Severe vitamin D deficiency has been shown to lead to rickets in children and osteomalacia in adults(1), while less severe vitamin D deficiency causes secondary hyperparathyroidism, increased bone turnover and bone loss(Reference Lips2–Reference Ooms, Lips and Roos4), as well as being associated with increased risk of several non-skeletal chronic diseases(Reference Zittermann5, Reference Holick6). Thus, ensuring adequate vitamin D status is important to human health and there is a consensus that serum 25-hydroxyvitamin D (25(OH)D) should be used to assess vitamin D status, as it reflects combined dietary supply and dermal production(Reference Cashman7).
Serum 25(OH)D was used as a functional indicator of vitamin D status by the recent Institute of Medicine (IOM) dietary reference intake (DRI) committee on Ca and vitamin D in North America(8), as well as by the UK and the European Union authorities(1, 9–11) during the 1990s, in establishing dietary requirements for vitamin D. To date, many, if not all, agencies briefed with establishing dietary requirements for vitamin D have used a cutoff of 25–30 nmol/l serum 25(OH)D as the lower threshold for vitamin D status (on the basis of rickets and osteomalacia)(1, 8–11). There has been an increasing body of data on the relationship between vitamin D status and a wide range of non-skeletal health outcomes, and some of these (mainly CVD or cancer outcomes) were reviewed in a comprehensive systematic review(12), commissioned by several US and Canadian federal government agencies, for use by the DRI committee during their deliberations. However, many of the studies of non-skeletal health effects provided often mixed and inconclusive results and led the DRI committee to question their reliability. The DRI committee instead prioritised bone health outcomes as the basis for establishing the new DRI values for vitamin D (and Ca)(8).
For individuals aged 1 year and older, the DRI committee choose serum 25(OH)D concentrations of 40 and 50 nmol/l as the median value (above which approximately half the population might meet vitamin D requirements for bone health and below which one-half might not) and that covering the needs of 97·5 % of a normal healthy population, respectively. These served as the target concentrations for an estimated average requirement and RDA for dietary vitamin D, respectively(8). The DRI committee then used data from nine vitamin D intervention studies of individuals aged 6 to >60 years, performed at the northern latitudes in Europe (>49·5°N) and Antarctica (78°S) during their respective winter seasons (with minimal UV blue (UVB) sun exposure) to establish regression equations of the simulated response of serum 25(OH)D concentration to total vitamin D intake. A similar analysis on data from randomised control trials (RCT) conducted in the latitude band 40 to < 49·5°N (all from the USA) yielded quite different regression equations, suggesting that UVB exposure during winter was not minimal(8). The estimated average requirement and RDA for vitamin D of 10 and 15 μg/d (20 μg/d for those >70 years), respectively, were derived from the >49·5°N/S RCT regression analysis to approximate conditions of minimal UVB sun exposure(8). The DRI committee, however, highlighted that the regression analysis had several assumptions and/or uncertainties: lack of age effect on the response of serum 25(OH)D to total vitamin D intake, large inter-study variance and uncertainties surrounding the predicted CI of the vitamin D intake–status relationship(8), which were used to estimate the DRI values for vitamin D. Furthermore, it is possible that European agencies briefed with the task of re-evaluation of DRI for vitamin D may decide to use a serum 25(OH)D target concentration other than 50 nmol/l, which may require a different regression model.
Thus, the aim of the present study was to use a systematic review approach to identify relevant RCT with vitamin D in children/adolescents, adults and older adults, and then apply meta-regression models (including that used by the DRI committee(8)) to the extracted data, as well as using individual data from two recent vitamin D RCT in the northern European adults and elderly(Reference Cashman, Hill and Lucey13, Reference Cashman, Wallace and Horigan14) during winter, to explore the most appropriate model of the vitamin D intake–serum 25(OH)D relationship. In addition, whether latitude influenced this relationship was investigated, as much of Europe resides between 40 and >70°N.
