In this article, we describe the multimodal longitudinal twin study BATSS (Babytwins Study Sweden). The study is based on the idea that combining the field of developmental cognitive neuroscience and twin research can advance knowledge about the origins of individual variation in brain and behavioral development in infancy. While there are existing studies using traditional psychological methods, such as questionnaires and direct observation of infant twins, there are no infant (<2 years) twin studies using a full array of state-of-the-art, advanced methodologies from developmental cognitive neuroscience, such as eye tracking and electroencephalography (EEG). These approaches can reveal unique aspects of infant brain and behavioral development.
The study of individual differences continues apace within the field of developmental science. Partly, this is due to the recognized need to predict who is likely to experience certain later outcomes (e.g., disease or developmental difficulties). Key constructs such as equifinality, multifinality, developmental cascades/pathways and probabilistic epigenesis all relate to individual differences in development (Cicchetti & Rogosch, Reference Cicchetti and Rogosch1996; Gottlieb, Reference Gottlieb2007; Waddington, Reference Waddington2014). In parallel with the theoretical developments, the gradual accumulation of reliable and valid psychological measures for infancy research has also contributed to the enhanced focus on individual differences in developmental science.
In older children and adults, twin studies have for decades been instrumental in our understanding of why people differ in their traits and disease and disorder outcomes (Polderman et al., Reference Polderman, Benyamin, De Leeuw, Sullivan, Van Bochoven, Visscher and Posthuma2015). By comparing the degree of similarity in MZ and DZ twins, one can quantify the relative contribution of genetic and environmental influences to single phenotypes. Furthermore, the multivariate extension of twin analyses allow for an array of analyses that explore, for example, the degree to which the same genetic influences and the same environmental influences affect two or more phenotypes or explain patterns of stability and change of the same phenotype over development. Twin studies can also shine light on the ‘heritability of the environment’ (i.e., when aspects of one’s environment are shaped by one’s genetic predispositions). Specifically, environments that are unique to each child can be incorporated into the twin design, and thus the role of genetic and environmental influences on a putative environment, such as stressful life events, can be estimated (Shakoor et al., Reference Shakoor, Zavos, Haworth, McGuire, Cardno, Freeman and Ronald2016).
In terms of the existing twin literature applying brain-based measures from developmental cognitive neuroscience, EEG studies of infant twins are nearly nonexistent. EEG measures electrical activity arising from synchronous neural activity in the brain from sensors placed on the skull. EEG studies have the potential to characterize early neurodevelopmental processes, and unlike eye tracking or other methods, it does not require any overt behavioral response. While EEG has limited spatial resolution, its temporal resolution is excellent, which is used in both time- and frequency-based analyses.
The lack of infant twin EEG studies is surprising given the widespread use of this method in developmental science generally, and the potential value of using noninvasive brain measures to identify heritable psychological traits associated with later neurodevelopmental conditions (e.g., autism; Bosl et al., Reference Bosl, Tager-Flusberg and Nelson2018). Studies of older children have found that EEG measures such as frequency band amplitude are highly heritable (van Baal et al., Reference van Baal, De Geus and Boomsma1996; Zietsch et al., Reference Zietsch, Hansen, Hansell, Geffen, Martin and Wright2007). However, to our knowledge, only one twin study of infant EEG exists (Orekhova et al., Reference Orekhova, Stroganova, Posikera and Malykh2003). This cross-sectional study, which included 95 twin pairs, provided evidence for genetic effects on the alpha frequency band in 7- to 12-month-olds, but also tentatively suggested that shared environment could play a role for other EEG measures at this age.
Similarly, we are not aware of any eye tracking studies of twins under the age of 2 years. Eye tracking uses infra-red light to measure the gaze direction of participants while observing stimuli. Eye tracking has been instrumental to our understanding of young infants’ attentional, perceptual and emerging cognitive abilities, in both the social and nonsocial domains (Gredebäck et al., Reference Gredebäck, Johnson and von Hofsten2010). Infants develop flexible gaze behavior earlier than many other behaviors (e.g., crawling), and hence, this method provides a unique window into preverbal development. Eye tracking can also be used to assess the size of the pupil, which has proven to be an informative measure as well (Nyström et al., Reference Nyström, Gliga, Nilsson Jobs, Gredebäck, Charman, Johnson, Bölte and Falck-Ytter2018).
One eye tracking study of 2-year-old twins conducted an analysis of eye movement and gaze characteristics in a relatively small sample (83 pairs; Constantino et al., Reference Constantino, Kennon-McGill, Weichselbaum, Marrus, Haider, Glowinski, Gillespie, Klaiman, Klin and Jones2017). They found that the tendency to prioritize the eyes versus the mouth during close-up face observation was highly heritable (broad heritability close to 90%). Further, many lower level aspects of eye movements, such as the timing and spatial direction of saccades, were also more similar in MZ than in DZ twins, although caution is needed in light of the small sample size. In light of the extensive use of eye tracking in developmental science generally, and specifically in studies of infants at risk of autism, there is considerable potential to employ this method in studies of young twins.
