INTRODUCTION
Leptospirosis is the most widespread zoonosis in the world but incidence of this disease is higher in tropical areas than in temperate countries [Reference Everard1]. Leptospires are bacteria belonging to the family Leptospiraceae, order Spirochaetales. These spirochetes are about 0·1 μm in diameter and 6–20 μm in length [Reference Faine2]. The genus Leptospira includes saprophytic (L. biflexa sensu lato) and pathogenic (L. interrogans sensu lato) bacteria [Reference Morey3] and the serological classification allows discrimination between more than 260 serovars of L. interrogans. Serovars that are antigenically related are grouped into serogroups but this classification is now challenged by a taxonomically more relevant genomic classification which distinguishes 13 pathogenic genomospecies [Reference Adler and de la Peña Moctezuma4]. Human infection most often occurs when mucous membranes or abraded skin are exposed to infected animal urine, contaminated water or soil, or infected animal tissue [Reference Faine2]. Many wild and domestic animals species have been identified as hosts of infecting leptospiral organisms and are able to maintain the leptospires in their kidneys and become chronic carriers, shedding the organisms in their urine [Reference Bahaman and Ibrahim5]. Therefore, although the organism has been recovered from rats, swine, dogs, cattle, and numerous wild animals [Reference Levett6], micromammals (particularly rats) remain the main chronic renal carriers of leptospires [Reference Pereira and Andrade7–Reference Collares-Pereira9].
We choose to present a limited number of tropical insular areas, selected according to three criteria: (i) island located in the tropics, (ii) land surface of <20 000 km2 and (iii) availability of published data on animal leptospirosis. Thus, this review deals with the following islands: Barbados, Martinique, Guadeloupe, Grenada and Trinidad in the Caribbean Sea; New Caledonia, Hawaii and French Polynesia in the Pacific Ocean; and La Réunion and Mayotte in the Indian Ocean (Table 1) [10–Reference Everard and Green13]. This review presents data on leptospirosis by island and by animal species chronologically (Tables 2 and 3) [Reference Gargominy14–Reference Wikramanayake, Dinerstein and Loucks24]. This data-gathering can be considered as a tool for those who work on leptospirosis in tropical islands. Knowledge on the animal reservoirs of Leptospira allows a better understanding of the epidemiology of the disease in these areas and also facilitates finding practical applications for control of the disease in humans.
Table 1. Presentation of the islands
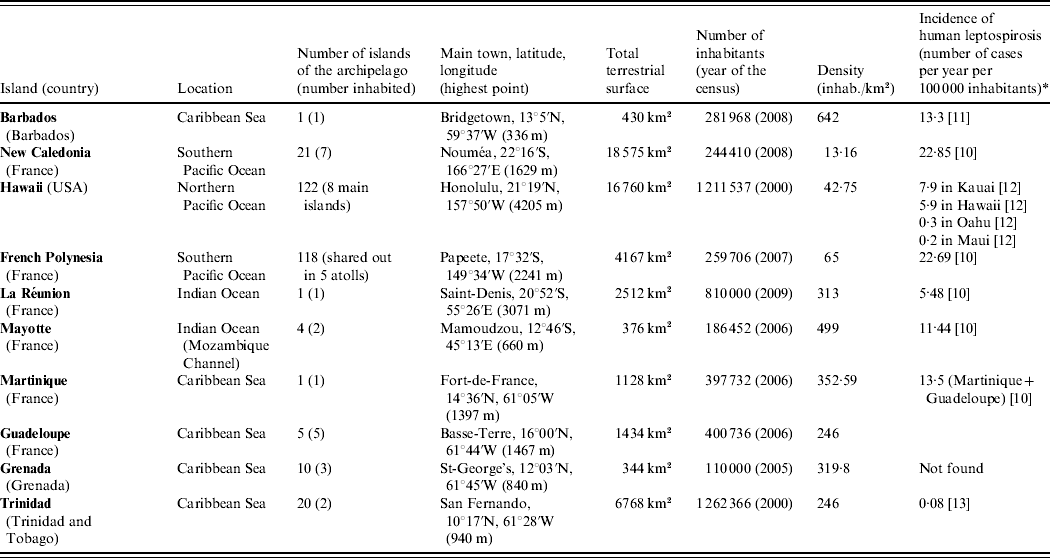
* Registered cases only.
Table 2. Insular repartition of the animal cited (class, order, family, Latin name and common name) and existence of studies on leptospirosis by species and island

• Presence of the species on the island.
* Presence of data on leptospirosis for this species in the island concerned.
Table 3. Main animal species studied for leptospirosis in the considered islands and results

ND, No data.
Absent, Species not present on this island.
All the areas described are tropical islands with a land surface area <20 000 km2. In these regions the climate has two contrasting seasons: a cool and dry season and a hot rainy season. Rainfall on the islands is principally orographic (mountain caused), with the resulting annual rainfall distribution closely following the topographic contours: amounts are greatest over the upper slopes and least on the leeward coast. Geologically, except for Barbados, all these islands are totally or partially of volcanic origin. Because of their small surface area and their isolation, for a given biogeographical area, islands have less species richness per surface unit than the mainland [Reference MacArthur and Wilson25]. Moreover, animal populations are often small because of the limited surface area which reduces the capacity of housing. Each tropical island has its own fauna, but all are characterized by a high density of invasive rodents of the family Muridae [Reference Lorvelec26, Reference Courchamp, Chapuis and Pascal27], rats (Rattus sp.) or mice (Mus musculus) [Reference Soubeyran22, Reference Stone, Stone and Stone23, Reference Howald28]. Several hunting or wild species have also been introduced by humans [Reference Lorvelec, Pascal and Pavis20, Reference Soubeyran22, Reference Pascal, Beauvais, Coléno and Jourdan29] and domestic animals (dogs, cats) and livestock (cattle, goats, pigs, sheep, horses) are present in all the islands [Reference Soubeyran22]. Except for Trinidad, which has a huge animal biodiversity, bats (order Chiroptera) represent generally the only endemic or indigenous terrestrial mammalian species of these ecosystems. On each island only a small part of the fauna has been studied for leptospirosis (Table 2) [15, 16, Reference Long18–Reference Stone, Stone and Stone23, Reference O'Connell30–Reference Germano32]. In this review, we use the species taxonomic level in its Linnean designation. In consequence, domestic animals or wild animals born of domestic forms, have the same Latin name as the wild ancestral species [33].