Methods
Research questions to be addressed by the present analysis and their rationale
The following key research questions were addressed in the present regression analysis:
(1) Does latitude (between 40 and 49·5°N compared to ≥ 49·5°N (and 78°S)) influence the response of serum 25(OH)D to increased vitamin D intake during winter? Rationale: The rationale for choosing RCT that finished at (or at least reported data from) the end of winter, as outlined by the IOM, was due to uncertainties about the contribution of sunlight to overall serum 25(OH)D concentration and that vitamin D requirements cannot be based on an accepted or ‘recommended’ level of sun exposure due to potential skin damage and cancer(8). Instead, the best remaining approach was to describe the relationship between total vitamin D intake and serum 25(OH)D levels under conditions of minimal sun exposure, as would be achieved in winter time(8), an approach we have also advocated in our recent studies of vitamin D dietary requirements(Reference Cashman, Hill and Lucey13–Reference Cashman, Fitzgerald and Viljakainen15). The IOM used the two latitude bands (namely, 40 to < 49·5°N and ≥ 49·5°N and 78°S) to test the assumption of minimal sun exposure during winter, which they found was met in RCT performed in the latter but not the former latitude region(8). As much of Europe resides between 40 and >70°N, the two latitude bands used by the IOM seemed appropriate to test in the present analysis. Finally, the choice of which months should be designated as winter (during which there is insufficient UVB sunshine to allow for dermal synthesis of vitamin D) may differ in these two latitude bands, particularly at the lower latitude range. Therefore, in addition to testing the DRI committee's definition of winter as September to June or part thereof(8), we also used September to April as a shorter winter period, as it is highly likely that there is UVB sunshine of sufficient strength in May and June to allow for dermal synthesis of vitamin D and thus could contribute significantly to achieved serum 25(OH)D concentration.
(2) What is the most appropriate model for the relationship between total vitamin D intake and achieved serum 25(OH)D? Rationale: The DRI committee used an integrated bone health outcome approach (incorporating Ca absorption, bone mineral density, risk of rickets and osteomalacia) to define the estimated average requirement-like serum 25(OH)D concentration (40 nmol/l) and 50 nmol/l as the concentration of 25(OH)D covering the needs of nearly all in the population(8). As mentioned previously, it is possible that European agencies briefed with the task of re-evaluation of population reference intake (PRI), or member state-specific equivalent dietary reference values, for vitamin D may decide to use a serum 25(OH)D target concentration other than 50 nmol/l. For example, should a European agency decide to use risk of rickets or osteomalacia as the health outcome used to establish their dietary reference value in preference to the more integrated bone health outcome approach used by the DRI, they may well use 30 nmol/l, or even the more precautionary 40 nmol/l(8), as the target 25(OH)D concentration on which to base their intake requirement value. Therefore, we wished to explore the vitamin D intake–serum 25(OH)D relationship under different meta-regression model constructs, particularly at different serum 25(OH)D concentrations.
Systematic review of vitamin D intake–status relationship
The methodology used in the systematic review and meta-regression in the present study follows the general methodology for a recent series of systematic reviews in relation to markers of nutrient status(Reference Hooper, Ashton and Harvey16) and in particular for our recent systematic review of existing and potentially novel functional markers of vitamin D status(Reference Seamans and Cashman17), with brief specific details as follows:
Inclusion criteria
Studies were RCT of vitamin D (D3 with or without Ca) supplementation in apparently healthy human subjects or in patients in whom there is no underlying reason for altered vitamin D metabolism or response to vitamin D supplementation that fulfilled all of the following characteristics: (1) vitamin D3 ≤ 2000 IU/d (50 μg/d; 1 μg = 40 IU) administered orally alone or with Ca on a daily basis (inclusion of vitamin D3 and not D2 was chosen on the basis that the IOM DRI committee used studies with vitamin D3 in their regression analysis of the northern European RCT (and Antarctica), on which DRI values were set(8), and there is still some debate as to the relative potency of vitamin D2 relative to vitamin D3(Reference Cashman18, Reference Lanham-New, Vieth and Heaney19); (2) reported serum or plasma 25(OH)D levels following supplementation in at least one intervention group and one control group; (3) no vitamin D metabolites (25(OH)D and 1,25(OH)2D) and analogues (e.g. α-calcidol) co-administered; (4) minimum duration of 6 weeks (on the basis that following initiation of vitamin D supplementation, serum 25(OH)D concentrations reach equilibrium after at least 6–8 weeks in adults and elderly subjects(Reference Harris and Dawson-Hughes20, Reference Viljakainen, Palssa and Kärkkäinen21)); (5) studies performed above 40°N (or 40°S) (these minimum latitudes were those used in RCT included in the regression analysis by the DRI committee(8)); (6) studies were carried out in children and adolescents (1–18 years), adults (18–64 years) and older adults (>64 years). (Infants and pregnant or lactating women are life-stage groups that have special considerations in relation to vitamin D and were excluded from the present analysis.)
