Introduction
Assessing the genetic diversity and structure of wildlife populations is significant for the development of effective conservation strategies. Such assessments facilitate the identification of groups with unique genetic identity, necessitating independent management approaches to ensure their preservation (Fallon Reference Fallon2007; Rivera-Ortíz et al. Reference Rivera-Ortíz, Solórzano, Arizmendi, del, Dávila-Aranda and Oyama2020). Despite the crucial role that genetic studies play in conservation and management processes, a substantial paucity of genetic information persists in wildlife populations. This deficiency is notably conspicuous in countries in the neotropics that, despite boasting a great diversity of bird species, presents a relatively low genetic characterisation in this group (Noreña et al. Reference Noreña, González Muñoz, Mosquera-Rendón, Botero and Cristancho2018).
Despite the neotropics having almost half the world’s entire bird richness (4,194 species) and including the top 10 most diverse countries, this region faces substantial ecosystem degradation and accelerated wildlife loss (Develey Reference Develey2021; Etter Reference Etter1993; Renjifo et al. Reference Renjifo, Amaya-Villarreal and Butchart2020; Tundisi and Matsumura-Tundisi Reference Tundisi and Matsumura-Tundisi2008; Victorino Reference Victorino2012). This is evident in countries like Colombia, Peru, and Brazil, which are considered the richest countries worldwide, yet 140, 122, and 166 bird species, respectively, are categorised as threatened (Angulo-Pratolongo Reference Angulo-Pratolongo2018; Develey Reference Develey2021; Renjifo et al. Reference Renjifo, Amaya-Villarreal, Burbano-Girón and Velásquez-Tibatá2016). Also, it has been reported that between 2002 and 2016, the number of species increasing their threat category was four times higher compared with those improving their conservation status, primarily attributed to habitat degradation and illegal trade (Renjifo et al. Reference Renjifo, Amaya-Villarreal, Burbano-Girón and Velásquez-Tibatá2016, Reference Renjifo, Amaya-Villarreal and Butchart2020).
Illegal wildlife trade remains a poorly managed issue, posing a significant risk to global biodiversity and biosecurity, while also providing a source of funding for criminal organisations (Rosen and Smith Reference Rosen and Smith2010). Estimates suggest that between 1998 and 2018, at least 421 million CITES-listed wildlife individuals were trafficked worldwide, with a disproportionate impact on developing nations facing economic challenges (Liew et al. Reference Liew, Kho, Lim, Dingle, Bonebrake and Sung2021). Birds are a prime target, with poaching affecting approximately 23% of all bird species (10,278 species) and 50% of threatened species. This is especially notorious in Latin America, where specimen extraction is prevalent (Scheffers et al. Reference Scheffers, Oliveira, Lamb and Edwards2019).
The Psittacidae family is severely impacted by habitat degradation and illegal trade with approximately one-third of Psittacidae species threatened globally (Vergara-Tabares et al. Reference Vergara-Tabares, Cordier, Landi, Olah and Nori2020). Parrots stand out as one of the most heavily traded birds globally, with approximately 321 CITES-listed species traded internationally (Chan et al. Reference Chan, Poon, Wong and Sin2021). Illegal trade represents the second most significant threat to parrot populations, particularly in the neotropics, where the Amazona genus is among the most impacted (Mercado et al. Reference Mercado, Asmussen, Rodriguez, Moran, Cardozo-Urdaneta and Morales2020; Tella and Hiraldo Reference Tella and Hiraldo2014). In Colombia, parrots account for 74–91% of confiscated birds, and the six Amazon species found in the country constitute 41% of all confiscated parrots (Mendivelso-Gamboa and Montenegro Reference Mendivelso-Gamboa and Montenegro2007; Restrepo-Rodas and Pulgarín-Restrepo Reference Restrepo-Rodas and Pulgarín-Restrepo2017). Similarly, in Venezuela, Amazon parrots are the most affected group by illegal trade, representing around 57% of all traded parrots in the country (Mercado et al. Reference Mercado, Asmussen, Rodriguez, Moran, Cardozo-Urdaneta and Morales2020). In Mexico, Amazon parrots along with macaws (genus Ara) are the most illegally traded parrots, with Amazona species representing 37% of all seized individuals (Tella and Hiraldo Reference Tella and Hiraldo2014). In Bolivia, Amazon parrots represent 15% of traded parrots, and the Turquoise-fronted Amazon Amazona aestiva is the fourth most traded species (Pires et al. Reference Pires, Schneider, Herrera and Tella2016).
The high and ongoing trade of parrots presents significant challenges for environmental agencies, where confiscated specimens with unknown classification and/or origin rapidly accumulate at reception centres. However, some specimens cannot be identified morphologically and the movement of parrots from various localities during poaching makes it challenging to trace the geographical origin, leading to biases in current translocation programmes (Restrepo-Rodas and Pulgarín-Restrepo Reference Restrepo-Rodas and Pulgarín-Restrepo2017). This poses a potential risk of artificially mixing genetically distant populations, which could result in outbreeding depression, homogenisation of genetic diversity, maladaptation to environmental conditions, and interference with the evolutionary process (Frankham et al. Reference Frankham, Ballou, Eldridge, Lacy, Ralls and Dudash2011; Gippoliti et al. Reference Gippoliti, Cotterill, Groves and Zinner2018; Oklander et al. Reference Oklander, Caputo, Solari and Corach2020). This issue is particularly prominent for groups with taxonomic uncertainties and whose current taxonomy requires re-evaluation (Gippoliti et al. Reference Gippoliti, Cotterill, Groves and Zinner2018). This is the case of the Amazon parrots, where taxa like A. ochrocephala and A. farinosa are considered species complexes with high genetic structure and variation between subspecies (Hellmich et al. Reference Hellmich, Saidenberg and Wright2021; Ribas et al. Reference Ribas, Tavares, Yoshihara and Miyaki2007; Wenner et al. Reference Wenner, Russello and Wright2012).
Decision-making regarding handling and disposition of confiscated individuals could be addressed using molecular tools, helping in their identification and reallocation, but such tools require a pre-existing genetic reference database (Coghlan et al. Reference Coghlan, White, Parkinson, Haile, Spencer and Bunce2012; Formentão et al. Reference Formentão, Saraiva and Marrero2021; Gonçalves et al. Reference Gonçalves, Oliveira-Marques, Matsumoto and Miyaki2015; Kim Reference Kim, Do, Duri, Yeo and Kim2020). Assessing the genetic structure of parrot populations has proven to be a valuable tool against illegal wildlife trafficking (Presti et al. Reference Presti, Guedes, Antas and Miyaki2015). This approach involves determining the potential origin of confiscated animals by analysing the genetic structure of populations with known origins to establish a reference database for comparison and inference (Oklander et al. Reference Oklander, Caputo, Solari and Corach2020). While some research projects have been conducted using this approach for neotropical parrots (Fernandes and Caparroz Reference Fernandes and Caparroz2013; Presti et al. Reference Presti, Guedes, Antas and Miyaki2015; Rivera-Ortíz et al. Reference Rivera-Ortíz, Arizmendi, Juan-Espinosa, Solórzano and Contreras-González2021), there is still a notable gap for one of the most trafficked parrot groups: the Amazon parrots.
The mitochondrial cytochrome oxidase subunit 1 (COI) gene is a widely employed molecular marker for assessing genetic structure and conducting biogeographical studies. This gene has revealed significant structural variations in neotropical birds, particularly among populations of geographically separated species (Mendoza et al. Reference Mendoza, Torres, Paz, Trujillo-Arias and López-Alvarez2016; Tavares et al. Reference Tavares, Gonçalves, Miyaki and Baker2011). Additionally, the COI gene has proven valuable in assessing phylogeographical differentiation within two Amazon parrots (Rocha et al. Reference Rocha, Rivera, Martinez, Prestes and Caparroz2014). However, it is crucial to be cautious when using mitochondrial markers for biogeographical inferences, as their applicability may vary among different species under study (Fuhrmann and Kaiser Reference Fuhrmann and Kaiser2021).