Two methods are commonly used to investigate leptospirosis in animals: the microscopic agglutination test (MAT) and the culture in a specific medium. The MAT is the gold standard test and is the one most utilized for the serological diagnosis of leptospirosis [34]. It is based on the use of agglutinating specific antisera and cross-absorption with homologous antigens. Authors can give the results of the MAT at the serogroup or at the serovar level. Serogroups corresponding to the serovars cited in this paper are given in Table 4 [Reference Faine2]. One limitation is that serological results depend on the number of serovars included in the panel [34], but another limitation of the MAT is the difficulty in setting a threshold of positivity which can range from 1:10 to 1:800, according to the authors and the location of the study [Reference Kazami35–Reference Kuriakose39]. In contrast, in vitro culture of Leptospira from kidney, blood or urine allows the serotyping of the isolated strains with certainty [Reference Wuthiekanun40] but this method is lengthy, of low sensitivity and notably limited by contaminants outgrowth.
Table 4. Relation between serovars cited in the text and serogroups (from [ Reference Faine2])

BARBADOS
Micromammals and mongooses
A study conducted during 1964–1965 [Reference Taylor, Turner and Everard41] on Rattus sp. in Barbados showed that 33% (32/98) of R. rattus and 35% (48/138) of R. norvegicus were seropositive for leptospirosis by MAT. In 1986–1987 and 1994–1995, Levett et al. [Reference Levett42] isolated leptospires by culture of kidneys, urine or blood from 19% (12/63) and 16% (16/100) of rats, respectively. In these studies, the prevalence of renal infection was higher in R. norvegicus than in R. rattus [Reference Taylor, Turner and Everard41, Reference Levett42], with 27% (37/138) and 15% (15/98) testing positive, respectively [Reference Taylor, Turner and Everard41]. Isolates identified in Rattus were serovars copenhageni (serogroup Icterohaemorrhagiae), arborea (Ballum) and bim (Autumnalis). R. norvegicus carried mostly leptospires from serogroup Icterohaemorrhagiae, whereas serogroup Autumnalis was mainly found in R. rattus [Reference Taylor, Turner and Everard41].
In 2002, Matthias & Levett [Reference Matthias and Levett21] showed that 28·2% (24/85) of mice (Mus musculus) and 40·7% (48/118) of mongooses (Herpestes auropunctatus) in Barbados had antibodies against Leptospira sp. In mice, the prevailing serovars assessed by serology (MAT) were arborea (Ballum) and bim (Autumnalis), whereas in mongooses the dominant serogroup was Autumnalis.
Primates
A survey conducted on a wild population of vervet monkeys Chlorocebus (Cercopithecus) aethiops revealed a seroprevalence to Leptospira of 29·9% (150/501). Serogroups identified were Ballum (61%), Icterohaemorrhagiae (16%), Autumnalis (15%), Pyrogenes, Panama, Pomona and Canicola (8% combined) [Reference Baulu, Everard and Everard43].
Amphibians
Everard & Gravekamp [Reference Everard44–Reference Everard46] showed that amphibians were carriers of leptospires and two pathogenic strains were grown from kidneys of toads Bufo marinus (family Bufonidae) and frogs Eleutherodactylus johnstonei (family Leptodactylidae). The most prevalent strain in amphibians was L. noguchii serovar bajan (Australis) [Reference Gravekamp45, Reference Everard46], followed by serovar bim (Autumnalis) [Reference Everard44–Reference Everard46].
Domestic carnivores
A serological survey showed that 62% (48/78) of asymptomatic (stray or domestic) dogs had a positive MAT titre, with the dominant serogroup being Autumnalis (45%), followed by serogroups Icterohaemorrhagiae and Australis (16% each), then Pomona (13%). However, in dogs presenting clinical signs of leptospirosis, the prevailing serogroup was Icterohaemorrhagiae [Reference Weekes, Everard and Levett47]. In this study, Leptospira grown from dogs' kidneys were principally serovars copenhageni (Icterohaemorrhagiae) and bim (Autumnalis) [Reference Weekes, Everard and Levett47, Reference Jones48].
Livestock
Levett et al. [Reference Levett, Whittington and Camus49] showed that 4·3% of sheep (1/23) and 9·3% of goats (4/43) were seropositive for leptospirosis and antibodies against serogroup Cynopteri were identified in both species [Reference Levett, Whittington and Camus49].
MARTINIQUE
Domestic carnivores
A serosurvey conducted in Martinique on dogs showed that the seroprevalence against leptospires was 76% (219/288) [Reference André-Fontaine50].
Livestock
Levett et al. [Reference Levett, Whittington and Camus49] showed that 25·7% (45/175) of cattle were seropositive for leptospirosis and that Sejroe was the most prevalent serogroup (44·4% of the positives), followed by Icterohaemorrhagiae (24·4%) and Autumnalis (17·7%) [Reference Levett, Whittington and Camus49]. In pigs, the seroprevalence was 39% (110/282), with a predominance of serogroups Icterohaemorrhagiae and Sejroe, followed by Australis and Cynopteri [Reference André-Fontaine50].
GUADELOUPE
Micromammals and wild carnivores (mongooses and racoons)
Michel [Reference Michel51] observed the renal carriage of the bacteria Leptospira in 16·6% (2/12), 36·8% (14/38) and 57·1% (8/14) of R. norvegicus, R. rattus and mice, respectively. MAT tests showed that seroprevalences in the racoon and the mongoose were similar with 48% (354/737) and 47% (8/17) positive, respectively [Reference Michel51, Reference Michel, Branger and Andre-Fontaine52]. The serovar arborea (Ballum) was predominantly found in kidneys of mice [Reference Michel51], while serogroup Icterohaemorrhagiae was isolated from R. rattus, and serogroups Icterohaemorrhagiae, Sejroe and Australis were isolated from mongooses [Reference Michel51, Reference Michel, Branger and Andre-Fontaine52].