Search strategy
In our recent systematic review of existing and potentially novel functional markers of vitamin D status(Reference Seamans and Cashman17), electronic searches were run (on Ovid MEDLINE (Ovid, http://www.ovid.com), EMBASE and Cochrane CENTRAL (http://www.thecochranelibrary.com)) from inception to 25 September 2007 by using a structured search strategy in the following format: ((vitamin D supplements) AND (supplementation or depletion studies in human subjects)) (details available online at http://www.ajcn.org/content/vol0/issue2009/images/data/ajcn.2009·27230D/DC1/AJCN_27230D_ST1.doc for full Ovid MEDLINE search strategy). As part of that systematic review, a vitamin D expert was contacted, reference lists of ten reviews drawn from electronic searches of reviews run on Ovid MEDLINE and all included studies were checked and additional articles collected and assessed for inclusion(Reference Seamans and Cashman17). For the present study, an updated search from 26 September 2007 to 30 November 2010, using the same structured search strategy, was performed. In addition, there have been two major systematic reviews performed in the area of vitamin D (and Ca) and bone(12, Reference Cranney, Horsley and O'Donnell22) and other health outcomes(12), as well as the IOM DRI report on Ca and vitamin D(8). For completeness, we cross-referenced the RCT that were both included and excluded in these reviews against those identified for inclusion or exclusion in the present study.
Data collection for updated search
Screening of titles and abstracts for collection, screening full-text articles for inclusion and data extraction (including quality assessment) from included studies were all performed by two independent reviewers. Both reviewers for the updated search were the same individuals who performed the original electronic searches(Reference Seamans and Cashman17).
Data synthesis
A flowchart showing the number of studies assessed (original and updated search) and included in the review is shown in Fig. 1. We extracted the number of participants included (and assessed) in each arm of each RCT, plus mean values and standard deviations of the baseline and final values in the treatment and control arms at each time point and for each vitamin D dose. In cases in which there were greater than or equal to two intervention arms and one common control group within an RCT, the various arms (up to and including 2000 IU/d) v. control were included so long as the arms fell into different dose range subgroups.

Fig. 1 Flow diagram for systematic review of vitamin D intake–status relationship. 25(OH)D, 25-hydroxyvitamin D; D2, vitamin D2.
Meta-regression of the response of serum 25-hydroxyvitamin D to total vitamin D intake
Weighted linear model meta-regression analyses of total vitamin D intake (i.e. habitual intake of the vitamin plus the supplemental dose) v. achieved serum or plasma 25(OH)D concentration (i.e. the concentration at the end of the winter sampling point) were performed in SPSS for Windows version 15.0 (SPSS, Inc., Chicago, IL, USA). As per the analysis by the IOM DRI committee, the regression analysis in the present study was performed on data from all RCT that were conducted during the winter period (September to June or for part thereof, as defined in the IOM DRI report(8)) stratified by >40 to < 49·5°N and ≥ 49·5°N and 78°S.
In situations in which the RCT did not assess and/or report the habitual vitamin D intake of the cohort(s) within their study, the appropriate age and sex group mean vitamin D intake value from the national nutrition survey relevant to the country in which the RCT was performed, where available (or where unavailable then from a published study in the relevant sex and age group) was used as a surrogate (Table 1). The habitual intake estimates were added to the supplemental vitamin D dose to generate total vitamin D intake estimates, which were then transformed to the natural log (Ln) before regression analysis, the approach used by the DRI committee(8). As per the DRI committee approach, the various vitamin D arms of an RCT were included as long as the arms fell into different dose range subgroups(8). In addition, the regression was set for a y 0 intercept of 0 nmol of 25(OH)D/l of serum, on the basis that the DRI committee suggested that this is consistent with the biological reality preventing a negative value for achieved serum 25(OH)D levels(8). In addition, regression models of achieved serum 25(OH)D concentration and total vitamin D intake were run without Ln transforming total intake, but limiting the total vitamin D intake data points to a maximum of 1400 IU/d, on the basis of Aloia et al. (Reference Aloia, Patel and Dimaano23), who in their recent analysis of sixty-four vitamin D RCT using a spline-fit approach showed that the slope response of serum 25(OH)D to increasing dose becomes constant at a dose of 1400 IU/d. Thus, at doses above this level, the response of serum 25(OH)D is more blunted and would not be best described by a linear fit model.
