration in carrying out the present study.
Financial support
This research received no specific grant from any funding agency or from commercial or not-for-profit sectors.
Conflict of interest
The authors declare that there are no conflicts of interest regarding this work.
Ethical standards
The authors assert that all procedures in this work that involved animals were approved by the Internal Committee for the Care and Use of Animals of the Autonomous National University of Mexico (Spanish acronym: UNAM).