Domestic carnivores
A recent MAT survey showed that 78·3% (83/106) of the Guadelupian dogs were seropositive against Leptospira [Reference André-Fontaine50].
Livestock
In 1973–1974, the dominant serogroup in cattle in Guadeloupe was Ballum (prevalence not shown) and the other serogroups found in cattle were Icterohaemorrhagiae, Bataviae, Australis, Pomona, and Sejroe [Reference Tissot, Mailloux and Corroller53]. A serosurvey in 2002–2003 showed that 14% (29/205) of cattle were serologically positive against Leptospira [Reference André-Fontaine50].
Levett et al. [Reference Levett, Whittington and Camus49] showed that 6·4% (13/203) of goats were seropositive for leptospirosis and Autumnalis, Cynopteri and Sejroe were identified as the infecting serogroups [Reference Levett, Whittington and Camus49].
A serological study in 27 pig farms in the 1990s in Guadeloupe showed that 93% of swine were positive [Reference Levillain54] but this seroprevalence fell to 35% (141/403) in 2002–2003 [Reference André-Fontaine50].
Equines
In 2002–2003, 61% (74/121) of horses were serologically positive against Leptospira [Reference André-Fontaine50].
GRENADA
Micromammals and mongooses
Utilizing kidney culture, Everard et al. [Reference Everard19] showed the renal carriage of serovar copenhageni (Icterohaemorrhagiae) in R. norvegicus, while serovars copenhageni (Icterohaemorrhagiae) and ballum (Ballum) were cultured from kidneys of R. rattus.
In 1971–1972 and in 1983, two serosurveys showed that 35% (152/432) [Reference Everard and Green13] to 36% (71/200) [Reference Everard19] of the Grenadian mongooses were seropositive by MAT and three serogroups were identified: Icterohaemorrhagiae was the dominant serogroup [Reference Everard and Green13, Reference Everard19] representing 37·5% (57/152) of the positives, then Pomona in 21·1% (32/152) of the positives and Canicola in 6·6% (10/152) of the positives [Reference Everard and Green13]. Leptospires were isolated from kidneys in 5·3% (10/190) of the mongooses and serovars copenhageni (Icterohaemorrhagiae), brasiliensis (Bataviae) and atchafalaya (Tarassovi) were identified [Reference Everard19].
Bats
In bats of the family Phyllostomidae, 8% (4/52) of Glossophaga sp. were found positive for leptospirosis, while 21% (13/61) of positives were found in Anoura sp. (13/61) [Reference Everard19]. Of the 121 cultures of bat kidneys none gave a positive result [Reference Everard19].
Amphibians
Everard et al. [Reference Everard19] reported 15% (10/66) seropositive in the toad B. marinus. Serovars navet (Tarassovi) and peruviana (Australis) were cultured from kidneys in two of these animals.
Livestock
Everard et al. [Reference Everard55] found 25% (80/324) of cattle to be seropositive for leptospirosis and Icterohaemorrhagiae was the dominant serogroup (28%), followed by Autumnalis (24%) and Hebdomadis and related serogroups Sejroe and Mini (12%) [Reference Everard55]. They also reported that 35% (45/130) of Grenadian pigs tested were seropositive, of which 35% were against serogroup Autumnalis and 32% against Icterohaemorrhagiae [Reference Everard55]. In sheep, 17% (18/108) were seropositive and Autumnalis was the predominant serogroup (33% of the positive sera). In goats, seroprevalence of leptospirosis was of 25% (11/44) and the dominating serogroup was Pyrogenes [Reference Everard55].
Chickens
Everard et al. [Reference Everard55] reported that 11% (19/175) of chickens were seropositive by MAT and antibodies found were mainly against serogroups Hebdomadis (42% of the positives) and Shermani (32%).
TRINIDAD
Micromammals and mongooses
Everard et al. [Reference Everard19] showed that 16% (5/32) of R. rattus were seropositive by MAT, while 43% (3/7) of R. norvegicus and 29% (2/7) of mice were seropositive. In Rattus sp. antibodies detected were directed against serogroups Icterohaemorrhagiae, Autumnalis, Hebdomadis and Javanica, while in mice, these authors found antibodies against Icterohaemorrhagiae only. Serovar copenhageni (Icterohaemorrhagiae) was isolated from the kidney of R. norvegicus and R. rattus, whereas serovars ballum (Ballum) and lanka (Louisiana) were isolated from kidneys of R. rattus only [Reference Everard19]. Everard et al. also showed that in the family Muridae, 24% (4/17) of the scaly-footed water rat Nectomys squamipes and 29% (2/7) of the rice rat Oryzomys capito were serologically positive. Twenty-five per cent (1/4) of the Trinidad spiny pocket mice Heteromys anomalus (family Heteromyidae) tested were positive. No antibodies against leptospires were found in Coues's climbing mouse Rhipidomys couesi (family Cricetidae) (0/2), nor the northern grass mouse Necromys urichi (family Cricetidae) (0/1) or the cane mouse Zygodontomys brevicauda (family Muridae) (0/1) [Reference Everard19].
In 1976, the proportion of seropositive Trinidadian mongooses ranged between 33·3% and 51·1% [Reference Everard and Green13], whereas in 1983, 48% (17/37) of the mongooses sampled were seropositive [Reference Everard19]. In both studies, MAT results showed that serogroup Canicola predominated in this species [Reference Everard and Green13, Reference Everard19], but Icterohaemorrhagiae and Pomona were also encountered [Reference Everard and Green13]. Canicola strains were isolated from the kidneys of mongooses [Reference Everard and Green13, Reference Everard19], with an infectivity rate of 4·7% (5/106) [Reference Everard and Green13].
Bats
On the eight species of bats caught by Everard et al. [Reference Everard19], four presented a seropositive result with the MAT method: Carollia perspicillata (family Phyllostomidae), with 11% (2/19) seropositive; Phyllostomus hastatus (family Phillostomidae), with 27% (13/48) seropositive; Pteronotus davyi (family Mormoopidae), with 13% (2/15) seropositive and Molossus major (family Molossidae) with 25% (5/20) seropositive. Serogroups identified in bats were: Autumnalis, Hebdomadis, Javanica, Panama, Pyrogenes, Tarassovi, and Cynopteri [Reference Everard19].