Table 1 Study characteristics of randomised controlled trials >49·5°N selected for the meta-regression analysis
(Mean values and standard deviations)

25(OH)D, 25-hydroxyvitamin D; CBPA, competitive binding protein assay; EIA, enzyme-linked immunoassay.
* Dose of vitamin D confirmed independently by analysis.
† Analytical methods for analysing circulating 25(OH)D levels: EIA; CBPA; HPLC.
‡ Intake estimated from Andersen et al. (Reference Andersen, Mølgaard and Skovgaard76).
§ An average latitude was taken from Helsinki, Finland (61°N) and Copenhagen, Denmark (55°N).
∥ Mean age is given where range is not available.
¶ Data was extracted from the ‘outpatient’ group of the study only.
** Intake estimate was obtained from Flynn et al. (Reference Flynn, Hirvonen and Mensink71).
Regression analysis of combined individual data from two winter-based randomised controlled vitamin D intervention trials in adults and older adults at latitudes >49·5°N
Regression analysis using the same model characteristics (total vitamin D intake with and without prior Ln transformation, setting y 0 intercept of 0 nmol of 25(OH)D/l of serum (Ln model) or allowing model predict y 0 intercept (non-Ln model)) was performed on individual data from two recently published randomised, double-blind, placebo-controlled, vitamin D3 intervention studies in northern European ( ≥ 52°N) 20- to 40-year-old adults (n 238(Reference Cashman, Hill and Lucey13)) and greater than 64-year-old adults (n 225(Reference Cashman, Wallace and Horigan14)), which estimated the dietary requirement for vitamin D in these population subgroups during late winter.
Results
In total, 2742 (2363 in our previous review(Reference Hooper, Ashton and Harvey17) and 379 in our updated search) titles and abstracts were screened (Fig. 1) and forty-four RCT were included(Reference Ooms, Lips and Roos4, Reference Cashman, Hill and Lucey13–Reference Cashman, Fitzgerald and Viljakainen15, Reference Lanham-New, Vieth and Heaney19, Reference Harris and Dawson-Hughes20, Reference Ala-Houhala, Koskinen and Koskinen24–Reference Viljakainen, Vaisanen and Kemi62), all of which met the inclusion criteria of the present study and provided extractable data on serum or plasma 25(OH)D. Details of the forty-four included studies (including some criteria of quality) have been presented in our previous systematic review(Reference Seamans and Cashman17) or in the Tufts systematic review group(12), the IOM DRI report(8) or are shown in Table 1. Of the forty-four studies, seven were in males and twenty-four in females (the remainder were mixed). Of the RCT, four were in children and adolescents (8–15 years), eleven in adults (18–64 years) and twenty-two in elderly (>65 years; some studies had more than one population subgroup). Among these, twenty-four studies gave vitamin D supplementation alone, twenty vitamin D plus Ca (some studies had both arms) and one study co-administered phylloquinone(Reference Bolton-Smith, McMurdo and Paterson30) and one alendronate(Reference Brazier, Kamel and Lorget32). In all, five studies gave ≤ 200, fifteen 201–400, thirty 401–1000 and six 1001–2000 IU/d of supplemental vitamin D (some studies provided multiple doses). Of the winter-based RCT, eleven studies were carried out in northern Europe (>49·5°N) and one in Antarctica (78°S), seven studies were in latitudes between 40 and 49·5°N, of which six were in the USA and one from Europe (Switzerland). Six of the twelve RCT in >49·5°N and four of the seven RCT at >40°N to < 49·5°N were included in the comprehensive systematic review by the Ottawa group(Reference Cranney, Horsley and O'Donnell22) and all had a Jadad score ≥ 3. The remaining RCT were not within the timeframe of that systematic review and thus do not have a Jadad score, but were all included in the IOM analysis(8).