Among the small number of infant twin studies using methods from cognitive neuroscience, most have used magnetic resonance imaging (MRI; for a review of these twin studies, see Maggioni et al., Reference Maggioni, Squarcina, Dusi, Diwadkar and Brambilla2020). In terms of differences in brain structure, evidence suggests that genetic factors are important, even very early in life. The magnitude of genetic influence appears to be higher for white matter than grey matter (Gilmore et al., Reference Gilmore, Schmitt, Knickmeyer, Smith, Lin, Styner, Gerig and Neale2010), and region specific (posterior grey matter volume is more heritable than grey matter in frontal areas; Gilmore et al., Reference Gilmore, Schmitt, Knickmeyer, Smith, Lin, Styner, Gerig and Neale2010, see also Duan et al., Reference Duan, Xia, Rekik, Wu, Wang, Lin, Gilmore, Shen and Li2020). Diffusion tensor imaging studies suggest significant heritability of early white matter, but substantial regional differentiation, which may reflect the different developmental or maturational timescales of different white matter tracts; for example, in terms of myelination, as well as time periods with more environmental susceptibility (Geng et al., Reference Geng, Prom-Wormley, Perez, Kubarych, Styner, Lin, Neale and Gilmore2012; Lee et al., Reference Lee, Steiner, Yu, Short, Neale, Styner, Zhu and Gilmore2017; Sadeghi et al., Reference Sadeghi, Gilmore and Gerig2017). To our knowledge, only one study has investigated functional MRI in young twins (Gao et al., Reference Gao, Elton, Zhu, Alcauter, Smith, Gilmore and Lin2014).
In contrast, there is a larger body of literature of infant twin studies assessing phenotypes such as temperament, sleep and cognition through questionnaires and observational measurements. Studies focused on language and communication in late infancy and toddlerhood show that shared environment accounts for most of the variance (∼60%) for measures including imitative ability (McEwen et al., Reference McEwen, Happé, Bolton, Rijsdijk, Ronald, Dworzynski and Plomin2007), language acquisition (Harlaar et al., Reference Harlaar, Hayiou-Thomas, Dale and Plomin2008), vocabulary (Hayiou-Thomas et al., Reference Hayiou-Thomas, Dale and Plomin2012; van Hulle et al., Reference van Hulle, Goldsmith and Lemery2004), and early verbal ability (Galsworthy et al., Reference Galsworthy, Dionne, Dale and Plomin2000). Modest heritability is shown for these type of measures in infancy and early childhood (Dale et al., Reference Dale, Dionne, Eley and Plomin2000; Ganger, Reference Ganger1998; Hayiou-Thomas et al., Reference Hayiou-Thomas, Dale and Plomin2012; McEwen et al., Reference McEwen, Happé, Bolton, Rijsdijk, Ronald, Dworzynski and Plomin2007; Robinson, Reference Robinson1999; van Hulle et al., Reference van Hulle, Goldsmith and Lemery2004). For nonverbal cognitive ability, most studies report low-to-moderate heritability in toddlerhood and early childhood (Bishop et al., Reference Bishop, Cherny, Corley, Plomin, DeFries and Hewitt2003; Cherny et al., Reference Cherny, Fulker, Emde, Robinson, Corley, Reznick, Plomin and DeFries1994; Matheny, Reference Matheny1980; Petrill et al., Reference Petrill, Saudino, Cherny, Emde, Fulker, Hewitt and Plomin1998; Petrill et al., Reference Petrill, Saudino, Cherny, Emde, Hewitt, Fulker and Plomin1997; Plomin et al., Reference Plomin, Emde, Braungart, Campos, Corley, Fulker, Kagan, Reznick, Robinson, Zahn-Waxler and Defries1993).
Studies of infant attachment report that shared environmental factors explain a large portion of the variation in the late infancy and toddlerhood period (Bakermans-Kranenburg et al., Reference Bakermans-Kranenburg, Van Uzendoorn, Bokhorst and Schuengel2004; Bokhorst et al., Reference Bokhorst, Bakermans-kranenburg, Pasco Fearon, Van Ijzendoorn, Fonagy and Schuengel2003; Fearon et al., Reference Fearon, Van IJzendoorn, Fonagy, Bakermans-Kranenburg, Schuengel and Bokhorst2006; Roisman & Fraley, Reference Roisman and Fraley2008). These results contrast with studies focusing on parent–child interaction in other contexts than attachment-eliciting situations, which suggest that genetic factors play a substantial role for variation in infant behavior during parent–child interaction, with little contribution from shared environmental factors (Deater-Deckard & O’Connor, Reference Deater-Deckard and O’Connor2000; DiLalla & Bishop, Reference DiLalla and Bishop1996).
Behavioral genetic research has also included socio-communicative traits. In the last decade, several studies have focused on early emerging behavioral traits linked to autism spectrum disorder (ASD). Overall, such autistic-like traits are moderately to highly heritable in toddlerhood (de Zeeuw et al., Reference de Zeeuw, van Beijsterveldt, Hoekstra, Bartels and Boomsma2017; Stilp et al., Reference Stilp, Gernsbacher, Schweigert, Arneson and Goldsmith2010). Very high heritability estimates have been found for some specific socio-communicative traits, such as attention to eyes and mouth in the second year of life (Constantino et al., Reference Constantino, Kennon-McGill, Weichselbaum, Marrus, Haider, Glowinski, Gillespie, Klaiman, Klin and Jones2017), as well as for socio-communicative behaviors rated by parents (Hawks et al., Reference Hawks, Marrus, Glowinski and Constantino2019), and reciprocal social behavior (Pohl et al., Reference Pohl, Jones, Marrus, Zhang, Klin and Constantino2019).