Didelphimorphia
Everard et al. [Reference Everard19] showed that in the order Didelphimorphia, 5% (1/22) of the black-eared opossums Didelphis marsupialis (family Didelphidae) and 4% (5/73) of the the murine opossums Marmosa mitis (=M. robinsoni, family Didelphidae) were found seropositive. Seven per cent (1/14) of the white-eared opossums Caluromys philander (family Caluromyidae) were seropositive. Serovars lanka (Louisiana) and ballum (Ballum) were cultured from kidneys of M. mitis and serovar ballum (Ballum) was isolated from C. philander. Serological research of leptospiral antibodies was negative in Marmosa fuscata (fuscatus) but renal cultures revealed the presence of serovar lanka (Louisiana) in this species [Reference Everard19].
Primates
Leptospiral antibodies were researched in Cebus sp. (family Cebidae) but revealed as negative [Reference Everard19].
Squamates and amphibians
Forty-two per cent (5/12) of the gold tegus Tupinambis nigropunctatus (order Squamata, family Teiidae) sampled were found positive by MAT, while all the lizards Ameiva ameiva (family Teiidae) (4/4) and all the iguanas Iguana iguana (family Iguanidae) (1/1) caught were seropositive [Reference Everard19]. Everard et al. [Reference Everard19] showed that 25% (20/80) of the marine toads B. marinus were seropositive but none (0/2) of the lesser tree frogs Hyla minuta (order Anura, family Hylidae) tested positive. Serovar autumnalis (Autumnalis) was isolated from the marine toad [Reference Everard19].
Domestic carnivores
In 1979, serological data reported that at least 55% of the stray dogs had been exposed to leptospires as opposed to only 12·5% of the cats. Agglutinins against serogroups Canicola, Icterohaemorrhagiae and Hebdomadis were found most frequently in these species [Reference Everard56]. Twenty per cent (10/50) of the sampled dogs carried leptospires in their kidneys [Reference Everard56]. Serovars isolated in dogs were portlandvere (Canicola), canicola (Canicola), copenhageni (Icterohaemorrhagiae) and georgia (Hebdomadis), whereas serovar canicola was isolated from one cat. A seroepidemiological survey was conducted in 2005 in different populations of Trinidadian dogs [Reference Adesiyun57]: among house dogs 7·7% (5/65) of the non-vaccinated animals were seropositive. The prevalence was the highest among hunting dogs with 25·5% (12/47) positive, while 20·4% (10/49) and 4·4% (5/113) of the farm and stray dogs, respectively, were seropositive. In the population of dogs suspected of leptospirosis, 48% (24/50) were seropositive. Nine serovars of L. interrogans were identified in this species. The most prevalent serovar was mankarso (Icterohaemorrhagiae), in 47·5% of the seropositive dogs (29/61). The other serovars were icterohaemorrhagiae RGA (Icterohaemorrhagiae 32·8%, 20/61), autumnalis (Autumnalis 41%, 25/61), copenhageni (Icterohaemorrhagiae 16·4%, 10/61), bratislava (Australis 13·1%, 8/61), georgia (Hebdomadis), ballum (Ballum) and wolffi (Sejroe) (1·6% each, 1/61) [Reference Adesiyun57].
Livestock
In 1985, MAT results reported that 92% (24/26) of cattle were seropositive with serogroup Hebdomadis predominating [Reference Everard55]. All of the ten ‘bufflypso’ (water buffaloes, Bubalus bubalis) tested were positive and the prevailing serogroup in these animals was Grippotyphosa [Reference Everard55]. In 2009, a larger study reported that 14·6% (33/226) of the water buffaloes were seropositive [Reference Adesiyun58].
Among swine, it was shown that 52% (64/122) of the sampled animals were serologically positive with 56% and 29% of those seropositive having antibodies against serogroups Icterohaemorrhagiae and Autumnalis, respectively [Reference Everard55].
Equines
MAT results showed that 76% (66/87) of horses and donkeys were seropositive [Reference Everard55]. Panama was the most frequently reported serogroup (23% of positive animals), followed by Icterohaemorrhagiae (15%), Canicola and Hebdomadis (9% each) [Reference Everard55].
Poultry and wild birds
Everard et al. [Reference Everard55] showed that 11% (16/144) of the chickens tested had a positive serological reaction against Leptospira. Fifty per cent of the reactions were against serogroup Shermani, while 25% were against serogroup Hebdomadis. Eight ducks and geese were also tested but were negative. No leptospiral antibodies were found in the American black vulture Coragyps atratus [Reference Everard55].
NEW CALEDONIA
Micromammals
In 1985–1986, a study based on culture showed that 61·1% (11/18) of rats (R. rattus, R. norvegicus, R. exulans) excreted leptospires in their urine [Reference Brethes59]. A complementary study identified the leptospires shed in urine of rats as belonging to serogroups Icterohaemorrhagiae and Canicola [Reference Brethes60].
Domestic carnivores
In 1985–1986, Brethes et al. [Reference Brethes60] reported that 59·25% (48/81) of canids in New Caledonia were seropositive, of which 39·6% (19/48) had antibodies against serogroup Icterohaemorrhagiae. In the particular area of Bourail (a ‘hot-spot’ of human leptospirosis in New Caledonia), 63% (29/46) of dogs were seropositive, of which 55% (16/29) were against Icterohaemorrhagiae. Predominance of serogroup Icterohaemorrhagiae in canids was confirmed in 1999 by the Laboratoire Territorial de Diagnostic Vétérinaire (LTDV) whose results reported serological evidence of a high circulation of serogroup Canicola in dogs [61].
Livestock
All cattle sampled (15 animals) in the area of Bourail in 1985–1986 were positive by MAT [Reference Brethes60]. A subsequent survey in 1990 on the entire New Caledonian cattle assessed the seroprevalence at 58·3% (204/350), with 74·6% (85/114) of the surveyed herds having at least one positive animal [Reference Thevenon62]. Serogroups Sejroe, Tarassovi and Pomona were circulating in New Caledonian cattle [Reference Brethes60, Reference Thevenon62], with a prevalence of 59·3%, 19·6% and 7·8% among the positive animals, respectively [Reference Thevenon62]. In 2007, the annual report of the LTDV confirmed the predominance of serovars hardjo (Sejroe) and sejroe (Sejroe) in cattle [10].