Meta-regression of the response of serum 25-hydroxyvitamin D to total vitamin D intake
The listing of winter-based RCT at ≥ 49·5°N and 78°S (n 12), identified through the search strategy and data collection of the present study, differed modestly from those (n 9) used in the IOM DRI committee's meta-regression analyses: seven RCT(Reference Cashman, Hill and Lucey13, Reference Cashman, Wallace and Horigan14, Reference Viljakainen, Palssa and Kärkkäinen21, Reference Ala-Houhala, Koskinen and Koskinen24, Reference Smith, Gardner and Locke58, Reference Viljakainen, Natri and Kärkkäinen61, Reference Viljakainen, Vaisanen and Kemi62) were common to both analyses, three RCT(Reference Schou, Heuck and Wolthers63–Reference van der Klis, Jonxis and van Doormaal65), which were used by the DRI committee, were excluded from the present analysis as they were too short in duration (4–5 weeks), whereas we included five RCT(Reference Barnes, Robson and Bonham26, Reference Honkanen, Alhava and Parviainen43, Reference Meier, Woitge and Witte50, Reference Mølgaard, Larnkjaer and Cashman51, Reference Pfeifer, Begerow and Minne55) that fit with the inclusion criteria of the present study, but were not included in the DRI committee's analysis (possibly because Ca was co-administered, but this has not been shown to influence the response of serum 25(OH)D to vitamin D in a meta-analysis(Reference Seamans and Cashman17) or experimentally in an intervention study(Reference McCullough, Bostick and Daniel66), or because the RCT was after the IOM's timeframe(Reference Mølgaard, Larnkjaer and Cashman51)). In the case of two RCT, combined data from 11-year-old girls in the RCT by Viljakainen et al. (Reference Viljakainen, Natri and Kärkkäinen61) and Mølgaard et al. (Reference Mølgaard, Larnkjaer and Cashman51), who were on the vitamin D intervention during September/October to March/April (a subset of the entire group) were presented recently by Cashman et al. (Reference Cashman, Fitzgerald and Viljakainen15).
We also checked the literature for any relevant studies between 1 December 2010 and 28 February 2011, but none of the three studies published during this time frame(Reference Cherniack, Florez and Hollis67–Reference Austin, Devine and Bruce69) could be included in the present regression analysis.
Weighted linear model meta-regression analyses of Ln total vitamin D intake v. achieved serum or plasma 25(OH)D concentration (and setting y 0 at 0 nmol/l serum 25(OH)D) from winter-time-only RCT stratified by latitude showed that in those performed at latitudes >40 to < 49·5°N or ≥ 49·5°N and 78°S, the interaction term between age and the Ln of total vitamin D intake (P = 0·922 and 0·472, respectively), as well as the main effect of age (P = 0·652 and 0·325, respectively) were non-significant. Therefore, because there was no age effect in the response of serum 25(OH)D level to Ln total intake of vitamin D, a single, combined regression analysis was carried out on the data from RCT at both latitude groupings separately.
With RCT at >40 to < 49·5°N (n 7), the present analysis yielded the predictive equation of achieved serum 25(OH)D in nmol/l = 12·6 Ln (total vitamin D intake) (Table 2). These RCT were conducted during the winter period, as defined broadly by the DRI committee as September to June or part thereof(8). The regression analysis was also run after omitting RCT whose end point was beyond April (n 2), which yielded the predictive equation of achieved serum 25(OH)D in nmol/l = 11·4 Ln (total vitamin D intake).
Table 2 Predictive regression equations of achieved winter serum 25-hydroxyvitamin D (s25(OH)D) as a function of natural log (Ln) and linear total vitamin D intake
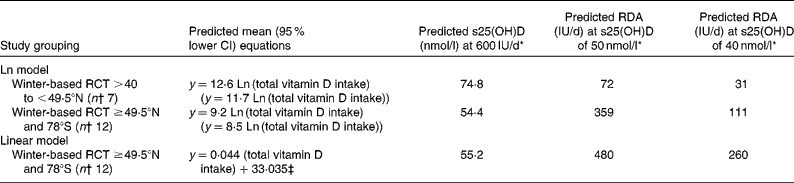
RCT, randomised controlled trials; y, achieved serum 25(OH)D (nmol/l) in winter.
* Using the 95 % lower CI regression equation.
† Refers to number of RCT.
‡ Maximum total vitamin D intake was limited to 1400 IU/d (35 μg/d).