Other areas of early psychopathology and neurodevelopment have been investigated. Hyperactivity was highly heritable in a study with preschool children (2−4 years; Price et al., Reference Price, Simonoff, Waldman, Asherson and Plomin2001) with no influences of shared environment. Similarly, the heritability of parent-rated self-control has been estimated to be substantial, with little evidence of shared environmental influences in studies with twins at 2 and 3 years of age (Gagne & Goldsmith, Reference Gagne and Goldsmith2011; Gagne & Saudino, Reference Gagne and Saudino2010, Reference Gagne and Saudino2016).
Emerging ADHD traits can also include executive functioning dimensions of temperament (i.e., effortful control and attention control). Temperament in infancy research is a broad but widely studied phenotype. Twin studies suggest that individual differences in infant and child temperament have a moderate to high heritability (Gagne et al., Reference Gagne, Vendlinski and Goldsmith2009; Planalp & Goldsmith, Reference Planalp and Goldsmith2020; Saudino, Reference Saudino2005), with higher genetic effects for negative aspects of temperament (e.g., anger; Gagne & Goldsmith, Reference Gagne and Goldsmith2011) than positive aspects (e.g., positive affectivity and soothability; Gagne et al., Reference Gagne, Vendlinski and Goldsmith2009; Goldsmith et al., Reference Goldsmith, Lemery, Buss and Campos1999; Saudino, Reference Saudino2005). Further, motor activity level shows moderate genetic and no shared environment effects (Goldsmith et al., Reference Goldsmith, Lemery, Buss and Campos1999); and significant genetic overlap with ADHD symptoms at 2 years (Ilott et al., Reference Ilott, Saudino, Wood and Asherson2010).
Temperament is linked to infant sleep, which is crucial for a healthy development. Sleep has been researched in a few twin studies of infants 6 months and older with moderate sample sizes and parent-reported measures. These studies suggest that early childhood daytime sleep may be driven by shared environmental factors to a substantial extent, whereas the variance in nighttime sleep may be more heritable (Brescianini et al., Reference Brescianini, Volzone, Fagnani, Patriarca, Grimaldi, Lanni, Serino, Mastroiacovo and Stazi2011; Dionne et al., Reference Dionne, Touchette, Forget-Dubois, Petit, Tremblay, Montplaisir and Boivin2011; Fisher et al., Reference Fisher, van Jaarsveld, Llewellyn and Wardle2012; Touchette et al., Reference Touchette, Dionne, Forget-Dubois, Petit, Pérusse, Falissard, Tremblay, Boivin and Montplaisir2013).
Finally, atypical sensory processing (e.g., hyperresponsivity to certain sensory signals; Nyström et al., Reference Nyström, Gliga, Nilsson Jobs, Gredebäck, Charman, Johnson, Bölte and Falck-Ytter2018) are increasingly seen as playing a role in a range of neurodevelopmental disorders, including ASD and ADHD (Dunn, Reference Dunn2002). However, its genetic and environmental influences early in life have not been extensively investigated, with only one twin study done in 1- to 3-year-olds reporting a moderate heritability for sensory defensiveness and a small contribution from shared environment (Goldsmith et al., Reference Goldsmith, Van Hulle, Arneson, Schreiber and Gernsbacher2006).
Taken together, while several areas of infant and early child development have been the focus of previous behavioral genetic research, there is considerable potential to expand understanding of the genetic and environmental influences on infant development, particularly in the areas of brain, attentional and behavioral development. Furthermore, some of the most influential studies have either begun at 24 months, thus missing the infancy years, or have been challenged by small samples of under 300 pairs. With BATSS, we took a multimethod approach to perform deep phenotyping of a wide range of traits at five months and followed a sample of 300 twin pairs longitudinally.
The purpose of this article is to describe the BATSS study. We will focus on describing the protocol and procedures, the feasibility of conducting a study of this kind, provide descriptive data about the sample, and discuss putative analysis steps going forward.
Materials and Methods
The Classic Twin Design
BATSS employs a classic twin design. Using twin data, the total variance in a trait can be partitioned into genetic variance, common environmental variance and nonshared environmental variance, which incorporates measurement error. MZ twins share 100% of their segregating DNA and DZ twins share on average 50% of their segregating DNA. All twins who are raised together are assumed to share all of their common environmental influences. Differences identified within MZ twin pairs are explained by nonshared environment. As such, when higher within-pair similarity is observed for MZ than DZ twins, this pattern is assumed to be due to the role of genetic influences on the trait under investigation (Polderman et al., Reference Polderman, Benyamin, De Leeuw, Sullivan, Van Bochoven, Visscher and Posthuma2015). Genetic influences are typically modelled as additive effects. Genetic influences can also include nonadditive genetic effects, which are indicated by DZ correlations that are less than half the MZ correlation. Such effects can be estimated in the classical twin design, although not simultaneously with shared environment, as these effects confound one another. In BATSS, the prespecified target sample size was 620 individuals (310 pairs) based on the size of previous twin studies of toddlers (e.g., Ronald et al., Reference Ronald, Edelson, Asherson and Saudino2010). Based on previous twin studies in Sweden, we expected roughly equal numbers of MZ and DZ same-sex twin participants.