This study aims to fill the existing knowledge gap concerning the phylogeographical structure of Amazon parrots and evaluate the utility of this information in addressing the growing threat of illegal poaching. Our primary goal was to assess the phylogeographical structure of six Amazon parrot species using mitochondrial sequencing to determine the probable origin of confiscated individuals and to identify juveniles and chicks taxonomically. The outcomes of this research offer a practical, cost-effective, and reliable tool that can enhance the accuracy of release programmes and help to mitigate the risks associated with intermixing distinct evolutionary lineages.
Methodology
Laboratory analysis
Sample collection
Tissue samples were sourced mainly from museum material from a variety of locations, as well as some field sampling efforts. We specifically targeted six Amazon parrot species: Amazona ochrocephala, A. amazonica, A. farinosa, A. autumnalis, A. festiva, and A. mercenaria. Seventy percent of the samples came from museum specimens collected between 1944 and 1988. Considering that these samples are 36–80 years old and that Amazon parrots have a notably long lifespan, which can exceed 60 years in captivity (Young et al. Reference Young, Hobson, Lackey and Wright2012), they most likely accurately represent current populations’ genetic structure. Additionally, we incorporated published sequences with known origins that were obtained from the GenBank and BOLD databases.
To address the taxonomic ambiguity within the Yellow-crowned Amazon A. ochrocephala, which is part of the “yellow-headed parrot species complex”, we included available sequences from related species. These related species included the Yellow-naped Amazon A. auropalliata, Yellow-shouldered Amazon A. barbadensis, and Turquoise-fronted Amazon A. aestiva, as they have been considered part of the same complex (Eberhard and Bermingham Reference Eberhard and Bermingham2004; Ribas et al. Reference Ribas, Tavares, Yoshihara and Miyaki2007). In total, our analysis included 140 sequences with known geographical origins, comprising our genetic reference database (Supplementary material Table S1).
In addition, we collected 156 blood samples from seized Amazon parrots with unknown origins confiscated in Colombia. These samples were obtained from tissue collections and live animals held by various institutions, including the Laboratory of Forensic Genetics of Wildlife Species at the Directorate of Criminal Investigation and INTERPOL, the Wild Animal Rescue and Rehabilitation Unit (URRAS) under the jurisdiction of the District Environment Secretariat of Bogotá, and the Regional Autonomous Corporation of Cundinamarca (CAR). Of these samples, 144 were obtained from adult individuals, while 12 were from young parrots whose taxonomic identification based on plumage characteristics was not feasible. For a comprehensive data set of all analysed sequences, please refer to Table S2.
DNA extraction
For fresh and well-preserved samples, we performed DNA extraction using the NucleoSpin Tissue Kit from MACHEREY-NAGEL (Düren, Germany), following the manufacturer’s instructions. For museum samples we followed the protocol described in Hoyos et al. (Reference Hoyos, Tusso, Bedoya, Manrique Gaviria and Bloor2017). After extraction, DNA concentration was quantified with an EzDrop 1000 Micro-Volume Spectrophotometer (Blue-Ray Biotech, Taipei, Taiwan), and purity was assessed based on absorbance ratios at 280:260 and 230:260. The samples were in a range of 1.6–1.9 for the 280:260 ratio, and 1.1–3.4 for the 230:260 ratio.
Polymerase chain reaction
We amplified a 658-bp segment of the COI gene via polymerase chain reaction (PCR). COI is demonstrated to be a suitable marker, given its established efficacy in species identification and the assessment of genetic diversity and structure in neotropical birds for both systematic and conservation purposes (Colihueque et al. Reference Colihueque, Gantz and Parraguez2021; Ham-Dueñas et al. Reference Ham-Dueñas, Canales-Del-Castillo, Voelker, Ruvalcaba-Ortega, Aguirre-Calderón and González-Rojas2020; Tavares et al. Reference Tavares, Gonçalves, Miyaki and Baker2011; Tizard et al. Reference Tizard, Patel, Waugh, Tavares, Bergmann and Gill2019). Moreover, COI has been employed in prior studies to investigate speciation and biogeography in Amazon parrot species (Rocha et al. Reference Rocha, Rivera, Martinez, Prestes and Caparroz2014).
We used the primers Psitt-COXIL1 (AACCAACCAYAAAGAYATYGGCAC) and Psitt-COXIH1 (CCBGGBAGRATRAGRATRTAHACTTC) for the majority of the samples. However, as most of the museum samples did not amplify using these primers (most likely due to DNA degradation), we employed the following internal primers: COIF (CCYGCHGGRGGAGGAGAYCCA), SITG1 (TCYGCYACMATAATYATCGC), SITG2 (TTCACAGTRGGRATAGAYGTAGAYAC), SITG3 (GCWCAYTTCCACTAYGTHCTATC), SITG4 (AAACTCYTCAYTAGAYATYGC), and COIA (GACTAYCCMGAYGCTTATACT). Psitt-COXIL1 and Psitt-COXIH1 primers were based on Kerr et al. (Reference Kerr, Lijtmaer, Barreira, Hebert and Tubaro2009), but were modified to improve efficiency for the Psittacidae family. Internal primers were designed specifically for this study.
PCR reactions were carried out in a 25 μL final volume, containing 30–100 ng of DNA, 12.5 μL of OneTaq® 2X Master Mix with Standard Buffer (Biolabs), 1.25 μL of the forward primer, 1.25 μL of the reverse primers, and adjusted with ultrapure water. All reactions were conducted using a Mastercycler PRO S 6325 Thermal Cycler (Eppendorf, Stevenage, UK). The PCR protocol included an initial denaturation step at 95°C for 3 minutes and 30 seconds, followed by 40 cycles of denaturation at 95°C for 30 seconds, annealing at 50°C (45–55°C for internal primers) for 35 seconds, extension at 72°C for 1 minute and 15 seconds, and a final elongation step at 72°C for 10 minutes. To verify amplification and primer specificity, PCR products were analysed using 1% agarose gel electrophoresis stained with EZ-vision.
Purification and sequencing
The PCR products were purified by combining 11 μL of ammonium acetate with 37.5 μL of 96% ethanol. This mixture underwent pellet precipitation for 15 minutes at room temperature, followed by centrifugation at 12,500 rpm and 2°C for 30 minutes. The resulting supernatant was carefully removed, and the pellet was then washed with 70 μL of 70% ethanol before another round of centrifugation for 15 minutes at 12,500 rpm and 2°C. Finally, the supernatant was discarded, and the pellet was eluted in 15–20 μL of ultrapure water. The purified products were sequenced on one or both strands when necessary (based on manual inspection of the chromatograms and review of quality values with a minimal threshold of 20) using Sanger sequencing technology with an ABI 3130 sequencer (Applied Biosystems, Waltham, USA).