In 1985–1986, 58·3% (21/36) of pigs were found to be seropositive for leptospirosis. By MAT, sera reacted principally against serogroup Pomona and secondly against Icterohaemorrhagiae [Reference Brethes59].
Antibodies against serovar hardjo (Sejroe) were found in the Rusa deer [Reference Brethes60].
Equines
In 1983, MAT results showed that the dominant serogroups in horses in New Caledonia were Canicola and Pomona [Reference Domenech and Lechapt63]. In 1986, the dominant serogroup was Icterohaemorrhagiae: 17/18 of the horses sampled in the area of Bourail were seropositive, of which nine were against Icterohaemorrhagiae [Reference Brethes60]. Icterohaemorrhagiae was still prevailing in horses in 1996 [64]. However, since 1996, inclusion of serogroups Pyrogenes and Hurtsbridge in the MAT panel of strains demonstrated the high circulation of these serogroups in positive horses, with a frequency of 29·3% and 18·8%, respectively, in 1996 [64]; 18·7% and 43·2%, respectively, in 1998 [65]; and 52·3% and 32·1%, respectively, in 1999 [61].
A serological survey conducted on the donkeys of Maré (Loyalty Islands) in 1999 proved that 97% (38/39) of the sampled animals had antibodies against Leptospira. The dominant serogroups were Hurtsbridge and Pyrogenes [61].
HAWAII
Micromammals and mongooses
In the 1950s and 1960s, the study of Wallace et al. [Reference Wallace, Gross and Lee66] on Hawaiian rats R. norvegicus, R. rattus, R. hawaiiensis (=R. exulans), mice and mongooses reported that 45% (558/1238) of these mammals had antibodies against Leptospira. A survey conducted between 1959 and 1961 on 1281 mammals (same species as cited above) [Reference Minette67] showed that mice and R. norvegicus populations were highly infected, with respectively 66·7% (26/39) and 32·4% (165/510) seropositive by MAT and 79·5% (31/39) and 60·2% (307/510) renal carriers. They were followed by the mongoose with 28·6% (36/126) seropositive, and 14·3% (18/126) renal carriers. The serological prevalence in R. rattus was lower with 19·7% (72/366) seropositive contrasting with the high rate of renal carriage (43·7%, 160/366) in this species [Reference Minette67]. Cultures of kidney tissues proved the renal carriage of serovar icterohaemorrhagiae (Icterohaemorrhagiae) in all the species [Reference Wallace, Gross and Lee66, Reference Minette67]. Serovar ballum (Ballum) was only recovered in R. rattus [Reference Wallace, Gross and Lee66]. One isolate of the serogroup Australis was obtained in R. norvegicus, while serogroups Canicola and Sejroe were isolated from the mongoose only [Reference Minette67]. Another survey was conducted in Hawaii between 1969 and 1973 on 2982 animals of the same species [Reference Shimizu68] and the following seroprevalences were found: 34·0% (419/1234) positive in R. rattus, 61·4% (137/223) in R. norvegicus, 17·8% (166/932) in R. exulans, 43·2% (41/95) in M. musculus and 28·8% (136/473) in H. auropunctatus [Reference Shimizu68]. Cultures of kidneys showed that serogroup Icterohaemorrhagiae was predominant in R. norvegicus (91·4% of positives, 85/93), while 58·7% (24/41) of the identified cultures recovered from mice were from serogroup Ballum and 59·7% (43/72) of those recovered from mongooses were from serogroup Sejroe. Serogroups Icterohaemorrhgiae and Ballum were isolated from all rat species and mice, but not from mongooses, while Sejroe was isolated only from mongooses [Reference Shimizu68].
Marine mammals
A serological study on the endemic monk seals of Hawaii Monachus schauinslandi (order Carnivora, family Phocidae) showed that leptospirosis was circulating in this population and that monk seals had positive titres against serovars bratislava (Australis), hardjo (Sejroe), icterohaemorrhagiae (Icterohaemorrhagiae) and pomona (Pomona) [Reference Aguirre69].
Livestock
Serovars hardjo (Sejroe) and bataviae (Bataviae) were identified by MAT in cattle on Kauai island in 1987 [Reference Katz, Manea and Sasaki70].
FRENCH POLYNESIA
Livestock
In 1988, Raust [Reference Raust71] published the results of a serological survey showing that 15·5% (23/148) of dairy cattle were seropositive and that the dominant serovar was hardjo (Sejroe) in 43% of those positive, followed by serovar tarassovi (Tarassovi) in 14% and serovar sejroe (Sejroe) in 10%. A health control conducted in 1997 in cattle confirmed the results of 1988 [72].
In 1988, 32% (37/115) [Reference Raust71] to 39% (140/360) [65] of pigs were seropositive by MAT. Both studies reported icterohaemorrhagiae (Icterohaemorrhagiae) as the most prevalent serovar in this species (22·6% of positive pigs for the former, 96% for the latter). The first study also identified pomona (Pomona, 18%), bratislava (Australis, 16·7%), canicola (Canicola, 10·8%), cynopteri (Cynopteri, 9·9%) and autumnalis (Autumnalis, 7·1%) as circulating serovars in pigs [Reference Raust71].
Equines
Only five horses were tested during the survey of Raust in 1988 [Reference Raust71], and all were seropositive. Serovars pomona (Pomona), australis (Australis) and icterohaemorrhagiae (Icterohaemorrhagiae) were identified in this species.
LA RÉUNION
Micromammals
In 2007, a serological survey on tenrecs Tenrec ecaudatus (order Lipotyphla, family Tenrecidae) showed a seroprevalence of 92% (34/37) in this species with all sera predominantly reacting against serogroup Icterohaemorrhagiae [Reference Sigaud73].