Study Protocol
The study protocol is provided in Table 1, which also includes full names of measures and abbreviations. Many of the experiments and instruments align with a longitudinal study of infants at elevated likelihood of ASD/ADHD (Jones et al., Reference Jones, Mason, Begum Ali, van den Boomen, Braukmann, Cauvet, Demurie, Hessels, Ward, Hunnius, Bolte, Tomalski, Kemner, Warreyn, Roeyers, Buitelaar, Falck-Ytter, Charman and Johnson2019). The main rationale behind this is to be able to assess the heritability of putative early ASD/ADHD markers (e.g., Nyström et al., Reference Nystrom, Jones, Darki, Bolte and Falck-Ytter2021). In addition, specific measures were added for this study, namely the ITC, SDQ, RBQ, three eye tracking tasks (emotional faces, dynamic singing faces, ANS) and one EEG task (form motion). Many eye-tracking and EEG tasks focus on social perception and attention, which is motivated by the abovementioned link to ASD, by the fact that many of these skills and processes emerge early in infancy, and because in older participants, evidence suggest that such socio-cognitive processes may be linked to unique etiological factors not shared with other aspects of cognition (Shakeshaft & Plomin, Reference Shakeshaft and Plomin2015).
Table 1. BATSS protocol

As many of the tasks and measures are well established, we do not describe them all in detail here, but additional information can be found elsewhere (Jones et al., Reference Jones, Mason, Begum Ali, van den Boomen, Braukmann, Cauvet, Demurie, Hessels, Ward, Hunnius, Bolte, Tomalski, Kemner, Warreyn, Roeyers, Buitelaar, Falck-Ytter, Charman and Johnson2019) or in the original works cited in Table 1. For other measures not described in detail elsewhere, we provide a brief description here.
Demographics and medical/psychiatric history
At the 5-month visit, we administered questionnaires and conducted interviews about basic background information such as date and place of birth, languages spoken in home, occupational and educational status, and family income. Information about the twins’ medical conditions was also collected (including twin specific birth complications), as well as the presence of developmental and psychiatric conditions in family and relatives. A set of items surveyed parents’ perceived similarity of their infant twins.
Eye-tracking tasks (not described elsewhere)
Dynamic faces
This eye tracking task consists of dynamic faces (n = 20, duration 4−12 s), divided into three conditions: singing, nonsinging nursery rhymes and silent. This task measures infants’ preference for eyes versus mouth (Constantino et al., Reference Constantino, Kennon-McGill, Weichselbaum, Marrus, Haider, Glowinski, Gillespie, Klaiman, Klin and Jones2017; Hunnius & Geuze, Reference Hunnius and Geuze2004) in the context of naturalistic face stimuli. This task was designed specifically for BATSS, but stimuli properties (e.g., duration) are similar to what has been used previously with infants.
Emotional faces
This task showed triplets of static faces in each stimulus (n = 12, duration 5 s), of which two were neutral and one had an emotional expression (happy). This task is intended to capture individual differences in attention to emotional facial stimuli, and was adapted from a longitudinal study of social attention.
Biological samples
Saliva
Saliva samples were collected by research assistants from all the infant twins using the DNA Genotek OG-575 (DNA Genotek Inc.) collection kit during the study visit.
Hair
A lock of hair was cut from the posterior vertex region of the head by parents, as close to the scalp as possible.
Nails
Samples of at least 4 mm in length were collected from fingernails and toenails. Hair and nail samples were collected to assess metabolic changes over time related to prenatal and early postnatal exposures to metals and/or other substances.
Recruitment and Testing Procedure
Recruitment and first telephone interview
Same-sex twin families were identified via the Swedish Population Registry (Folkbokföringsregistret, hosted by the Swedish Tax Agency). In total, 1068 families with same-sex twins were invited to join the study via letters. If families agreed to participate during a follow-up telephone call, a screening interview was performed. To be included in the study, the twins had to hear Swedish at home from at least one parent and live with at least one biological parent. Parents also needed to be willing to share information about medical and psychiatric history in the family, demographic background and delivery. Reasons for exclusion were hearing or vision impairments, premature birth (defined as prior to week 34 of gestation), epilepsy or seizures, medical conditions that were likely to affect brain development, ability to participate in the study, and the presence of known genetic syndromes. In a few cases, we included and tested twins with ambiguous medical conditions (12 pairs with twin-to-twin transfusion syndrome reported by parents, one twin with low birth weight, one twin with spina bifida, and two twins with seizures at birth; these are included in the total sample described in this article). The study was approved by the Regional Ethics Board in Stockholm, and parents provided informed written consent to participate in the research.
Five month in-person visit at the lab
At age 4−5 months, a letter was sent home to the parents with information about the visit, printed questionnaires and directions for completing online questionnaires (see Table 1 for overview).
The 5-month visit was typically performed in one day, lasting approximately five hours. At least two research assistants were present, each having the responsibility for specific tasks. During the visit, the twins were called by their actual names in communication with their parents, and each twin had their own personal ‘schedule’. This enabled the research assistants to keep track of the tasks performed and minimized the risk of mixing the twins up. If the research assistants had difficulty telling them apart, due to the twins being very alike and/or dressed similarly, stickers with the numbers ‘1’ and ‘2’ were placed on their abdomens. The twins performed different tasks at the same time and we generally encouraged both parents to be present during the visit, so that each child could be accompanied by one caregiver in all tasks. When occasionally it was not possible for both parents to attend the visit (i.e., only one parent being able to take leave from work or in single parent households), we had a third research assistant present acting as a ‘stand-in’. If this was the case, we always informed the parent(s) prior to the visit, to verify that this was acceptable to them. Occasionally, other relatives and friends could be present and to assist in such cases.
