Calcium plays an important role in bone health. Many people use supplemental calcium because they do not secure adequate amounts from diet alone. However, ingesting excess supplemental calcium disturbs iron, magnesium, phosphorus and zinc metabolism(Reference McDowell1). We previously reported that an AIN-93G-based diet containing 2·5 % calcium induced moderate iron deficiency, increased hepatic copper accumulation and decreased renal copper in rats. The high-calcium diet did not have any significant effect on calcium concentrations in the plasma and the tissues including the kidney(Reference Takasugi, Matsui and Yano2). Some studies support the finding that excess calcium increased hepatic copper and decreased renal copper in rats(Reference Koba, Matsui and Yano3–Reference Kimura, Matumura, Hatsuda, Teophanides and Anastassopoulou5). These reports indicate that excess calcium disturbed copper metabolism. Copper absorbed from the intestine is mainly deposited in the liver and then supplied to other tissues(Reference Linder, Wooten and Cerveza6). Saari(Reference Saari7) reported that renal copper concentration decreases in response to copper status even with a marginal copper deficiency. Therefore, increased accumulation of hepatic copper may suppress supply to other tissues in rats given excess calcium and result in a reduction of renal copper concentration.
Several studies have shown that calcium interferes with iron absorption in rats(Reference Amine and Hegsted8–Reference Wienk, Marx and Lemmens10) and man(Reference Dawson-Hughes, Seligson and Hughes11–Reference Whiting18). Furthermore, a high-calcium diet induces iron deficiency in rats(Reference Takasugi, Matsui and Yano2, Reference Prather and Miller19). Many researchers reported that iron deficiency increases hepatic copper concentration(Reference Johnson and Gratzek20–Reference Yokoi, Kimura and Itokawa23). Copper is released from the liver by biliary excretion and efflux to blood with ceruloplasmin. Tran et al. (Reference Tran, Ashraf and Jones24) reported that iron deficiency decreases ceruloplasmin activity and plasma copper concentration and suggested that iron deficiency suppresses the hepatic efflux of the copper–ceruloplasmin complex. Therefore, a secondary iron deficiency may increase hepatic copper concentration by interfering with copper efflux to blood in animals given excess calcium.
We investigated the effect of iron supplementation on the tissue distribution of copper in rats given a high-calcium diet. We also measured copper concentrations in plasma and bile, and plasma ceruloplasmin activity in rats given the high-calcium diet. The present experiment suggested that iron deficiency, induced by excess calcium, increased hepatic copper concentration by suppressing copper transport into circulation without affecting its biliary excretion. Furthermore, the secondary iron deficiency was not related to a decrease in renal copper concentration.
Experimental methods
Animals and diets
Twenty-eight 4-week-old male Wistar rats were purchased from SLC Japan (Shizuoka, Japan) and cared for according to the Guide for the Care and Use of Laboratory Animals (Animal Care Committee, Kyoto University). The rats were individually housed in stainless steel cages in a temperature-, humidity- and light-controlled room (24°C, 60 %, 12 h light–dark cycle). All rats were fed the AIN-93G diet during a 1-week adaptation period. The animals were divided into four groups of seven rats: the control group given the AIN-93G diet (0·5 % calcium) and three groups given a high-calcium diet (2·5 % calcium) without iron supplementation (HCa), with a low level of iron supplementation (LFe) or with a high level of iron supplementation (HFe) for 4 weeks. Calcium carbonate was added to the AIN-93G diet at the expense of maize starch in the high-calcium diet (Table 1). All groups were allowed free access to their respective experimental diets and demineralised water. Wienk et al. (Reference Wienk, Marx and Lemmens10) reported that high calcium carbonate reduced whole-body retention of iron by 185 μg/animal per d. Based on these results, we estimated that an intraperitoneal injection of 1–2 mg iron/animal per week for 4 weeks could recover the iron insufficiency induced by the high-calcium diet. Thus, the LFe and HFe groups were intraperitoneally injected with 1 and 2 mg iron/animal in the form of iron dextran (ferric hydroxide dextran complex; Sigma, St Louis, MO, USA), respectively, at 1-week intervals from day 4 of the experimental period. The control and the HCa groups were injected with physiological saline in the same way. We measured feed intake daily and body weight gain weekly during the experimental period. The bile sample was collected at the end of the experiment with an indwelling catheter (22G; Terumo, Tokyo, Japan) under sodium pentobarbital anaesthesia (Somnopentyl; Pitman-Moore, NJ, USA). After the collection of bile samples, a blood sample was obtained from the abdominal aorta with a heparinized plastic syringe and a heparinized needle. The haematocrit was measured by centrifugation of the blood in a capillary tube (VC-HO75H; Terumo, Tokyo, Japan). The plasma samples were separated by centrifugation at 2500 g for 30 min at 4°C. The testis, kidney, liver and spleen were collected. The plasma, bile and tissue samples were stored at − 20°C until analysis.