Domestic carnivores
Two serosurveys conducted in a dog pound in 1977–1979 [Reference Moutou74] and 1978–1983 [Reference Mollaret, Mailloux and Debarbat75] showed that 40% (58/142 and 60/150, respectively) of the stray dogs were seropositive by MAT. In the former study, serogroups Canicola and Icterohaemorrhagiae were found in 69% (40/58) and 26% (15/58), respectively, of the seropositive dogs [Reference Moutou74] while in the latter study 16% of those seropositive had antibodies against Icterohaemorrhagiae [Reference Mollaret, Mailloux and Debarbat75].
Livestock
In 1978–1979, two simultaneous serological studies showed similar results with 29% (452/1582) [Reference Debarbat, Mollaret and Mailloux76] and 32% (337/1063) [Reference Moutou74] of cattle having a positive serological titre. Serogroups Hebdomadis and Sejroe each represented 25% of the seropositive reactions [Reference Moutou74, Reference Debarbat, Mollaret and Mailloux76], serogroup Icterohaemorrhagiae accounted for 12–13% [Reference Mollaret, Mailloux and Debarbat75, Reference Gares77], Pomona 12% [Reference Debarbat, Mollaret and Mailloux76], Autumnalis 10–12% [Reference Moutou74, Reference Debarbat, Mollaret and Mailloux76], Ballum 5% [Reference Debarbat, Mollaret and Mailloux76], Australis, Bataviae and Grippotyphosa 4·5% each, and Canicola 0·5% [Reference Debarbat, Mollaret and Mailloux76]. In La Réunion, serogroups Sejroe and Hebdomadis were recognized as a major cause of abortion in dairy cattle [Reference Gares77].
A sampling conducted in 1979 at a slaughter-house revealed a limited circulation of leptospires in swine, with 5% (3/57) of pigs seropositive and circulation of serogroups Autumnalis and Hebdomadis [Reference Moutou74]. Currently, field data indicate a high seroprevalence rate in reproduction swine: a serological follow-up of 13 pig farms between 2001 and 2008 showed that each year 6–29% of the tested sera were positive (Dr P. André, personal communication).
Equines
At the end of the 1970s, there were four riding schools in La Réunion, accounting for about 150 horses. In this equine population, 10–20 cases of leptospirosis occurred throughout the year [Reference Gares77]. In 1979, two serological surveys [Reference Moutou74, Reference Debarbat, Mollaret and Mailloux76] revealed that 69% (100/145) to 71% (121/171) of the horses were seropositive. Eleven different serogroups were serologically identified in horses and the predominant serogroup was Autumnalis (30–34% of positive reactions), while Icterohaemorrhagiae was found in 14–18% of positive animals [Reference Moutou74, Reference Debarbat, Mollaret and Mailloux76]. In 1983, Mollaret et al. [Reference Mollaret, Mailloux and Debarbat75] confirmed that 12% of horses were serologically reactive against serogroup Icterohaemorrhagiae. Moutou [Reference Moutou74] pointed out that the prevailing serogroup differed among the riding school of origin: Icterohaemorrhagiae in the riding school of St-Denis, Australis in the riding schools of St-Gilles and Tampon, Ballum in horses of Bras Panon. Nevertheless, in 1990, following a clinical outbreak of leptospirosis in the riding school of Tampon, 22 horses were tested. All were seropositive for Icterohaemorrhagiae (Dr A. Michault, personal communication). Thus if leptospirosis is highly prevalent in horses in La Réunion without systematic clinical expression of the disease, serogroup Icterohaemorrhagiae could be responsible for clinical outbreaks.
MAYOTTE
Micromammals and wild fauna
The 19 rats sampled in 1991 were all seronegative by MAT [Reference Charton78].
In the same year, the circulation of serovar hardjo (Sejroe) was shown in two out of ten tenrecs T. ecaudatus and in the only fruit bat Pteropus seychellensis (order Chiroptera, family Pteropodidae) caught. Antibodies against serogroup Pyrogenes and serovar wolffi (Sejroe) were also found in the tenrec, while antibodies against serovar icterohaemorrhagiae (Icterohaemorrhagiae) were found in small Indian civets Viverricula indica (order Carnivora, family Viverridae) [Reference Charton78].
Domestic carnivores
MAT results showed the circulation of serovar icterohaemorrhagiae (Icterohaemorrhagiae) in dogs [Reference Charton78].
Livestock
At the beginning of the 1990s, zebus, goats and dogs were highly infected, with 85% (34/40), 70% (7/10) and 83% (5/6) seropositive, respectively. In zebus, serovars identified by MAT were canicola (Canicola), grippotyphosa (Grippotyphosa), sejroe (Sejroe), each accounting for 23·5% of the seropositives, then ballum (Ballum, 11·7%), Pyrogenes (8·8%), wolffi (Sejroe, 5·8%) and australis (Australis, 2·9%). In goats, serovars were icterohaemorrhagiae, wolffi (each accounting for 28·6% of seropositives), canicola, ballum and grippotyphosa (14·3% each) [Reference Charton78].
DISCUSSION
Origin of the serovars
Introduction of animal species in a region induces introduction of simultaneous pathogens. So, originally, the presence of leptospiral serovars circulating on each island was linked with the history of the human colonization and the shipping importations of animals by the Europeans [Reference Lorvelec, Pascal and Pavis20, Reference Howald28, Reference Matisoo-Smith79]. Nevertheless, serovars circulating on a colonized island are different from those of the colonizing country. Even if no study has compared mainland and tropical islands, we know that serovars carried by rats, mice and hedgehogs (Erinaceus europaeus) in New Zealand are not the same as those carried by the same species in Great Britain, the country from where they were imported during colonization [Reference Hathaway, Blackmore and Marshall80]. Thus, the few serogroups of leptospires circulating in animals on an island are specific to the animals which have colonized the island and could maintain themselves in this typical environment. Serovars present on tropical islands are generally circulating worldwide but each island represents a unique ecosystem, the limited panel of serovars found in each insular area is absolutely island specific.