We started with performing the tasks that were most demanding for the infants, namely EEG (approximately 11 min), and the Mullen Scales of Early Learning (MSEL; approx. 10−15 min). Later in the visit, we performed Eye Tracking (several experiments in two separate sessions; approximately 6 and 10 min, respectively) and Parent–Child Interaction (PCI), a playtime with the mother and one twin at a time that was video recorded for subsequent behavioral coding (approximately 15 min). However, the order of tasks was not entirely fixed and varied depending on the moods and needs of the twins.
We took breaks when needed to let the twins rest and eat. In the middle of the day, we had a longer break for lunch and carried out parent report interviews (about demographic details such as education and employment, as well as medical and psychiatric history in relatives; see Table 1). The parents received gift vouchers as compensation for participating.
Follow-up questionnaire packages (14−36 months)
The twins were followed up through online questionnaires at 14, 24 and 36 months (see Table 1 for a full overview of included questionnaires). At these ages, we sent a letter with detailed instructions about the questionnaires and log-in information, together with a gift voucher. If the questionnaires were not completed after two weeks, we sent a brief reminder via email. If the questionnaires were not completed after a month, we initially sent a reminder letter, but this routine was later changed to a phone call to assist the parents with any questions or technical issues. The last date for the parents to complete the questionnaire before the time point was considered passed was at 15 months for the 14-months’ time point and 30 months for the 24-months’ time point; for the 36-months’ time point there was no last date for completion. The exact time point of questionnaire competition was recorded, allowing for further age-based selection and analysis if needed.
Sample collection and genotyping
Saliva samples were collected from all the infant twins using the DNA Genotek OG-575 collection kit during the study visit. All saliva samples were stored and processed for DNA extraction at the Karolinska Institutet Biobank. DNA extraction was done using Chemagen kits based on magnetic bead separation using the automated Hamilton ChemagicSTAR® platform. DNA quantity and quality was checked before proceeding to genotyping. The twins were genotyped using the Illumina Infinium Global Screening Array version 3 Infinium Assay using standard protocols at the SNP&SEQ Technology Platform at Uppsala University.
Results
Recruitment Summary
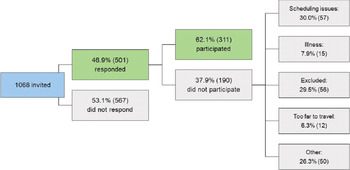
In total, 1068 families were invited to participate in BATSS. Because we recruited from the Swedish Population Registry, these individuals represent most same-sex twins born in the targeted area during the period in question (Supplementary Figure S1). In total, 46.9% of the invited families responded to our recruitment materials and could be interviewed by telephone. A total of 567 (53.1%) of the invited families did not participate, even though brief reminder letters were sent to most of them. Figure 1 outlines the categories of reasons given by families who did not participate.

Figure 1. Overall recruitment statistics
Sample Characterization
Table 2 summarizes the main characteristics of the final BATSS sample, as well as MZ and DZ subsamples. As can be seen, the total sample consists of 311 twin pairs, of whom 177 are MZ and 134 are DZ. All twin pairs in the BATSS sample were same-sex, with 151 pairs being female and 160 male. All pairs lived in the greater Stockholm and Uppsala area in Sweden upon recruitment, with 27.6% living in central Stockholm. Supplementary Table S1 summarizes response rates for online questionnaires.
Table 2. Participant characteristics 1
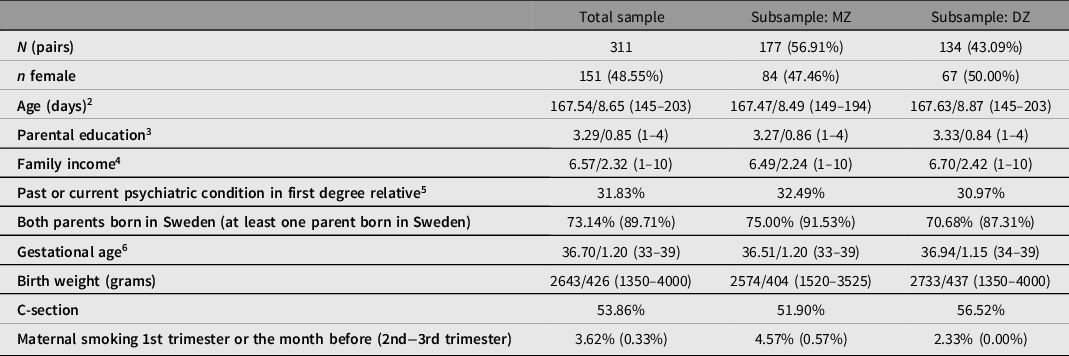
1 n for individual variables vary slightly (298–311 pairs).
2 Age at 5 months assessment. Mean/SD [min max]; this format applies generally unless otherwise specified.
3 Education level on a scale from 1 to 4, where 1 = Primary, 2 = Secondary, 3 = Undergraduate (≤3 years) and 4 = Postgraduate level (>3 years).