Data analysis
Phylogenetic analyses
To assess the genetic structure within and between Amazon parrot species, we conducted phylogenetic analyses by aligning sequences with known origins. The alignment was carried out using the MUSCLE algorithm (Edgar Reference Edgar2004) available in MEGA 11 (Kumar et al. Reference Kumar, Nei, Dudley and Tamura2008). For the inference of the phylogenetic hypothesis, we employed a Bayesian species tree approach, utilising the software BEAST 2.6.7 (Drummond and Rambaut Reference Drummond and Rambaut2007). The configuration of analysis parameters was made through the software BEAUTI 2. To determine the most appropriate substitution model, we utilised JmodelTest 2.1.1 (Posada Reference Posada2008) and employed the Bayesian information criterion (BIC) for model selection. Two independent Markov Chain Monte Carlo (MCMC) runs were conducted, each consisting of 50,000,000 generations with samples collected at intervals of 1,000 generations including a burn-in of 20%. The results of these two analyses were subsequently merged using the LogCombiner 2. For the assessment of sampling, we employed Tracer 2.7.2, calculating mean and 95% highest posterior density (95% HPD) values and ensuring that effective sample size (ESS) values exceeded 200 for all parameters (Rambaut et al. Reference Rambaut, Drummond, Xie, Baele and Suchard2018). Maximum credibility tree was obtained, along with corresponding support values expressed as posterior probabilities (PP). This was achieved using TreeAnnotator 2.6.6, and the resulting tree was visualised using FigTree 1.4.4. In addition, we applied a maximum likelihood approach to infer the phylogenetic hypothesis, using IQ-tree version 1.6.12 (Minh et al. Reference Minh, Schmidt, Chernomor, Schrempf, Woodhams and von Haeseler2020). To identify the optimal substitution model, we used the Model Finder option. Following this, we generated a consensus tree based on 100,000 bootstrap replicates to assess support for the tree topology. The consensus tree, along with the associated bootstrap values, was also visualised using FigTree 1.4.4.
Haplotype networks
The establishment of genealogical relationships among haplotypes is a widely used strategy for studying genetic structure and discerning phylogeographical patterns of differentiation (Fernandes and Caparroz Reference Fernandes and Caparroz2013; Rivera-Ortíz et al. Reference Rivera-Ortíz, Arizmendi, Juan-Espinosa, Solórzano and Contreras-González2021). This approach has been previously applied to assess genetic structure and ascertain probable origins in macaws (Fernandes and Caparroz Reference Fernandes and Caparroz2013; Rivera-Ortíz et al. Reference Rivera-Ortíz, Arizmendi, Juan-Espinosa, Solórzano and Contreras-González2021). Haplotypes were inferred using DnaSP version 6.12.03 (Rozas et al. Reference Rozas, Ferrer-Mata, Sanchez-DelBarrio, Guirao-Rico, Librado and Ramos-Onsins2017) and the genealogical lineage relations were established using the haplotype networks generated with the TCS algorithm in PopART version 1.7 (Cruz et al. Reference Cruz, Funkler, Zani, Wagner, Andretta and Segura2021). Geographical associations of haplotypes were plotted using PopART (Cruz et al. Reference Cruz, Funkler, Zani, Wagner, Andretta and Segura2021). To better delimit the phylogeographically differentiated groups, we applied the Bayesian Analysis of Population Structure (BAPS) algorithm, using RhierBAPS version 1, implemented in the Rstudio version 4.4.1 environment (Tonkin-Hill et al. Reference Tonkin-Hill, Lees, Bentley, Frost and Corander2018).
Genetic distances and structure
To calculate the genetic distances between species and intra-specific phylogeographical groups, we computed the P uncorrected distances using the MEGA 11, with 1,000 bootstrap repetitions (Rodrigues et al. Reference Rodrigues, Brandão, Bitencourt, Jucá-Chagas, Sampaio and Schneider2016). Significant genetic differences between phylogeographical groups were tested using an Analysis of Molecular Variance (AMOVA) and differentiation coefficients (Fst) with Arlequin 3.5.2.2 (Excoffier et al. Reference Excoffier, Laval and Schneider2005). Correction for multiple testing was done for pairwise F ST P values applying a false discovery rate (FDR) adjustment using the Benjamini–Hochberg correction in Rstudio version 4.4.1 with the stats (version 3.6.2) package “p-adjust” function (Jacobs et al. Reference Jacobs, De Noia, Praebel, Kanstad-Hanssen, Paterno and Jackson2018).
Identification and estimation of probable geographical origin
Seized individuals’ identification and the estimation of their probable geographical origin was performed by COI sequences comparison with the reference database of individuals with known origins. This inference was based on the relationships observed between haplotypes as has been carried out previously in similar studies (Fernandes and Caparroz Reference Fernandes and Caparroz2013; Rivera-Ortíz et al. Reference Rivera-Ortíz, Arizmendi, Juan-Espinosa, Solórzano and Contreras-González2021). To visually illustrate the impact of illegal trade on the previously identified geographical genetic groups, we generated new haplotype networks using PopART, which included sequences from individuals with known origins as well as sequences from seized individuals.
Results
The Bayesian phylogenetic tree provided strong support for the differentiation among the six studied Amazon parrot species (see Figure 1). The mean genetic distance observed among these species was 5.3%, with values ranging from 3.5% to 6.4% (Table S3). These results validate COI barcoding as a reliable marker to distinguish between Amazon parrot species from Colombia.
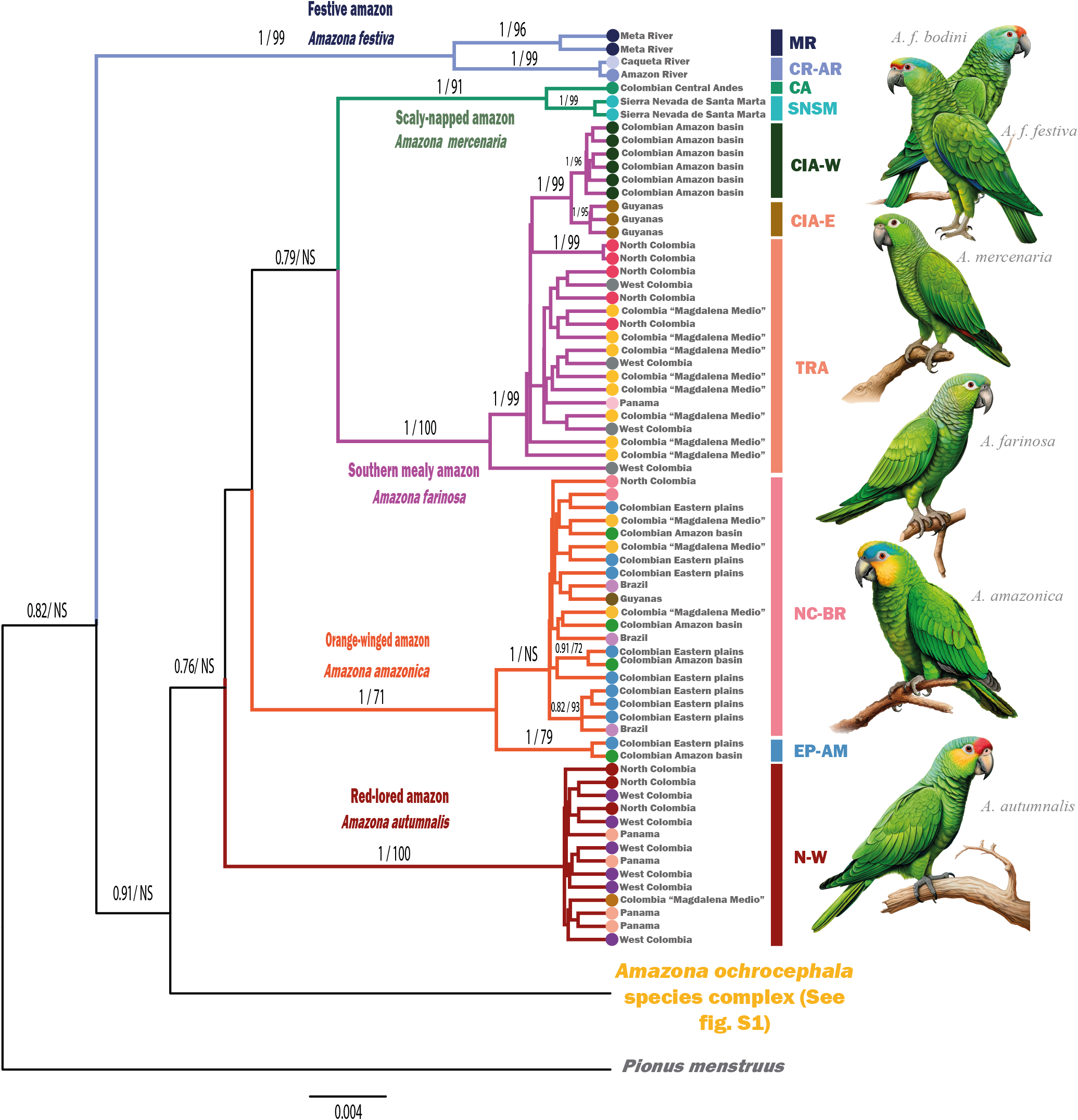
Figure 1. Consensus cytochrome oxidase subunit I (COI) gene tree of the six Amazona species found in Colombia, along with their corresponding support values as posterior probability (PP) (left) and bootstrap support (right). Only support values higher than 0.7/70 are shown. The geographical locality of the samples is denoted by coloured circles at the tips of the tree. Amazona festiva: MR: Meta River, CR-AR: Caquetá River to Amazon River; A. mercenaria: CA: Central Andes, SNSM: Sierra Nevada de Santa Marta; A. farinosa: CIA-W: Cis-Andean West, CIA-E: Cis-Andean East, TRA: Trans-Andean; A, amazonica: NC-BR: North Colombia to Brazil, EP-AM: Eastern Plains to Amazon Basin; A. autumnalis: N-W: north to west South America. The analysis of Amazona ochrocephala species complex (See fig. S1).