A single, combined regression analysis was also carried out with data from winter-time-only (April, the latest end date) RCT at latitudes ≥ 49·5°N and 78°S, and resulted in the predictive equation of achieved serum 25(OH)D in nmol/l = 9·2 Ln (total vitamin D intake) (Table 2 and Fig. 2). Using the combined regression predicted 95 % lower CI of y = 8·5 Ln (total vitamin D intake) and inputting a total intake value of 600 IU/d (the RDA for those aged 1–70 years(8)) would predict an achieved serum 25(OH)D of 54·4 nmol/l. Using the same equation, but in reverse, to predict the total intake of vitamin D that would achieve a serum 25(OH)D of 50 nmol/l (the concentration that would meet the needs of 97·5 % of the population(8)), the required total intake of vitamin D dropped dramatically to 359 IU/d.

Fig. 2 Response of serum 25-hydroxyvitamin D (25(OH)D) level to total intake of vitamin D in northern latitudes in Europe (>49·5°N) and Antarctica (78°S) during their respective winter seasons, when effective sun exposure for endogenous vitamin D synthesis is minimal. Mean responses (white lines) with 95 % CI using a weighted linear meta-regression model following either a natural logarithmic transformation (dark gray shading, curvilinear model) or no transformation (pale gray shading, linear model) of total vitamin D intake data. The maximum total intake data point in the linear model was < 1400 IU/d (35 μg/d). A line is plotted at 50 nmol/l serum 25(OH)D for illustrative purposes.
As an alternative to the curvilinear relationship arising from the Ln-transformed intake data, if non-transformed total vitamin D intake (and limiting it to a maximum of 1400 IU/d on the basis of Aloia et al.(23)) was used, a more linear relationship resulted (Fig. 2). The interaction term between age and total vitamin D intake (P = 0·213), as well as the main effect of age (P = 0·196) were non-significant, allowing for a single, combined regression analysis that resulted in the predictive equation of achieved serum 25(OH)D in nmol/l = 0·044 (total vitamin D intake)+33·035. The 95 % lower CI predictive regression values from the linear intake–status relationship predicted an achieved serum 25(OH)D of 55·2 nmol/l, at a total intake value of 600 IU/d. The lower CI regression equations predicted the total intake of vitamin D that would achieve a serum 25(OH)D of 50 nmol/l of 480 IU/d (Table 2).
Using the Ln and linear lower CI regression equations, it was predicted that the total intake of vitamin D would achieve a serum 25(OH)D of 40 nmol/l at 111 and 260 IU/d, respectively.
Regression analysis using the combined data from two vitamin D randomised control trials
The predicted RDA estimates for vitamin D at two target serum 25(OH)D concentrations (i.e. 40 and 50 nmol/l) using the 95 % lower CI meta-regression analysis with group means (n 8) and regression analysis with 95 % lower CI and 95 % range of individual combined data (total n 463; maximum total vitamin D intake = 1310 IU/d; hence, all data were included) from the two RCT with adults(Reference Cashman, Hill and Lucey13) and older adults(Reference Cashman, Wallace and Horigan14) are shown in Table 3. The RDA estimates from the meta-regression and regression of individual data, which both used the 95 % lower CI, were dramatically lower than that from the regression model of individual data that used the 95 % range.
Table 3 Predicted RDA estimates using linear regression models of group means and individual data from two winter-based vitamin D randomised controlled trials (RCT) at ≥52°N
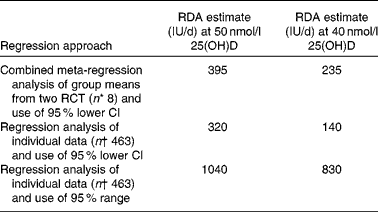
25(OH)D, 25-hydroxyvitamin D.
* Refers to eight group means from two RCT (young adults, Cashman et al.(Reference Cashman, Hill and Lucey13); and older adults, Cashman et al.(Reference Cashman, Wallace and Horigan14)).
† Refers to number of combined individuals within the two RCT.
After seeing the magnitude of the difference in the requirement estimates arising from use of 95 % lower CI and 95 % range, we also went back and for comparative purposes applied a 95 % range to the linear meta-regression analysis of twelve winter-based RCT at >49·5°N, although caution is warranted when applying a 95 % range when the number of data points are relatively low (n 30). This analysis predicted that the total intake of vitamin D that would achieve a serum 25(OH)D of 50 nmol/l was 930 IU/d (v. 480 IU/d with the 95 % lower CI).