4 Family income per month. Scale 1–10 where 1 = <20K, 2 = 20–30K, 3 = 30–40K, 4 = 40–50K, 5 = 50–60K, 6 = 60–70K, 7 = 70–80K, 8 = 80–90K, 9 = 90–100K and 10 = >100K (SEK).
5 Reported past/current ASD, ADHD, intellectual disability, panic or anxiety disorder, depression, bipolar disorder, schizophrenia and/or admittance to psychiatric care of mother, father or full siblings of the whole sample. Classified binarily (1 = yes to at least one, 0 = no to all).
6 Gestational age of the whole sample as defined by the number of completed gestational weeks. One pair was born in week 33 + 6 (weeks + days).
To further assess the representativeness of our sample, we compared socioeconomic status of our sample with the population of Stockholm and Sweden as a whole, using statistics from Statistiska Centralbyrån (‘Statistics Sweden’, SCB; Table 3).
Table 3. Socioeconomic status

Note:
1 Mothers’ highest level of completed education (primary (≤10 years of education on ISCED levels 1–2), secondary (≤3 years of education on ISCED levels 3–4) and tertiary (>0 years of education on ISCED levels 5–8)). Education levels represent the ISCED levels of education (UNESCO Institute for Statistics, 2012).
2 Mothers’ yearly income (low: ≤299K SEK, medium: 300–499K SEK and high: ≥500K SEK), mean age 34 years.
3 For comparison, we have included data showing the income and education of the population of Stockholm county and Sweden, compiled 2019 (women aged between 25–44 years; source Statistiska Centralbyrån, SCB).
Molecular Genetic Analyses
Quality control and imputation
In 117 pairs of MZ twins (whose zygosity was confirmed earlier via a simplified genetic analysis; Hannelius et al., Reference Hannelius, Gherman, Mäkelä, Lindstedt, Zucchelli, Lagerberg, Tybring, Kere and Lindgren2007), only one of the infants in the pairs were genotyped with the Illumina Infinium array. This array consists of 730,059 markers, of which 97.8% had a sample call rate of 98% with an average SNP call rate per sample of 99.3%. Quality control (QC) for the raw genotyping at the individual and marker level was completed using PLINK v1.90. Thereafter, we imputed both autosomal and X chromosomes using IMPUTE2 followed with additional QC (Howie et al., Reference Howie, Donnelly and Marchini2009; van Leeuwen et al., Reference van Leeuwen, Kanterakis, Deelen, Kattenberg, Slagboom, De Bakker, Wijmenga, Swertz, Boomsma, van Duijn, Karssen and Hottenga2015). There were 518,570 markers remaining after genotyping QC and 7,507,876 markers after imputation QC.
Polygenic scores
Polygenic scores were calculated using PRS-CS (Ge et al., Reference Ge, Chen, Ni, Feng and Smoller2019) for a range of development-related and psychopathological traits (ADHD, Demontis et al., Reference Demontis, Walters, Martin, Mattheisen, Als, Agerbo, Baldursson, Belliveau, Bybjerg-Grauholm, Bækvad-Hansen, Cerrato, Chambert, Churchhouse, Dumont, Eriksson, Gandal, Goldstein, Grasby, Grove and Bækvad-Hansen2019; ASD, Grove et al., Reference Grove, Ripke, Als, Mattheisen, Walters, Won, Pallesen, Agerbo, Andreassen, Anney, Awashti, Belliveau, Bettella, Buxbaum, Bybjerg-Grauholm, Bækvad-Hansen, Cerrato, Chambert, Christensen, Churchhouse, Dellenvall, Demontis, De Rubeis and Børglum2019; bipolar disorder, Stahl et al., Reference Stahl, Breen, Forstner, McQuillin, Ripke, Trubetskoy, Mattheisen, Wang, Coleman, Gaspar, de Leeuw, Steinberg, Pavlides, Trzaskowski, Byrne, Pers, Holmans, Richards and Abbott2019; major depressive disorder, Howard et al., Reference Howard, Adams, Clarke, Hafferty, Gibson, Shirali, Coleman, Hagenaars, Ward, Wigmore, Alloza, Shen, Barbu, Xu, Whalley, Marioni, Porteous, Davies, Deary and McIntosh2019; schizophrenia, Ripke et al., Reference Ripke, Walters and O’Donovan2020; IQ, Savage et al., Reference Savage, Jansen, Stringer, Watanabe, Bryois, De Leeuw, Nagel, Awasthi, Barr, Coleman, Grasby, Hammerschlag, Kaminski, Karlsson, Krapohl, Lam, Nygaard, Reynolds, Trampush and Posthuma2018; educational attainment, Lee et al., Reference Lee, Wedow, Okbay, Kong, Maghzian, Zacher, Nguyen-Viet, Bowers, Sidorenko, Karlsson Linnér, Fontana, Kundu, Lee, Li, Li, Royer, Timshel, Walters, Willoughby, Yengo, Alver, Bao and Cesarini2018; physical height, Yengo et al., Reference Yengo, Sidorenko, Kemper, Zheng, Wood, Weedon, Frayling, Hirschhorn, Yang and Visscher2018). The distribution of the different polygenic scores and the correlational pattern between them were expected and are shown in Supplementary Figure S2. Genetic ancestry can affect the validity of polygenic score analyses, because the base rate of specific SNPs can vary across populations. To control for this one needs to quantify genetic ancestry. Therefore, a principal component analysis (PCA by EIGENSOFT 7.2.1) was performed based on the BATSS and two reference datasets — HapMap Phase III (HapMap3), representing individuals with genetic ancestry from Asia, Africa and Europe, and SweGen, representing the genetic ancestry of the Swedish population (Ameur et al., Reference Ameur, Dahlberg, Olason, Vezzi, Karlsson, Martin, Viklund, Kähäri, Lundin, Che, Thutkawkorapin, Eisfeldt, Lampa, Dahlberg, Hagberg, Jareborg, Liljedahl, Jonasson, Johansson and Gyllensten2017; Gibbs et al., Reference Gibbs, Belmont, Hardenbol, Willis, Yu, Yang, Ch’ang, Huang, Liu, Shen, Tam, Tsui, Waye, Wong, Zeng, Zhang, Chee, Galver, Kruglyak and Tanaka2003) — to analyze population stratification of our twin sample in comparison to the reference datasets (using R3.6.3). Visual inspection of the results of the PCA confirmed that the sample was largely homogenous in terms of Swedish/European ancestry, with only a minority of mixed genetic ancestry or non-European genetic ancestry (for comparison, see questionnaire-based data in Table 1).