The case of vaccinated animals
The most commercially available vaccines against leptospirosis are for dogs and are directed against serogroups Icterohaemorrhagiae and Canicola. Consequently, the presence of seropositive domestic dogs [Reference André-Fontaine50] and the presence of both these serogroups in high proportions in populations of healthy dogs [Reference Brethes60, 61] could be partly explained by the vaccination measures currently practised in the majority of the presented islands. Nevertheless, a study in Trinidad showed that vaccination did not have any significant effect on Leptospira infection as similar prevalence of infections were detected for both vaccinated (5·3%) and non-vaccinated dogs (7·7%) [Reference Adesiyun57]. Moreover, Hathaway et al. [Reference Hathaway, Blackmore and Marshall80] showed that agglutinins induced by the vaccine disappear within weeks of administration [Reference Klaasen81]. Consequently, the seropositive dogs detected in the different studies were essentially due to exposure to field serovars of Leptospira sp.
Carrier state and immune response
In Hawaii the rate of renal infection in R. norvegicus, R. rattus and M. musculus is significantly higher than the serological prevalence in each species [Reference Minette67]. The same observation was reported in the rodent population of Terceira Island (Azores) [Reference Collares-Pereira9] and in R. norvegicus caught in Brazil [Reference Pereira and Andrade7, Reference Tucunduva de Faria38]. Duration of immunity is not known in field rats, but after infection in carrier animals, leptospires are subsequently cleared from all organs except the renal tubules [Reference Athanazio82]. Thus, in the absence of re-infection, carrier animals may be serologically negative, thus the carrier state may not be detected in MAT-positive animals. In contrast, other studies showed that the serological prevalence in rats is higher than the renal carriage [Reference Vanasco8]. Consequently, serology is often not clear, as MAT-negative bacteriologically proved carriers may be encountered [Reference Faine2].
Diversity of hosts and serovars in insular areas
In insular areas of volcanic origin like La Réunion, Mayotte, Hawaii, Martinique, Guadeloupe and French Polynesia, the mammalian diversity is generally poor and leptospires have a limited choice in mammalian hosts compared to the larger choice offered by continental countries like Guyana [Reference Matthias83], Peru [Reference Bunnel84], Brazil [Reference Lilenbaum85], or larger islands, e.g. New Zealand [Reference Hathaway, Blackmore and Marshall80] or Australia [Reference Milner86]. In consequence, bacteria concentrate themselves in abundant species, susceptible but generally non-sensitive, living most frequently in an anthropic environment, and which are perfect to play the role of reservoir and spreader of bacteria. On these islands, this role is played most frequently by alien species, e.g. rats and mice, or even mongooses and dogs.
Almost all knowledge on leptospirosis is related to infection in mammals but the finding of Leptospira in amphibians and reptiles [Reference Gravekamp45, Reference Everard46], which live in moist or wet environments, and birds [Reference Everard55], leads to questions about the role of these species, if any, in the carriage and maintenance of foci of leptospirosis.
Comparison with mainland
The seroprevalence of leptospirosis in animals seems to be higher in small islands than in mainland or larger islands but the number of circulating serovars is lower. In fact, the diversity of serovars in a region may be correlated on the one hand directly with the faunistic diversity of the area (number of potential hosts) and on the other with its environmental diversity [Reference Vanasco8]. For example, in Australia, which can be considered as the nearest ‘mainland’ from New Caledonia, the prevalence of leptopsirosis in the dog population is 1·9% (18/956) [Reference Zwijnenberg87], which is markedly inferior to the prevalence in New Caledonian dogs (59·25%) [Reference Brethes60]. Nevertheless, although only two serovars are described in the New Caledonian canids [Reference Brethes60, 61], 11 are found in Australian dogs [Reference Zwijnenberg87]. An other example can be found in Trinidad which has a greater mammal species diversity (about 100 mammalian species) than the neighbouring island of Grenada (15 mammalian species): 80 isolates of L. interrogans were reported in Trinidad to infect humans, domestic and wild animals, and only 20 were reported in Grenada [Reference Everard88]. The hypothesis is reinforced by the situation in the temperate Azorean islands (North Atlantic ocean) where three serovars are described in the four rodents and insectivorous mammal species present, while six serovars are counted among the 21 micromammals in Portugal [Reference Michel51, Reference Collares-Pereira89].
Adaptation of the serovars to insular ecosystems
When a serovar is introduced within a new ecosystem, it finds an ecological niche that may be different from the one it uses in its native environment. Indeed, one animal species, living in two different countries/islands within two different ecosystems, may offer two distinct ecological niches for leptospires [Reference Hathaway, Blackmore and Marshall80, Reference Martiny90]. Generally, in a geographical region an equilibrium is established in which there is an ‘adaptation’ of a serogroup to a reservoir species [Reference Adler and de la Peña Moctezuma4, Reference Moutou74]. Thus, the Indian mongoose (H. auropunctatus) is considered as a reservoir for serogroups Sejroe, Icterohaemorrhagiae and Canicola in Hawaii [Reference Minette67, Reference Shimizu68], serogroup Sejroe in Oahu island [Reference Higa and Fujinaka91], serogroups Icterohaemorrhagiae, Sejroe and Australis in Guadeloupe [Reference Michel51, Reference Michel, Branger and Andre-Fontaine52], serogroup Canicola in Trinidad [Reference Everard and Green13, Reference Everard19] and serovars copenhageni (Icterohaemorrhagiae), atchafalaya (Tarassovi) and brasiliensis (Bataviae) in Grenada [Reference Everard19]. Moreover, in La Réunion, Moutou [Reference Moutou74] reported that the dominant serogroup identified by serology in horses differed according to the riding school in which the animals lived, i.e. according to the geographical zone of the island.
Lastly, it should be noted that phenomena of speciation by adaptation to a particular host in a small biotope can lead to the appearance of new serovars, e.g. serovar bim (Autumnalis) in dogs on Barbados [Reference Jones48] or atchafalaya (Tarassovi) in Grenadian mongooses [Reference Everard19], or even serovar lanka (Louisiana) in Trinidad [Reference Everard19].