Discussion
The new IOM DRI values for vitamin D(8) relied heavily on data from eight northern European (plus one from Antarctica) winter-based vitamin D RCT and thus would appear to be highly relevant to Europe in terms of a re-evaluation of its vitamin D dietary reference values (i.e. population reference intakes; PRI) and indeed those of its constituent member states/regions. Using the systematic review approach of the present study, we identified and extracted data from eleven relevant European-based RCT (plus one RCT from Antarctica) with vitamin D3, which were conducted at latitudes ≥ 49·5°N during winter, in line with the approach used by the IOM DRI committee. Despite some differences in the final collection of RCT included in the present regression analysis relative to that in the DRI committee's analysis (as outlined previously), the 95 % lower CI predictive equations of achieved 25(OH)D v. Ln total vitamin D intake were very close.
The DRI committee choose to apply the curvilinear Ln model to the intake–status data from the RCT to account for the more blunted response of serum 25(OH)D to high intakes of vitamin D(8). This non-linear response of serum 25(OH)D to vitamin D intake is to be expected on the basis of metabolic kinetics. Heaney et al. (Reference Heaney, Armas and Shary70) showed that the relationship between serum vitamin D3 and 25(OH)D concentrations is biphasic due to the fact that hepatic 25-hydroxylase becomes saturated and the reaction switches from first to zero order. Even though the lower limit CI predictive equations arising from the Ln model overshoots the target serum 25(OH)D mark of 50 nmol/l at the 600 IU/d vitamin D intake level (e.g. 56 nmol/l in the DRI analysis(8), 54 nmol/l in the present analysis), the committee used this intake estimate to allow for some uncertainties and limitations within the analysis(8). Should one choose to use 50 nmol/l as the target concentration of serum 25(OH)D in these equations, then the dietary requirement estimate is 313 IU/d (359 IU/d in the present analysis), and alternatively, if one uses 40 nmol/l, it is only 99 IU/d (111 IU/d in the present analysis). The Ln model has a steep decline in achieved serum 25(OH)D concentrations at total vitamin D intakes, particularly at the lower end of intakes, and at zero intake, the achieved serum 25(OH)D was 0 nmol/l due to a forcing of the model to avoid a negative predicted value for achieved serum 25(OH)D levels(8). However, it is also worth considering whether it is likely that someone with no vitamin D intake during winter (if that were possible) might still have a serum 25(OH)D concentration greater than zero as a consequence of tissues stores? For example, the adult subject with lowest total vitamin D intake (24 IU/d) in our vitamin D intervention study in 20- to 40-year-old adult group(Reference Cashman, Hill and Lucey13) had a winter serum 25(OH)D of 31·6 nmol/l.
Others, including ourselves(Reference Seamans and Cashman17), have reported serum 25(OH)D response estimates to vitamin D supplementation from RCT based on a linear analysis(Reference Cranney, Horsley and O'Donnell22, Reference Heaney, Davies and Chen41), but with doses up to 2000 IU/d(Reference Cranney, Horsley and O'Donnell22) and even 10 000 IU/d(Reference Heaney, Davies and Chen41). This clearly does not take account of the smoothening in the response of serum 25(OH)D to higher intakes of vitamin D. Therefore, in the present analysis, we also performed a linear analysis of the intake–status data, but excluded intake data points (n 2) in excess of 1400 IU/d on basis of Aloia et al. (Reference Aloia, Patel and Dimaano23), who showed that the response slope of serum 25(OH)D becomes constant at this level. In Ireland and the UK, as well as in several other European member states(Reference Flynn, Hirvonen and Mensink71), the 95th percentile of total vitamin D intake in national nutrition surveys is generally less than 600 IU/d; thus, a range of 0–1400 IU/d brackets the nutritional intake of vitamin D seen in the population. Using an intake of 600 IU/d in the lower limit CI predictive linear regression equation, the predicted serum 25(OH)D concentration was 55 nmol/l – similar to that from the Ln models, and providing further support to the DRI committee's findings. However, an intake of 260 and 480 IU/d was required to achieve serum 25(OH)D concentrations of 40 and 50 nmol/l, respectively (2·3- and 1·4-fold higher than that predicted from the Ln model, respectively).