Discussion
The main aim of this article was to describe the BATSS study, focusing on its methodology, feasibility and participant characteristics. As reviewed in the introduction, while previous research studies have included infant twins, very few studies have taken a multimethod approach, and there are no well-powered twin studies employing eye tracking and EEG. Further, few twin studies of infants have included a comprehensive, longitudinal protocol spanning behavior and brain measures and covering psychological constructs from early basic perception to later language skills. By introducing eye tracking and EEG methodologies, the current study can help us understand the etiological factors behind a range of informative processes ranging from low level perception and attention to complex behaviors. The multilevel, longitudinal protocol, which includes both these novel methods and more classical assessment approaches, has the potential to provide a more complete picture of cognitive and brain development than has been possible before.
The study shows that it is possible to recruit a relatively large number of infant twin families and assess them at a steady pace within a medium-sized metropolitan area (greater Stockholm area ∼2 million people; Supplementary Figure S1). The relatively high final inclusion rate (approximately 29% of the population in the targeted area; Figure 1), despite requiring in-person visits to the lab, suggests high motivation among infant twin parents to participate in research in Sweden. It is notable that initially, nearly 50% of the target population were interested in participating (Figure 1), but some were ultimately not able due to scheduling issues or not fulfilling inclusion criteria. In addition, while 53.1% of the targeted population did not respond to our materials, this does not necessarily mean that all these families were negative to participation. Taken together, we conclude that the motivation to participate in research is very high among parents of infant twins in Sweden: At least about half of the population is interested in participation in principle. This may reflect general interest in research and an appreciation of the fact that twins are particularly valuable for science (Sweden has a strong tradition of twin research of older children and adults; e.g., Anckarsäter et al., Reference Anckarsäter, Lundstrom, Kollberg, Kerekes, Palm, Carlstrom, Långström, Magnusson, Halldner, Bölte, Gillberg, Gumpert, Råstam and Lichtenstein2011; Zagai et al., Reference Zagai, Lichtenstein, Pedersen and Magnusson2019), but it can also reflect other factors such as generous parental benefits in Sweden, which allow parents to participate with few practical or economic negative consequences.
Importantly, the study shows that it is possible to conduct multimethod assessments within cognitive neuroscience (brain-based measurements, direct behavioral assessments, questionnaires, biosamples) from two infant twins within one day. Again, this may reflect the generous parental benefits in Sweden allowing both parents to be on leave at the same time, and thus both present during testing. However, it is manageable to conduct the research with only one parent, and extra research assistants. Taken together, the BATSS study demonstrates high feasibility of comprehensive infant twin studies in other areas with similar societal and geographical characteristics.
There was a slight bias toward MZ twins in the sample (Table 1). This may reflect true differences in rates of MZ versus DZ among same-sex twins, but is likely to reflect higher interest in participating in research in MZ twin families. There was a near-equal distribution of females versus males, both in the total sample and in the separate MZ and DZ groups. While the age range (4.8−6.7 months, mean 5.5 months) is relatively narrow, we will take age into account in data analyses (e.g., use as covariate). Indeed, this age range is marked by considerable development across many domains of cognitive and brain development.
The MZ and DZ groups were similar in terms of age, parental education, income, psychiatric family history, and region or country of birth of parents. Because twin analyses build on comparisons between MZ and DZ twins, it simplifies analysis and interpretation that these groups do not differ on background variables. Further, MZ and DZ groups were similar in terms of gestational age and frequency of C-section. MZ twins were around 200 g lighter at birth compared to DZ twins (p = .001). While this difference was marginal at the descriptive level, it could be important to include birth weight as a covariate in analyses.
Compared to the mean of the general Stockholm population, the sample had a higher average level of formal education and somewhat higher average income (Table 3). This difference is consistent with studies of older twins in Sweden (e.g., Taylor et al., Reference Taylor, Rosenqvist, Larsson, Gillberg, D’Onofrio, Lichtenstein and Lundström2020) and should be taken into account in any findings from the study. Little is known about how socio-economic status may moderate heritability estimates in early infancy (for an example of this phenomenon in young children, see Turkheimer et al., Reference Turkheimer, Haley, Waldron, d’Onofrio and Gottesman2003), but as with all twin samples, the results should be considered in light of the sample characteristics.