We utilised the reference database of species to address the taxonomic identity of 12 confiscated Amazon parrot chicks that could not be identified morphologically. Our analysis revealed that 10 of these parrots belonged to the Yellow-crowned Amazon A. ochrocephala, while the remaining two were identified as Orange-winged Amazon A. amazonica (Table S1).
Beyond the observed inter-specific differentiation, we identified a significant intra-specific genetic structure in specific Amazon parrot species, as reflected in both the phylogenetic tree and haplotype network analysis (Table S1).
Southern Mealy Amazon Amazona farinosa
Phylogeographical and haplotype analyses
This parrot exhibits two major clades and three distinct phylogeographical groups (Figures 1 and 2A). The first major clade corresponds to individuals of the A. f. farinosa subspecies located in the Cis-Andean region (Figures 1 and 2). This clade further divides into two phylogeographical groups. The first group comprises parrots from the Amazon basin in the south-west of Colombia (CIA-W), while the second (CIA-E) includes individuals from the eastern part of South America, specifically from Guyana. The second major clade and the third phylogeographical group consist of specimens of A. f. inornata subspecies found in the Trans-Andean region, from the west and north of Colombia to Panama and in the Middle Magdalena region in eastern Colombia (TRA). Additionally, the haplotype network and haplotype map reveal some haplotypes associated with the Northern region, Middle Magdalena, and western Colombia (Figure 2A and C).
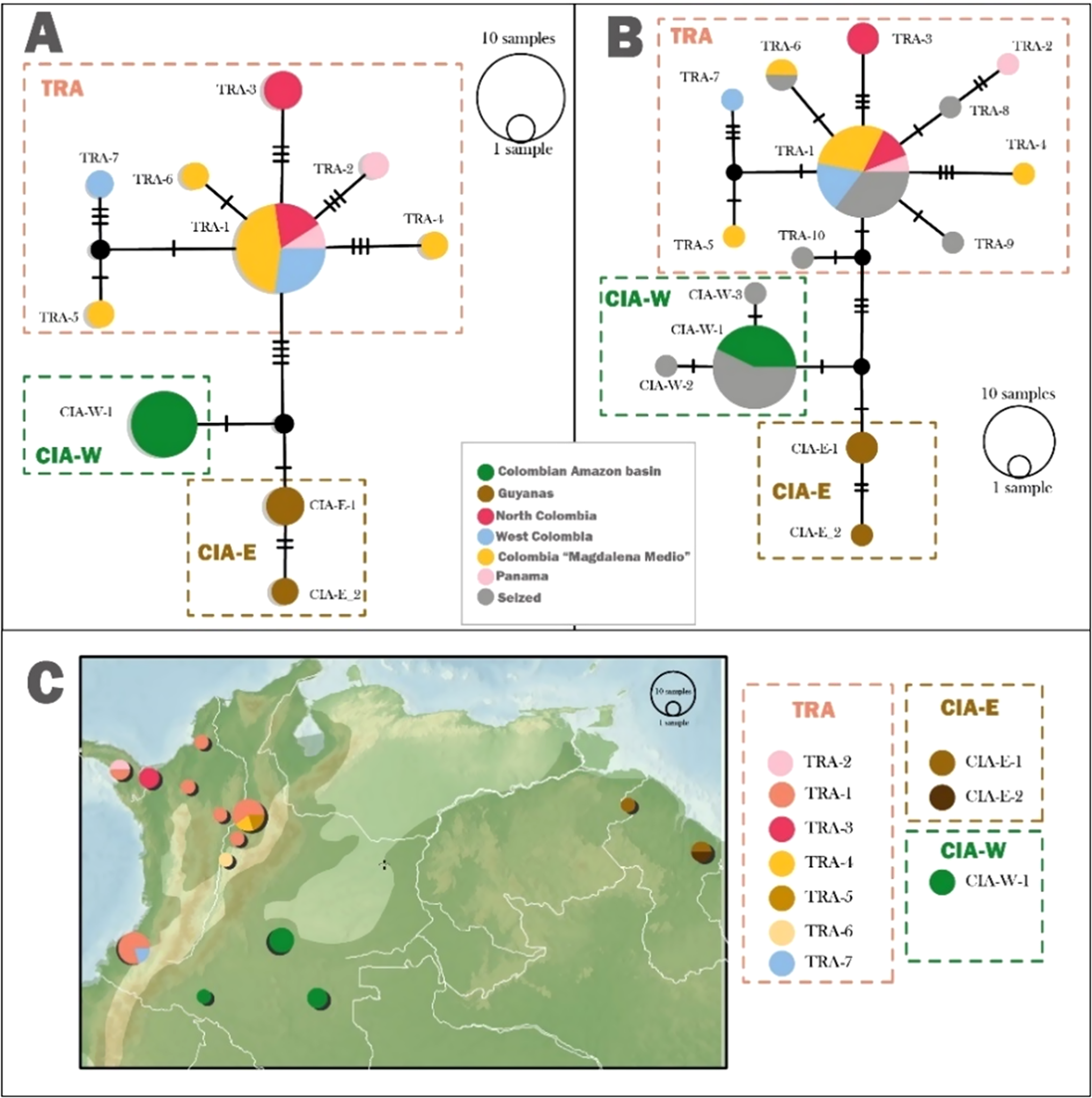
Figure 2 . (A) Haplotype network of the Southern Mealy Amazon Amazona farinosa samples with known geographical origins. (B) Haplotype network of the Southern Mealy Amazon including sequences from seized individuals in Colombia. Hatch marks indicate the number of mutations separating each haplotype. Coloured boxes represent the haplotypes grouped under a population according to RhierBAPS analysis. (C) Haplotype map for the Southern Mealy Amazon. The distribution of the species is indicated by the shaded areas. The size of the circles is proportional to the number of samples.
Genetic distances and structure analyses
The genetic distance between the Trans-Andean (western region passing the Andes Mountain range) group and the two Cis-Andean (eastern region passing the Andes Mountain range) groups was 0.9% (CIA-W) and 1% (CIA-E), respectively. The distance between the two Cis-Andean populations was lower, at 0.4%. The AMOVA test confirmed a significant difference among the three groups (P <0.001), with 74% of the variance observed between populations. The F ST values indicated high levels of genetic differentiation between the TRA population and both Cis-Andean populations (F ST = 0.7) and a higher F ST value (0.8) between the CIA-W and CIA-E groups. These differences were statistically significant in all pairwise comparisons (P <0.05) (Table 1).