Table 1 Composition of the experimental diets

* Containing 0·5 % calcium.
† Containing 2·5 % calcium.
‡ AIN-93G mineral premix.
§ AIN-93 vitamin premix.
∥ Calcium carbonate was added at the expense of maize starch.
Determination of calcium, iron and copper concentrations
The mineral analyses were done on fresh weight tissues. The tissue, bile and plasma samples were transferred into nitric acid-washed tubes with trace element-grade concentrated nitric acid and perchloric acid (Wako Chemicals, Osaka, Japan). These samples were wet-ashed at 180°C on a heating block until decoloration. After the acid was evaporated, the ash was completely dissolved in 0·1 mol/l nitric acid at 80°C. For mineral determinations, the sample solutions were diluted with 0·1 mol/l nitric acid to appropriate concentrations. For calcium determination, the final dilution was made in a 28·5 mmol/l strontium chloride solution. The calcium, iron and copper concentrations in these sample solutions were measured with an atomic absorption spectrophotometer (AA-6600F; Shimadzu, Kyoto, Japan). Bovine liver (1577B, National Institute of Standards and Technology) was wet-ashed and analysed for mineral concentrations to verify accuracy. Analysed values for calcium, iron and copper fell within the respective certified ranges for the bovine liver reference material.
Determination of ceruloplasmin activity
The plasma ceruloplasmin activity was measured with p-phenylenediamine as a substrate according to the method of Rice(Reference Rice25). In brief, 1·0 ml substrate solution (containing 9·2 mmol/l p-phenylenediamine in 0·1 mol/l acetate buffer, pH 5·2) was added to 0·1 ml of each plasma sample and mixed. The mixture was incubated at 37°C for 15 min and the reaction was terminated by the addition of 5·0 ml 0·02 % (w/v) sodium azide solution. Thereafter, the absorbance (A) was determined at 540 nm with a spectrophotometer (U-2000A; Hitachi, Tokyo, Japan). Each plasma sample was also added to the substrate solution after the addition of the sodium azide solution and the absorbance (B) was determined at 540 nm. The absorptivity at 540 nm of the pigment produced by the oxidation of p-phenylenediamine is 1·910. The plasma ceruloplasmin activity (international units) was expressed as μmol pigment/min per litre plasma under a specified condition. Therefore, plasma ceruloplasmin activity (U/l) was calculated as (A − B) × 1000/(1·91 × 15 × 0·1).
Statistics
Data are expressed as mean values with their standard errors. One-way ANOVA and the post-hoc Tukey's studentized range test were used to detect significant differences among the groups using the General Linear Model procedure of SAS (SAS Institute Inc., Cary, NC, USA). Differences were considered significant at P < 0·05.
Results
Feed intake and body weight gain
Feed intake did not differ among the groups (Table 2). Body weight gain and feed efficiency (weight gain/feed intake) were significantly (P < 0·05) lower in the HCa group than in the control group. Iron supplementation did not restore body weight and feed efficiency in rats given the high-calcium diet.
Table 2 Effects of iron supplementation on feed intake and body weight gain in rats given excess calcium*
(Mean values with their standard errors for seven rats per group)
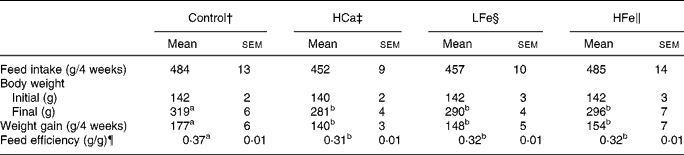
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* For details of procedures and diets, see Experimental methods and Table 1.
† Rats given a control diet containing 0·5 % calcium and injected with physiological saline weekly.
‡ Rats given a diet containing 2·5 % calcium and injected with physiological saline weekly.
§ Rats given a diet containing 2·5 % calcium and injected with 1 mg iron as iron dextran weekly.
∥ Rats given a diet containing 2·5 % calcium and injected with 2 mg iron as iron dextran weekly.
¶ Feed efficiency (g/g) = weight gain (g)/feed intake (g).
Calcium concentration in plasma and tissues
The calcium concentration in plasma, liver, kidney, testis and spleen did not differ between the HCa and control groups (Table 3). Iron supplementation did not affect the calcium concentration in plasma and tissues other than kidney. The calcium concentration in the kidney was significantly (P < 0·05) higher in the LFe group than in the control and HFe groups.
Table 3 Effects of iron supplementation on haematocrit, plasma ceruloplasmin activity and mineral concentrations in several tissues of rats given excess calcium*
(Mean values with their standard errors for seven rats per group)

a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* For details of procedures and diets, see Experimental methods and Table 1.
† Rats given a control diet containing 0·5 % calcium and injected with physiological saline weekly.