Evolution of seroprevalence
Few studies report a follow-up of the seroprevalence of leptospirosis in animal species. A survey was conducted between 1959 and 1961 on five species of wild mammals in Hawaii [Reference Minette67] and another survey on the same species was conducted between 1969 and 1973 [Reference Shimizu68] (see earlier results): comparison between the two studies shows that (i) serogroups of Leptospira sp. isolated by culture in each animal species were the same but the relative distribution of the serovars per species was different and (ii) the serogroup Sejroe was emergent in the mongoose. Furthermore, the respective densities of the rodents and mongooses have changed in Hawaii, with an increase of the populations of R. rattus, R. exulans and mongooses, while the populations of R. norvegicus and mice decreased. In consequence, although in the 1960s R. norvegicus and the mouse were the main reservoirs of leptospires in Hawaii, in 1973 R. rattus represented the main bacterial reservoir. Therefore, the epidemiology of the disease had changed in Hawaii, switching from a peridomestic animal reservoir (R. norvegicus and mouse) to a more rural reservoir (R. rattus and mongoose) [Reference Shimizu68].
Different examples show that the seroprevalence of leptospirosis in one species seems to be quite stable over time. In 1971–1972 and in 1983, two serosurveys proved that the seroprevalence assessed by MAT in mongooses in Grenada did not evolve over 10 years with a prevalence of 35% [Reference Everard and Green13] and 36%, respectively [Reference Everard19]. Moreover, prevalence of antibodies did not change much in Trinidadian mongooses over 6 years, with 33·3–51·1% seropositive in 1976 [Reference Everard and Green13], whereas in 1983, 48% of the mongooses sampled were seropositive [Reference Everard19]. In La Réunion two serosurveys conducted in the same dog pound at two distinct periods (1977–1979 [Reference Moutou74] and 1978–1983 [Reference Mollaret, Mailloux and Debarbat75]) showed that 40% (58/142 and 60/150, respectively) of the stray dogs were seropositive by MAT. Similarly for French Polynesia the seroprevalence in cattle did not evolve between 1988 and 1997 [65].
Nevertheless, an exception can be found in the population of pigs in La Réunion in which the seroprevalence seemed to increase significantly over 30 years going from 5% of pigs seropositive in 1979 to 6–29% at 7 years follow-up conducted between 2001 and 2008. Three hypothesis can be put forward: (i) the survey of Moutou [Reference Moutou74] underestimated the prevalence of the disease in swine, either because of a too small sample size or because the animals sampled were too young; (ii) the disease has greatly evolved in La Réunion, with a ‘burst’ occurring during the last 30 years; (iii) changes in the methods of farming, going from small family pig farms to battery industrial breeding farms could have induced an evolution in the prevalence of leptospirosis in pigs. Thus, higher animal density could favour the maintenance and transmission of the disease inside farms, and the gathering of fattening animals born in different reproductive farms, or in a growing farm could favour the spread of the disease between sites. Moreover the seroprevalence and consequences of the disease are different when considering breeding sows or grower animals [Reference Boqvist92].
Meteorological factors
In tropical regions, high rainfall is the main climatic factor of maintenance of leptospires in the environment and of their transmission to exposed animals and humans [Reference Yanagihara93, Reference Tassinari94]. A survey conducted in Hawaii between 1969 and 1973 showed that the seroprevalence rates in rodents and mongooses were higher on the Eastern coast (where rainfall is high) than on the Western part of the island [Reference Shimizu68]. In North America, a statistical positive correlation was also demonstrated between prevalence of infection in dogs and rainfall [Reference Ward95]. Moreover, in 2002–2003 and 2003–2004 the Caribbean region had two successive years of the El Niño phenomenon, which resulted in an increase in rainfall and probably in a proliferation of rodents which modified the epidemiology of human leptospirosis in Guadeloupe. In consequence, not only was there an increase in the total number of human cases observed in this island, but also the number of cases due to serogroup Ballum, a mouse-associated serogroup [Reference Vanasco8, Reference Collares-Pereira9] increased [Reference Hermann Storck96].
Nevertheless, cyclones do not appear to be linked with an increase in the number of human cases in La Réunion (Dr A. Michault, personal communication), nor in Guadeloupe [Reference Hermann Storck96]. It is likely that these intense climatic phenomena are responsible for the leaching of the environmental reservoirs and the destruction of the habitats of the micromammals considered as reservoirs [Reference Hermann Storck96].
CONCLUSION
This paper reviews the current knowledge on animal leptospirosis in small tropical islands and shows that the specificity of the host–serovar relation is greatly dependent of a specific insular ecosystem. However, the interpretation of the serological results and comparison between islands might be hazardous for two main reasons: (i) data are mainly stemmed from seroepidemiological surveys that include a variable number of species and individuals, and (ii) methods of analysis and thresholds of positivity differ between studies.
Nonetheless, leptospirosis appears endemic in the majority of the animal species. If the status of domestic or peri-domestic (rats, mongooses, mice) animals against leptospirosis has been well studied in insular areas, the wild fauna has been investigated less so. The interest of the scientific community in animal leptospirosis in these regions is modest thus far, and available data are often poor, mainly due to the fact that research is concentrated on the human disease. This paper stresses the need for more research in this field and highlights that studies on fauna have to be done at the island scale. Identification of the prevailing serovars and of their animal reservoirs is essential to understand the particular epidemiology of leptospirosis on each island and advise measures of prevention for humans. Furthermore, the economic cost of human and animal leptospirosis in these islands is not negligible [Reference Goarant97]. Because molecular tools are more powerful than serology and because they allow the establishment of stronger epidemiological links between strains circulating in animals and those inducing disease in humans, the use of genotyping techniques needs to be incorporated into epidemiological studies of Leptospira sp. in insular areas in order to generate more meaningful and translational data.
ACKNOWLEDGEMENTS
We are very grateful to Pierre Aubry (Emeritus Professor at the Medicine Faculty of Antananarivo, Madagascar), Margarida Collares-Pereira (Institute of Hygiene and Tropical Medicine, New University of Lisbon, Portugal) and Cyrille Goarant (Pasteur Institute, New Caledonia) for providing several cited papers. We thank François Moutou (AFSSA, Paris), Michel Pascal (INRA, Rennes), Pascale Bourhy (Centre National de Références des Leptospiroses, Pasteur Institute, Paris), Vincent Porphyre (CIRAD, La Réunion) and Paul André (Veterinarian, La Réunion) for their scientific advice, and Hélène Delatte (CIRAD, La Réunion) for support.
Political and population information on countries has been extracted from Wikipedia via the internet and is not necessarily accurate.
DECLARATION OF INTEREST
None.