Table 1. Pairwise comparison of the genetic distance and F ST values between the genetic and phylogeographical clades identified in Amazon parrots. AV = Andean valleys; CA: Central Andes; CAM = Central America; CIA-E: Cis-Andean East; CIA-W: Cis-Andean West; CR-AR: Caquetá River to Amazon River; EP-E = eastern part of South America; EP-W = western part of South America; MR: Meta River; NA = not available; NV = North Venezuela; NW= Northwest; SNSM = Sierra Nevada de Santa Marta’s Mountain; TRA = Trans-Andean

Estimation of the probable geographical origin
We utilised the reference genetic database to determine the most likely origin of 20 confiscated individuals. Among them, 75% matched haplotypes that had already been documented in the reference database, while five individuals presented new haplotypes (Figure 2B). These new haplotypes were closely related to established groups, allowing them to be assigned to specific geographical regions. Half of the confiscated parrots were assigned to the TRA, and the remaining 50% were assigned to the Cis-Andean region in CIA-W (Table S2).
Festive Amazon Amazona festiva
Phylogeographical and haplotype analyses
This parrot was divided into two genetic groups. A first group of A. f. festiva from the Caquetá River in the Colombian Amazon basin and the mouth of the Amazon River in eastern Brazil (CR-AR). A second group included A. f. bodini in the Meta River (MR) (Figures 1 and 3C-D).

Figure 3. Haplotype network constructed using sequences of individuals with known geographical origins (top) and including seized individuals in Colombia (bottom) for (A) the Red-lored Amazon Amazona autumnalis, (C) Festive Amazon Amazona festiva, and (F) the Scaly-naped Amazon Amazona mercenaria. Haplotype map plot for the (B) Red-lored Amazon, (D) Festive Amazon, and (E) the Scaly-naped Amazon. The size of the circles is proportional to the number of samples. Hatch marks indicate the number of mutations separating each haplotype. Coloured boxes represent the haplotypes grouped under a population according to RhierBAPS analysis
Genetic distances and structure analyses
The genetic distance between these two groups was 1.9%. The AMOVA test indicated a marginally non-significant value of P = 0.06, with 84% of the variance observed between populations.
Estimation of the probable geographical origin
Upon analysing two seized parrots, both were assigned to the MR locality (Table S2).
Scaly-naped Amazon Amazona mercenaria
Phylogeographical and haplotype analyses
This species exhibited two distinct phylogroups and haplotypes. One of the haplotypes was associated with an individual from the Central Andean Mountains (CA), while the second haplotype corresponded to parrots in the Sierra Nevada de Santa Marta’s Mountain (SNSM) (Figure 3E-F).
Genetic distances and structure analyses
The genetic distance between both phylogroups was 0.6%. However, due to the limited sample size, we were unable to conduct the RhierBAPS analysis or AMOVA test for this species.
Estimation of the probable geographical origin
None of the available seized samples corresponded to the Scaly-napped Amazon.
The yellow-headed species complex
Phylogeographical and haplotype analyses
The phylogenetic tree and haplotype network analysis revealed a notable structure within the yellow-headed parrot species complex (see Figure S1 and Figure 4A). This complex consists of four major groups.

Figure 4. (A) Haplotype network of the yellow-headed parrot species complex constructed using samples with known geographical origins. (B) Haplotype network of the yellow-headed parrot species complex, including sequences from seized individuals in Colombia. The size of the circles is proportional to the number of samples. Hatch marks indicate the number of mutations separating each haplotype. Coloured boxes represent the haplotypes grouped under a population according to RhierBAPS analysis. (C) Haplotype map plot of the yellow-headed parrot species complex. The shadowed part of the map represents the distribution of the species. Precise locality information was unavailable for samples of A. aestiva from Brazil.
The first major group (I) includes specimens from the Trans-Andean region of Colombia to Central America and is subdivided into two subgroups. The first subgroup consists of the Yellow-naped Amazon A. auropalliata located in Central America (CAM), while the second subgroup includes the Panama Amazon A. o. panamensis subspecies, ranging from Panama to northern Colombia. This clade is further divided into two phylogeographical clades: one including specimens from the Andean valleys of Colombia (AV), and the second one with specimens from the north-west region of South America, spanning Colombia and Panama (NW).
The second major group (II) includes individuals of the A. o. ochrocephala subspecies and exhibited paraphyly, sharing haplotypes with the Turquoise-fronted Amazon A. aestiva located in the southern region of the continent, from Para state in Brazil to northern Argentina. The RhierBAPS analysis identified two clades within this phylogroup (Figure 4). However, most samples from A. aestiva retrieved from GenBank lack precise information regarding their geographical origin, since they correspond to captive individuals. Hence, it is not possible to determine whether these two genetic groups support a consistent phylogeographical structure. Therefore, we consider all the southern samples as a single genetic group (Table S2).
The third group (III) consists of A. o. ochrocephala individuals from the Cis-Andean region of Colombia to Guyana and was further subdivided into two clades according to RhierBAPS. These populations exhibited a geographical overlap in the central part of the eastern plains of Colombia (Figure 4C). The first clade (OL-E) was associated with individuals in the eastern part of South America, while the second clade (OL-W) comprised specimens from the western region of Colombia to the Amazon basin.
The final genetic group (IV, NV) corresponds to a single haplotype sequence from the Yellow-shouldered Amazon A. barbadensis. The species is found exclusively in the north-west of South America in Venezuela. In summary, the yellow-headed parrot species complex consists of four major clades, further subdivided into seven phylogeographical groups, each defined by distinct geographical patterns.
Genetic distances and structure analyses
We observed a mean genetic distance of 1.49% (ranging from 0.4% to 2.1%) between the seven phylogeographical groups. Higher genetic distances were found between the Cis Andean, Trans-Andean, and southern groups (Table 1). As there was only one sequence from the A. barbadensis (NE) clade, genetic differentiation analysis was limited to the Cis Andean, Trans-Andean, and Southern populations. The AMOVA test showed a significant difference between the proposed phylogeographical groups (P <0.001), with 88.8% of the variance observed between groups. High genetic differentiation (F ST >0.8) was measured between most groups. Moreover, all pairwise comparisons were statistically significant (P <0.05), except for CAM vs NW, CAM vs AV, and CAM vs EP-E, which were marginally significant (P = 0.06).
Estimation of the probable geographical origin
Given the significant phylogeographical structure among parrots, we utilised the genetic reference database to determine the likely origin of 95 confiscated Yellow-crowned Amazons A. ochrocephala from Colombia. Each individual was assigned to one of the previously described groups. We found that 81 (85%) of the seized individuals had haplotypes already documented in the reference database, while only 14 individuals had new haplotypes (Figure 4B). Among the confiscated parrots, 61% were assigned to the Middle Magdalena Andean valleys group, 29% to the eastern plains-east region, 5% to the northern region of the country, and 4% to the eastern plains-western region (Table S2).
Orange-winged amazon Amazona amazonica
Phylogeographical and haplotype analyses
The species exhibited a less pronounced genetic structure. The phylogenetic and RhierBAPS analyses (Figures 1 and 5) identified two genetic groups. However, the first one (NC-BR) included samples from all studied geographical regions, ranging from north Colombia to the southern part of Brazil (Figure 5A). The second group (EP-AM) included two samples from the eastern plains and Amazon basin from Colombia. However, several samples from the eastern plains and Amazon basin were also included within the NC-BR group (Figure 5C).