‡ Rats given a diet containing 2·5 % calcium and injected with physiological saline weekly.
§ Rats given a diet containing 2·5 % calcium and injected with 1 mg iron as iron dextran weekly.
∥ Rats given a diet containing 2·5 % calcium and injected with 2 mg iron as iron dextran weekly.
¶ Values given on a fresh weight basis.
Haematocrit and iron concentration in tissues
The HCa group had significantly (P < 0·05) lower haematocrit than the control group (Table 3). The haematocrit did not differ among the control and the iron-supplemented groups given the high-calcium diet. The HCa group had a significantly (P < 0·05) lower (approximately 50–70 % of control) iron concentration in the liver, kidney and testis than the control group.
The iron concentration in the tissues other than the spleen did not differ among the control and LFe groups. Moreover, the iron concentration in the spleen was significantly (P < 0·05) higher in the LFe group than in the control group. The HFe group had a significantly (P < 0·05) higher iron concentration in the liver, testis and spleen than the other groups, but the iron concentration in the kidney did not differ among the control, LFe and HFe groups.
Copper concentration in plasma, bile and tissues, and plasma ceruloplasmin activity
The HCa group had significantly (P < 0·05) lower plasma and renal copper concentrations and significantly (P < 0·05) higher hepatic copper concentration than the control group (Table 3). The copper concentration in the plasma and the liver did not differ among the control, LFe and HFe groups. However, renal copper concentration was significantly (P < 0·05) lower in the LFe and HFe groups than in the control group. The copper concentration in the testis, spleen and bile did not differ among the groups. The plasma ceruloplasmin activity was significantly (P < 0·05) lower in the HCa group than in the control group. The ceruloplasmin activity did not differ among the control and iron-supplemented groups given the high-calcium diet.
Discussion
The present experiment indicated that the high-calcium diet did not affect the calcium concentration in the plasma and tissues, which was consistent with the findings of our previous study(Reference Takasugi, Matsui and Yano2). These results suggested that calcium homeostasis was maintained even in rats fed with the diet containing calcium at a level 5-fold greater than its requirement. Miura et al. (Reference Miura, Matsuzaki and Suzuki26) reported that a high-calcium diet containing 1·5 % calcium markedly increases faecal and urinary calcium excretion and has no effect on the calcium concentration in the serum and the kidney. It appears that faecal and urinary calcium excretion was markedly increased in the rats given the high-calcium diet, which resulted in the maintenance of body calcium levels.
Excess calcium is well known to suppress the availability of iron in rats(Reference Takasugi, Matsui and Yano2, Reference Koba, Matsui and Yano3, Reference Amine and Hegsted8–Reference Wienk, Marx and Lemmens10, Reference Prather and Miller19) and man(Reference Dawson-Hughes, Seligson and Hughes11–Reference Whiting18). The present experiment also suggested that the high-calcium diet decreased iron availability. Especially, the hepatic iron concentration in the HCa group was approximately one-half of that observed in the control group. Some researchers(Reference Kaganda, Matsuo and Suzuki27–Reference Strube, Beard and Ross29) also reported that hepatic iron concentration in iron-deficient rats is approximately one-half of that observed in rats fed a normal diet. Haematoctrit also decreased in the HCa group. Therefore, we assumed that the HCa group had a moderate iron deficiency.
The purpose of the present study was to examine whether the disruption of copper metabolism induced by excess calcium resulted from a secondary iron deficiency. Therefore, we investigated whether iron supplementation ameliorated the disruption of copper metabolism induced by a high-calcium diet. It was assumed that oral administration of iron would interact with other minerals in the gastrointestinal tract. Thus, iron was administered intraperitoneally. The lower dose of iron supplementation restored iron concentrations in most tissues, whereas the high level of iron supplementation increased iron concentrations in most tissues. The present results suggested that iron supplementation ameliorates iron deficiency induced by excess calcium.
The high-calcium diet decreased body weight gain and feed efficiency, which was not restored by iron supplementation. The present results suggest that the suppression of weight gain and feed efficiency does not result from a secondary iron deficiency. Goto & Sawamura(Reference Goto and Sawamura30) reported that excess calcium reduces the absorption of fat and protein. Therefore, excess calcium may suppress weight gain and feed efficiency by interfering with the absorption of these nutrients.
In agreement with previous reports(Reference Takasugi, Matsui and Yano2, Reference Koba, Matsui and Yano3, Reference Kimura, Matumura, Hatsuda, Teophanides and Anastassopoulou5), the high-calcium diet did not affect the copper concentration in the testis and spleen but increased copper concentration in the liver when the animals were not supplied with iron. However, iron supplementation restored the high concentration of hepatic copper induced by the high-calcium diet, suggesting that secondary iron deficiency stimulated the hepatic copper accumulation in rats given the high-calcium diet.