Within the framework of the classical twin design, both univariate and multivariate analyses will be conducted. Given the young age of the participants, it is of particular interest to compare heritability and estimates of environmental influence for the different types of measures, to understand which aspects of development are under relatively higher versus lower genetic and environmental influence. Further, extending the classic twin design to include measures of the environment (such as the parent’s behavior during parent–child interaction) will also help us understand to what extent the child’s genetic predispositions affect their social environment (e.g., DiLalla & Bishop, Reference DiLalla and Bishop1996; Kennedy et al., Reference Kennedy, D’Onofrio, Quinn, Bölte, Lichtenstein and Falck-Ytter2017). It is also of primary interest to understand the genetic landscape across different phenotypes (multivariate twin modelling) to understand to what extent development within and across domains is influenced by a set of shared versus unique genetic and environmental factors. Ultimately, it will also be beneficial, to both the behavioral genetic and development science fields, to account for our longitudinal design in multivariate models to study genetic and environmental continuity and change in cognitive and brain development across the first few years of life.
In addition to comparing similarity in MZ versus DZ twins (classic twin analyses), we will be able to capitalize on MZ differences within the sample, based on the logic that if MZ twins are different, it must reflect differences in nonshared environment (but see Jonsson et al., Reference Jonsson, Magnusdottir, Eggertsson, Stefansson, Arnadottir, Eiriksson, Zink, Helgason, Jonsdottir, Gylfason, Jonasdottir, Jonasdottir, Beyter, Steingrimsdottir, Norddahl, Magnusson, Masson, Halldorsson, Thorsteinsdottir and Stefansson2021). This design can be powerful in identifying (or falsifying) potential causal pathways linked to environmental exposures. For example, research has shown atypical concentration of certain metals in the teeth of children with autism already in infancy (Arora et al., Reference Arora, Reichenberg, Willfors, Austin, Gennings, Berggren, Lichtenstein, Anckarsäter, Tammimies and Bölte2017). We can use similar analyses to check whether any differences in metal concentration in nails or hair in MZ twins correlate with differences in development within MZ pairs.
The sample has been genotyped and polygenic scores have been calculated for all individuals who provided DNA samples and who passed our quality control stages. Therefore, our study is in a position to be able to contribute to an understanding of how the common genetic architecture in neurodevelopmental conditions, such as autism and ADHD, and traits and conditions that affect people in later life, such as educational attainment and schizophrenia, influence cognitive and brain development in the first months of life, long before these conditions or traits have emerged (for a recent example of this approach in infancy research, see Gui et al., Reference Gui, Mason, Gliga, Hendry, Begum Ali, Pasco, Shephard, Curtis, Charman, Johnson, Meaburn and Jones2020). Polygenic score analyses will be conducted in the sample, and we hope the sample can also be used more broadly to advance understanding of the genetic basis of neurodevelopmental, cognitive and psychiatric phenotypes. At present, molecular genetic research on infancy lags far behind research on older samples (Papageorgiou & Ronald, Reference Papageorgiou and Ronald2013, Reference Papageorgiou and Ronald2017); the potential to work with the genetic data within the sample represents another opportunity afforded by the BATSS dataset.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/thg.2021.34.
Acknowledgments
The authors thank all participating families, as well as research assistants Joy Hättestrand, Johanna Kronqvist, Sofia Jönsson, Anna Kernell, Carolin Schreiner, Sophie Lingö, Angelinn Liljebäck, Isabelle Enedahl, Matthis Andreasson, Lisa Belfrage, Mattias Savallampi, Isabelle Ocklind and Hjalmar Nobel Norrman. The authors thank Professor Paul Lichtenstein for general support.
Author Contribution
Conceptualization: TFY with contributions from AR, SB, KT; Methodology: TFY, KT, AR, MT, SB, LW; Formal analysis: LH, KT, DL, CW, IH; Data curation: TFY, LH; Investigation: MS, LH, LM; Writing (original draft): TFY with contributions from AR, MS, AMP, LH, CW; Writing (review and editing): all authors; Supervision: TFY with contributions from AR, MT. Visualization: DL, LH, AMP; Project administration: TFY with contributions from LH, MS; Funding acquisition: TFY, AR, KT, SB.
Financial Support
The work leading to these results were supported by Stiftelsen Riksbankens Jubileumsfond (NHS14-1802:1; Pro Futura Scientia [in collaboration with SCAS]); the Swedish Research Council (2018-06232); Knut and Alice Wallenberg foundation; and the European Union (Initial Training Network BRAINVIEW; 642996). LT and DL were supported by the Swedish Foundation for Strategic Research FFL18-0104 and China Scholarship Council. We acknowledge the KI Biobank for handling the biological samples, SNP&SEQ Technology platform at Uppsala University for genotyping and the Swedish National Infrastructure for Computing (SNIC) at UPPMAX, partially funded by the Swedish Research Council through grant agreement no. 2018-05973” for computations.
Conflict of Interest
The authors declare no conflict of interest related to this article. SB discloses that he has in the last 3 years acted as an author, consultant or lecturer for Medice and Roche. He receives royalties for textbooks, diagnostic and intervention tools from Hogrefe Publishers.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.