Figure 5. (A) Haplotype network of the Orange-winged Amazon Amazona amazonica constructed using samples with known geographical origins. (B) Haplotype network of the Orange-winged Amazon including information from seized individuals. Hatch marks indicate the number of mutations separating each haplotype. The size of the circles is proportional to the number of samples. (C) Haplotype map for the Orange-winged Amazon. The distribution of the species is shown in the shaded areas. Coloured boxes show haplotypes grouped under a population according to RhierBAPS analysis
Genetic distances and structure analyses
No significant differences were found between the Trans-Andean and Cis-Andean regions (AMOVA test P value = 0.4). These results indicate a lack of genetic structure for this parrot species.
Estimation of the probable geographical origin
We analysed 26 seized Orange-winged Amazon samples of unknown origin and found that the genetic diversity and structure of the species are notably underestimated (Figure 5B). Adding seized individuals to the analyses nearly doubled the number of haplotypes identified from 12 to 23. Only 35% of the seized individuals shared haplotypes with samples with known locality. Furthermore, 53% of the samples were associated with the genetic NC-BR group, making their geographical interpretation complex and potentially biased. Only one sample (4%) was related to the EP-AM group, suggesting a higher likelihood of origin in the Cis-Andean region of Colombia. Unexpectedly, 42% of the samples formed a new genetic group (UNK) according to the RhierBAPS analysis. Given the position of the haplotypes within this group in the network analysis and their close relation to the EP-AM group, it is likely that these samples also come from the Cis-Andean region in Colombia (Figure 5B).
The Red-lored Amazon Amazona autumnalis
Phylogeographical and haplotype analyses
This parrot did not exhibit a significant phylogeographical structure. In the phylogenetic analysis (Figure 1), only one clade was observed, and RhierBAPS analysis (Figure 3A and B) predicted a single population (N-W).
Genetic distances and structure analyses
AMOVA test revealed no significant differences between west and north samples (P value = 1) and the mean genetic divergence between this locality was low (0.03%).
Estimation of the probable geographical origin
We analysed 13 samples of seized Red-lored Amazons, but their inclusion did not result in a noticeable change in the genetic diversity or phylogeographical structure. We found only one new haplotype, and RhierBAPS consistently grouped all samples into a single population. Consequently, due to the lack of genetic structure in this species, we were unable to make any geographical inferences.
Discussion
We identified significant inter- and intra-specific genetic structure, correlated with the geographical distributions for several of the studied species. These findings align with prior research on neotropical birds, which has also demonstrated high intraspecific genetic structure associated with biogeographical differences (Tavares et al. Reference Tavares, Gonçalves, Miyaki and Baker2011). We identified well-supported relationships among the studied species. However, it is crucial to note that our analysis only encompassed one mitochondrial gene and a subset of the species within the diverse Amazona genus. Our objective in conducting this analysis was to focus on the intraspecific phylogeography of the species, whereas more comprehensive evolutionary hypotheses can be found in earlier studies (Kolchanova et al. Reference Kolchanova, Komissarov, Kliver, Mazo-Vargas, Afanador and Velex-Valentin2021; Ribas et al. Reference Ribas, Tavares, Yoshihara and Miyaki2007; Russello and Amato Reference Russello and Amato2004; Smith et al. Reference Smith, Merwin, Provost, Thom, Brumfield and Ferreira2023; Tilston-Smith et al. Reference Tilston-Smith, Thom and Joseph2024; Urantoẃka et al. Reference Urantoẃka, Mackiewicz and Strzaał2014).
The substantial genetic structure of certain species underscores the necessity for appropriately managing seized individuals and implementing a guided release process to prevent artificial mixing between divergent lineages. Such mixing can lead to outbreeding depression and genetic homogenisation (Frankham et al. Reference Frankham, Ballou, Eldridge, Lacy, Ralls and Dudash2011; Gippoliti et al. Reference Gippoliti, Cotterill, Groves and Zinner2018; Oklander et al. Reference Oklander, Caputo, Solari and Corach2020). These findings hold particular relevance in regions like the neotropics, characterised by high biodiversity and widespread illegal exploitation of wildlife (Mota-Rojas et al. Reference Mota-Rojas, Strappini, Whittaker, Ghezzi, Gonçalves Titto and Calderón-Maldonado2023). Consequently, there is a considerable number of seized individuals requiring appropriate management. Unfortunately, in various instances, such as in Colombia, the release of individuals often occurs without knowledge of their geographical origin or even outside their natural distribution range (Restrepo-Rodas and Pulgarín-Restrepo Reference Restrepo-Rodas and Pulgarín-Restrepo2017).
Here we have validated the use of mitochondrial COI sequencing as a reliable marker to allocate seized individuals to Amazon parrot species. The use of mitochondrial sequencing for species and geographical origin inference has been performed in other organisms with high levels of poaching to fight against illegal trade and to improve management of seized individuals (de Carvalho Reference de Carvalho2013; Fernandes and Caparroz Reference Fernandes and Caparroz2013; Hatten et al. Reference Hatten, Fitriana, Prigge, Irham, Sutrisno and Abinawanto2023; Ishida et al. Reference Ishida, Georgiadis, Hondo and Roca2013; Kongrit et al. Reference Kongrit, Markviriya, Laithong and Khudamrongsawat2020; LaCasella et al. Reference LaCasella, Jensen, Madden Hof, Bell, Frey and Dutton2021; Ruiz-García et al. Reference Ruiz-García, Leguizamón, Vásquez, Rodríguez and Castillo2010). This method is particularly useful in cases where external characteristics are unavailable, such as in chicks, eggs, and products (de Carvalho Reference de Carvalho2013; Formentão et al. Reference Formentão, Saraiva and Marrero2021; Gonçalves et al. Reference Gonçalves, Oliveira-Marques, Matsumoto and Miyaki2015).
It is important to notice that mitochondrial genetic assessment is a relatively simple, inexpensive, and standardised technique that can be easily implemented as a routine test, particularly in developing countries (Bellagamba et al. Reference Bellagamba, Velayutham, Cozzi, Caprino, Vasconi and Busetto2016; Deagle et al. Reference Deagle, Jarman, Coissac, Pompanon and Taberlet2014). Our research also underscores the utility of museum samples in constructing reference databases, offering a means to reduce both cost and time associated with sampling from distant geographical regions. The promotion of increased utilisation of museum materials in wildlife conservation studies is encouraged, as they serve as a viable and readily accessible source of information (Nakahama Reference Nakahama2021). We anticipate that our research can serve as a reference point in the development of tools aimed at enhancing the management of other traded species in the neotropics. This, in turn, would contribute to the refinement of management protocols for seized wildlife, fostering improved conservation practices.
However, it is important to note that this delimitation analysis is based on mitochondrial DNA data. While this method can identify genetically distinct phylogroups, it may not fully represent the genetic structure of the nuclear genome. This discrepancy arises because the mitochondrial genome is exclusively maternally inherited, sensitive to population size and introgression effects, and lacks recombination (Rubinoff et al. Reference Rubinoff, Cameron and Will2006). Therefore, further studies using nuclear markers are recommended to complement our findings.
Southern Mealy Amazon Amazona farinosa
In the past, the Mealy Amazon had a distribution in Central and South America. However, a phylogenetic study in 2012 revealed significant differentiation within the group, leading to the split of the species into the Northern Mealy Amazon A. guatemalae and the Southern Mealy Amazon A. farinosa. This research also identified two phylogeographical subclades within the South American species, corresponding to the Trans-Andean subspecies A. f. inornata and the Cis-Andean subspecies A. f. farinosa + A. f. chapmani (forming a paraphyletic clade) (Wenner et al. Reference Wenner, Russello and Wright2012). A subsequent study in 2020 confirmed these findings and identified an additional management unit in the Atlantic Forest in southern Brazil (Hellmich et al. Reference Hellmich, Saidenberg and Wright2021). However, previous studies only included samples of A. f. farinosa from the eastern part of South America, lacking the western distribution, now included in this study.