Biliary excretion of copper is quantitatively the most important excretory route(Reference Hambidge31). We found that a high-calcium diet and iron supplementation did not affect biliary copper concentration. Yu et al. (Reference Yu, West and Beynen32) reported that an iron deficiency increases biliary and hepatic copper concentration. The iron deficiency induced by excess calcium may be moderate compared with dietary iron deficiency in the report of Yu et al. (Reference Yu, West and Beynen32). The present results indicate that excess calcium-induced high copper accumulation in the liver does not result from a reduction of biliary copper excretion.
Ceruloplasmin is a serum α-2 glycoprotein that binds more than 95 % of the total copper found in plasma(Reference Takahashi, Ortel and Putnam33). This protein binds to six atoms of copper in the liver and is secreted into circulation as a holoprotein with oxidase activity(Reference Sato and Gitlin34). The high-calcium diet decreased the ceruloplasmin activity and the plasma copper concentration, which was restored by iron supplementation. Meyer et al. (Reference Meyer, Durley and Prohaska35) reported that hepatic copper content markedly increases in aceruloplasminaemic mice without affecting copper absorption and biliary copper excretion. This increase is also observed in aceruloplasminaemic human subjects(Reference Morita, Ikeda and Yamamoto36). Tran et al. (Reference Tran, Ashraf and Jones24) reported that iron deficiency decreases ceruloplasmin activity and plasma copper concentration. These results suggest that secondary iron deficiency induced by excess calcium suppresses copper efflux from the liver to circulation in the form of ceruloplasmin complex, which effectively increases copper accumulation in the liver. Tran et al. (Reference Tran, Ashraf and Jones24) also indicated that the iron deficiency does not affect the expression of ceruloplasmin mRNA in the liver nor ceruloplasmin protein concentration in the plasma but increases its activity in the plasma. It is not clarified how iron deficiency prevents the efflux of copper–ceruloplasmin complex. However, high intake of ascorbate reduced the ceruloplasmin activity in the plasma of man without changing ceruloplasmin protein concentration(Reference Jacob, Skala and Omaye37). Therefore, the changes in redox state may decrease the efflux of copper as ceruloplasmin complex from the liver in the iron-deficient rats induced by excess calcium.
Copper is capable of generating hydroxyl radicals and thereby increases oxidative stress(Reference Gunther, Hanna and Mason38). In patients with Wilson's disease(Reference Ferenci39) and the Long-Evans Cinnamon rat(Reference Terada and Sugiyama40), a model of Wilson's disease, excessive hepatic copper accumulation results in increased oxidative stress. Although the hepatic copper accumulation observed in the rats fed with excess calcium was lower than that reported in the Long-Evans Cinnamon rats(Reference Terada and Sugiyama40), the hepatic copper accumulation induced by excess calcium might result in increased oxidative stress.
The present experiment showed that the high-calcium diet decreased copper concentration in the kidney but not in the other tissues, and iron supplementation did not restore the low kidney copper concentration. Although ceruloplasmin is the major copper-containing protein in the plasma(Reference Takahashi, Ortel and Putnam33), this protein is unlikely to transport copper from the liver to other tissues as aceruloplasminaemic mice show normal copper concentrations in tissues other than the liver(Reference Meyer, Durley and Prohaska35). Owen(Reference Owen41) reported that copper deficiency severely decreases copper concentration in the spleen more than in the kidney. Because the reduction of copper concentration was observed in the kidney, but not in the other tissues, including the spleen of the rats given excess calcium, we suggest that the reduction of renal copper was not due to a decrease in copper supplied from the liver. Additionally, iron deficiency increases hepatic copper concentration but does not affect copper concentration in the kidney, spleen and plasma(Reference Yu, West and Beynen32). Therefore, we can assume that excess calcium directly affects the kidney and decreases copper concentration in the kidney.
To our knowledge, there are no studies that have reported the effect of excess dietary calcium on copper homeostasis in man; however, excess dietary calcium is well known to inhibit iron absorption in man(Reference Dawson-Hughes, Seligson and Hughes11–Reference Whiting18). Therefore, secondary iron deficiency induced by high supplemental calcium may disturb copper homeostasis also in man. The changes in copper may result in increased oxidative stress that could have pathological consequences. A detailed clinical examination of the effects of excess calcium in man will help clarify this effect on copper homeostasis.
In conclusion, the present experiment suggested that iron deficiency induced by excess calcium increased hepatic copper concentration by suppressing the hepatic release of copper into the circulation without affecting its biliary excretion. The secondary iron deficiency was not related to a decrease in the renal copper concentration in rats given excess calcium.
Acknowledgements
The authors have no conflict of interest in this paper. No financial support has been received for the study. S. T. and T. M. contributed equally to the present study and H. Y. provided valuable advice concerning the present study.