Clearly, the shape of the intake–status relationship has an important bearing on the predicted RDA estimates for vitamin D at serum 25(OH)D target concentrations ≤ 50 nmol/l. However, and maybe more importantly, irrespective of whether a Ln or linear model is applied in these meta-regression analyses, estimates of 359 or 480 IU/d vitamin D requirements, respectively, to cover the needs of 97·5 % of the population in terms of maintaining serum 25(OH)D >50 nmol/l does not fit well with our estimates from experimental studies that suggest that 988–1120 IU/d would be required(Reference Cashman, Hill and Lucey13, Reference Cashman, Wallace and Horigan14). The use of CI in meta-regression analyses provides some estimate of the variability about the fitted response line, but does not provide any estimate of the variability between individuals in terms of dietary intake of vitamin D needed to achieve a serum 25(OH)D concentration (i.e. an estimate of the range). This was illustrated in the present study, wherein RDA estimates from either the meta-regression or regression of individual data that used the 95 % lower CI were dramatically lower than the model that used the 95 % range. The former variability term gives 95 % surety that the average serum 25(OH)D level in the adult population is above 50 nmol/l at a certain intake of vitamin D, whereas the latter can be used to take account of inter-individual variability on intake required to reach a chosen serum 25(OH)D cutoff. The importance of this inter-individual variability term (95 % range) can also be seen if one compares the estimates from our RCT in young adults(Reference Cashman, Hill and Lucey13) and older adults(Reference Cashman, Wallace and Horigan14), which incorporated the range, and suggest that 346 IU/d of vitamin D are required to keep winter time serum 25(OH)D levels >25 nmol/l in 97·5 % of the population, whereas the model with lower 95 % CI predicts that 0 IU/d intake will suffice. We have reported previously that if one tests the former estimate within the nationally representative UK National Diet and Nutrition Survey databases of adults and older adults, there was a 11–18·4 % prevalence of serum 25(OH)D below 25 nmol/l during late winter/early spring in those with intakes below 346 IU/d and only 0–2·9 % prevalence in those with intakes above this estimate(Reference Cashman and Kiely72), suggesting that this intake did indeed protect the vast majority of the adult population from vitamin D deficiency.
The present regression analysis also predicted a higher response of serum 25(OH)D to total vitamin D intake at lower (40–49·5°N) than at higher latitudes ( ≥ 49·5°N/S), in line with the DRI committee's findings(8). Although the reason(s) for this could not be explored in the present analysis, it may relate to differences in the capacity for dermal synthesis during extended winter in these different regions and/or stores of vitamin D arising from previous summer UVB sun exposure. Nevertheless, it may be relevant to Europe, much of which resides between 40 and >70°N. The present analysis had a number of limitations arising from the available data on which to base the meta-regression. Most of the subjects in the twelve RCT that met with the inclusion criteria of the present study were Caucasians, and thus does not reflect the ethnic diversity that exists in many European member states. Recent data from national nutrition and health surveys in the USA and Canada clearly show that risk of serum 25(OH)D concentrations below 30 and 50 nmol/l was higher in non-white than in white persons(Reference Looker, Johnson and Lacher73, Reference Whiting, Langlois and Vatanparast74). The DRI committee highlighted the need for a greater understanding of how skin pigmentation influences vitamin D synthesis, and highlighted that South Asian and Middle Eastern immigrant groups may be a particular concern(8). Finally, the quality of vitamin D intake data in the meta-regression analysis may be a limitation, as in some cases national intake vitamin D data had to be used as a surrogate in those studies that did not measure/report intake data. Furthermore, there can be considerable differences in food compositional data for vitamin D across countries(75).
In conclusion, although the relation of serum 25(OH)D to vitamin D intake is critical to the establishment of dietary requirements for vitamin D, the model used to describe this relationship needs to be configured to take into account important considerations such as target serum 25(OH)D concentration, range of intakes of vitamin D within the population and inter-individual variability. There may be additional benefit from use of individual data from vitamin D RCT, if these were available, to augment the meta-analyses approach.
Acknowledgements
The present study was carried out with partial financial support from the Commission of the European Communities, specific RTD Programme ‘Quality of Life and Management of Living Resources’, within the 6th Framework Programme (contract no. FP6-036196-2 EURRECA: EURopean micronutrient RECommendations Aligned). This paper does not necessarily reflect the views of the Commission and in no way anticipates future policy in this area. K. M. S. and K. D. C. assessed the studies for inclusion, extracted data and assessed validity; K. M. S., A. P. F. and K. D. C. conducted meta-regression and regression; K. M. S. and K. D. C. tabulated data; K. M. S., M. K. and K. D. C. wrote the manuscript. The authors declare no conflicts of interest.