Consistent with previous studies, we found significant genetic differentiation between Trans-Andean and Cis-Andean mealy amazon populations. Furthermore, we observed genetic differentiation between eastern populations of A. f. farinosa in Guyana and those in the western Amazon basin of Colombia, suggesting a new conservation management unit for the species. However, additional sampling is necessary to assess the genetic structure of these parrots across their entire distribution range. In particular, areas like Venezuela and northern and central Brazil are currently understudied.
Our findings emphasise the importance of considering the geographical origin of seized mealy amazon parrots during their release. We validate the use of COI sequencing as a reliable tool to identify the most likely geographical origin of these parrots. Our data indicate the presence of multiple trade networks in Colombia, with an equal impact on the Trans-Andean and Cis-Andean populations.
Festive amazon Amazona festiva
The taxonomy of this parrot species is still subject to debate as it has been divided into two main lineages: the Southern Festive Amazon (festiva) and the Northern Festive Amazon (bodini). Classification of these two clades into species or subspecies is debated between taxonomists and based on morphological and plumage differentiation (del Hoyo and Collar Reference del Hoyo and Collar2015; Hilty Reference Hilty2021). These parrots exhibit riparian behaviour, with A. f. bodini being distributed from the Orinoco River drainage to eastern Colombia in the Meta River. A. f. festiva is found from the Amazon River drainage to the Amazon basin of Colombia (Caquetá River), Peru, and Ecuador (Ayerbe-Quiñones Reference Ayerbe-Quiñones2018; Donegan et al. Reference Donegan, Ellery, Pacheco, Verhelst and Salaman2018). Our analysis revealed significant genetic differentiation between the two lineages, with a genetic distance of 1.9%. This genetic distance is slightly lower than that reported in a previous study comparing the sister species Red-spectacled Amazon A. pretrei and Tucumán Amazon A. tucumana, which has 2.2% distance based on COI mtDNA analysis (Rocha et al. Reference Rocha, Rivera, Martinez, Prestes and Caparroz2014). Only two samples from the seized individuals were included, both of which were assigned to the Meta River locality (bodini lineage).
Scaly-naped Amazon Amazona mercenaria
This parrot species is distributed along the middle and northern South American Andean Mountains, but currently there is little available information on its biology, ecology, and genetics. Despite the small sample size, our study describes a potential phylogeographical divergence between samples from the Central Andes and the Sierra Nevada de Santa Marta, a geographically isolated population in the north part of Colombia. The Sierra Nevada de Santa Marta is a critical centre of endemism, and its isolation from other mountain systems has likely led to important processes of speciation, resulting in genetic divergence for this species (Roach et al. Reference Roach, Urbina-Cardona and Lacher2020). Moreover, samples from the east and west Andean Mountains should be studied as these Andean Mountain systems in the country are known to promote speciation and genetic structure processes in bird species (Mendoza et al. Reference Mendoza, Torres, Paz, Trujillo-Arias and López-Alvarez2016). While no seized individuals of this species were included, as it is the least traded Amazon parrot in the country (Restrepo-Rodas and Pulgarín-Restrepo Reference Restrepo-Rodas and Pulgarín-Restrepo2017), our findings highlight the need to establish a more robust genetic reference database to inform guided release in trade cases.
The yellow-headed species complex
This species complex exhibited a substantial phylogeographical structure and non-monophyletic relationships between the described species and subspecies. Similar discrepancies have been identified in previous studies, leading to this group being described as a “taxonomic headache” (Eberhard and Bermingham Reference Eberhard and Bermingham2004).
Our analysis revealed four major clades and seven separated phylogeographical groups that should be considered as independent management units for conservation purposes. The first clade consists of individuals from the Trans-Andean region and was further divided into three phylogeographical clades. The first one consisted of samples from the A. o. panamensis subspecies, spanning northern Colombia to Panama. The second clade included A. o. panamensis parrots from the Colombian Andean Valleys, while the third clade included samples of the Yellow-napped Amazon A. auropalliata from Central America. The existence of this Trans-Andean clade was also reported in previous multi-locus phylogenetic analyses, which also included the Yellow-headed Amazon A. oratrix (Eberhard and Bermingham Reference Eberhard and Bermingham2004; Ribas et al. Reference Ribas, Tavares, Yoshihara and Miyaki2007; Urantoẃka et al. Reference Urantoẃka, Mackiewicz and Strzaał2014). However, the separation of A. o. panamensis from the northern region and Andean Valleys had not been previously documented.
The second clade grouped individuals of the A. o. ochrocephala subspecies from Brazil and the Turquoise-fronted Amazon A. aestiva from northern Brazil to Argentina. These paraphyletic relationships have also been previously reported in studies using multi-locus analysis, including the A. o. xantholaema subspecies and specimens of A. o. nattereri subspecies from Bolivia and southern Peru (Eberhard and Bermingham Reference Eberhard and Bermingham2004; Ribas et al. Reference Ribas, Tavares, Yoshihara and Miyaki2007; Urantoẃka et al. Reference Urantoẃka, Mackiewicz and Strzaał2014). However, a more recent study using ultra-conserved elements shows a strong differentiation of this species from the other parrots of the yellow-headed species complex, supporting the hypothesis that the current mitochondrial genetic structure is most likely a product of past introgression processes (Caparroz et al. Reference Caparroz, Seixas, Berkunsky and Collevatti2009b; Smith et al. Reference Smith, Merwin, Provost, Thom, Brumfield and Ferreira2023).
The third clade consisted of specimens of the A. o. ochrocephala subspecies from the Cis-Andean region of Colombia, extending from the eastern plains and the Amazon basin to the east in Guyana. This lineage has also been reported in previous multi-locus analyses, including specimens of A. o. ochrocephala from Venezuela (Eberhard and Bermingham Reference Eberhard and Bermingham2004; Ribas et al. Reference Ribas, Tavares, Yoshihara and Miyaki2007; Urantoẃka et al. Reference Urantoẃka, Mackiewicz and Strzaał2014). This study additionally found that this clade splits into two sub-clades, indicating divergence between the west and east samples, but with geographical overlap in the central eastern plains of Colombia.
The final clade included the Yellow-shouldered Amazon A. barbadensis, found in the northern region of Venezuela. This was the most basal group within the complex. The phylogeographical position of these species within the complex has been debatable. Russello and Amato (Reference Russello and Amato2004) using three mitochondrial and three nuclear genes, as well as Urantoẃka et al. (Reference Urantoẃka, Mackiewicz and Strzaał2014) using seven mitochondrial genes, described it as part of the complex, closely related to A. o. ochrocephala. However, Eberhard and Bermingham (Reference Eberhard and Bermingham2004) using four mitochondrial loci, placed A. barbadensis outside the complex, but as its sister species. More recently, using ultra-conserved elements, Smith et al. (Reference Smith, Merwin, Provost, Thom, Brumfield and Ferreira2023) placed this species as the sister of A. versicolor (not part of the yellow-headed species complex), but remaining in a clade encompassing A. ochrocephala, A. auropalliata, Tres Marías Amazon A. tresmariae, and A. aestiva. Further, Tilston-Smith et al. (Reference Tilston-Smith, Thom and Joseph2024) also using genomic ultra-conserved elements in their taxonomic revision, place this species again as part of the yellow-headed species complex.
The significant phylogeographical structure observed among different regions for the Yellow-crowned Amazon parrots suggests that the release of confiscated individuals should be undertaken with caution to avoid mixing different lineages. This is a challenging task, given that the Yellow-crowned Amazon is the second most commonly traded parrot in Colombia, accounting for 24.8% of all seized parrots (Restrepo-Rodas and Pulgarín-Restrepo Reference Restrepo-Rodas and Pulgarín-Restrepo2017). Current translocation protocols for this parrot do not consider the potential geographical origin of individuals, and previous attempts to differentiate between the Trans-Andean and Cis-Andean subspecies based on morphological and morphometric features have proven to be unreliable (Jaramillo-Castaño Reference Jaramillo-Castaño2020). In contrast, this study shows the potential of using COI to differentiate the geographical origin of seized Yellow-crowned Amazon parrots. Our results also reveal that parrots in Colombia are collected from multiple geographical locations in the country, suggesting the existence of multiple trade networks that help to explain the high levels of illegal trade reported. Importantly, we found a higher incidence of extraction in the Middle Magdalena Andean valleys (61%) and the eastern plains-east region (29%), while the northern and eastern plains-western region were less affected (5% and 4%).
Orange-winged Amazon Amazona amazonica
Our study represents the first attempt to examine genetic structure in the Orange-winged Amazon. However, samples with known geographical origin revealed a lack of significant structure in this species. This is consistent with the current taxonomy, which establishes a single subspecies (A. a. amazonica) distributed from northern Colombia to the south-east of Brazil (Forshaw Reference Forshaw2010). While some authors recognise a second subspecies (A. a. tobagensis) distributed on Trinidad and Tobago Island, we were unable to include samples from this location. However, haplotype diversity and genetic structure significantly increased when samples from seized individuals were included in the analysis. The inclusion of these samples nearly doubled the number of haplotypes and revealed additional genetic structuration. As a result, the genetic diversity and structure in this parrot remain uncertain.
It is still inaccurate to infer the geographical origin of seized individuals in this group. Only 46% of the samples could be assigned to a geographical region, primarily associated with the Cis-Andean region of Colombia. These results evidence a management challenge since the Orange-winged Amazon is the third most commonly seized parrot in Colombia (Restrepo-Rodas and Pulgarín-Restrepo Reference Restrepo-Rodas and Pulgarín-Restrepo2017). Thus, it is necessary to explore more reliable alternative molecular markers, potentially more variable between populations, such as other mitochondrial fragments (control region [CR]) or microsatellites (Presti et al. Reference Presti, Guedes, Antas and Miyaki2015; Rivera-Ortíz et al. Reference Rivera-Ortíz, Arizmendi, Juan-Espinosa, Solórzano and Contreras-González2021; Urantowka et al. Reference Urantowka, Hajduk and Kosowska2013). Moreover, a more comprehensive sampling across the distribution area is required, as our data suggest that a significant portion of the genetic diversity of this species was not captured in the reference database.
Red-lored Amazon Amazona autumnalis
With no prior genetic studies in the Red-lored Amazon, our results indicate a single genetic group in its distribution from western and northern Colombia to Panama. However, populations in the rest of Central America and the northern part of Ecuador remain unexplored, and a more extensive sampling of the middle Magdalena region and the eastern part of Colombia is needed. Analysis including seized samples of the species supports the lack of phylogeographical structuration.
Conclusions
Our study revealed that the translocation process of Amazon parrots should not proceed without prior definition of their geographical origin. This is crucial due to the genetic structure observed in wild populations and the heterogeneity in the origins of seized individuals. Furthermore, other highly traded parrot species, such as the Blue-headed Parrot Pionus menstruus and the Scarlet Macaw Ara macao, exhibit a phylogeographical structure, showing genetic differentiation between Trans-Andean and Cis-Andean regions (Ribas et al. Reference Ribas, Tavares, Yoshihara and Miyaki2007; Schmidt et al. Reference Schmidt, Aardema and Amato2020).
In Colombia, the current environmental law encourages the use of genetic tests to assess genetic variability during the management of seized wildlife as a preventive measure aimed at avoiding genetic contamination of natural populations (Choperena Palencia and Mancera Rodríguez Reference Choperena Palencia and Mancera Rodríguez2016; Ministerio de Ambiente vivienda y Desarrollo Territorial 2010). However, these recommendations are not consistently enforced. Therefore, we strongly urge environmental entities to enforce the approximation of geographical origin before making release decisions for seized parrots.
Our results validate the use of COI barcoding for guiding the translocation of several Amazon parrot species. The species A. amazonica did not show genetic structure with this marker and further testing using more variable markers may be needed. Previous studies have employed sequencing of the CR, a mitochondrial informative marker due to its higher variability. Nonetheless, in the case of Amazon parrots, the use of CR sequencing would need to be carefully evaluated because some species in this genus exhibit CR duplication, which can affect sequencing quality and data analysis (Eberhard et al. Reference Eberhard, Wright and Bermingham2001; Eberhard and Wright Reference Eberhard and Wright2016; Schirtzinger et al. Reference Schirtzinger, Tavares, Gonzales, Eberhard, Miyaki and Sanchez2012). Microsatellites (nuclear DNA) are another potential marker that has been used to assess genetic structure and identify the potential origin of seized parrots (Presti et al. Reference Presti, Guedes, Antas and Miyaki2015; Rivera-Ortíz et al. Reference Rivera-Ortíz, Arizmendi, Juan-Espinosa, Solórzano and Contreras-González2021). However, microsatellite analysis is not always more effective for geographical identification in Psittacidae (Caparroz et al. Reference Caparroz, Miyaki and Baker2009a; Presti et al. Reference Presti, Guedes, Antas and Miyaki2015). It is also crucial to note that our sampling for some taxa was sparse. Therefore, we strongly recommend conducting additional studies with more comprehensive sampling efforts to better elucidate the phylogeography and genetic structure of these species.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S0959270924000339.
Acknowledgements
Thanks to Professor Gary Stiles for granting us access to the museum samples of the ornithological collection of the Instituto de Ciencias Naturales (ICN) at the Universidad Nacional de Colombia (UNAL). We would also like to extend our thanks to the Laboratory of Forensic Genetics of Wildlife Species at the Direction of Criminal Investigation and Interpol, the Wild Animal Rescue and Rehabilitation Unit (URRAS), and the Regional Autonomous Corporation of Cundinamarca (CAR) for granting us access to the seized parrot samples. Thanks to the sequencing service of the Genetics Institute SSigmol and Paul Bloor. Thanks to Carlos Moreno Torres and the Facultad de Medicina Veterinaria y de Zootecnia (UNAL). Samples of the parrots with known and unknown origins were obtained from the Colección Ornitológica del Instituto de Ciencias Naturales (ICN-MHN-Or), Zoological Collection, Instituto de Ciencias Naturales, Universidad Nacional de Colombia and Banco de AND y Tejidos de la Biodiversidad (BTBC), Instituto de Genética, Universidad Nacional de Colombia. Samples were processed under the “Permiso Marco de Recolección de especímenes de especies silvestres de la Diversidad Biológica con fines de investigación científica no comercial Resolución 0255-2014”, given by the Autoridad Ambiental de Licencias Ambientales ANLA to the Universidad Nacional de Colombia, subscribed by the Grupo Biodiversidad y Conservación Genética de la Universidad Nacional de Colombia. This study was funded by the Research Direction of the Universidad Pedagógica y Tecnológica de Colombia under the grant code SGI: 3315 and the Interadministrative Agreement No. 3131 of 2023 signed between the Corporación Autónoma Regional de Cundinamarca – CAR – and the Facultad de Medicina Veterinaria y de Zootecnia of the Universidad Nacional de Colombia: “Aunar esfuerzos entre la Corporación Autónoma Regional de Cundinamarca – CAR – y la Universidad Nacional de Colombia – Facultad de Medicina Veterinaria y Zootecnia – Unidad de Rescate y Rehabilitación de Animales Silvestres (URRAS) para el desarrollo de actividades de manejo, conservación y atención de la fauna silvestre en la jurisdicción de la CAR”.








