Iodine excess exists in many countries and may pose possible harm to public health. More than adequate or excessive iodine intakes have been reported in thirty-four countries worldwide( Reference Zimmermann 1 ). Excessive iodine intakes mainly result from iodised salt, seafood with a high content of iodine, iodine-rich meat and milk and iodine-rich drinking-water( Reference Zimmermann 1 ). The key health problems caused by iodine excess are hypothyroidism, thyroiditis, hyperthyroidism and goitre( Reference Hans 2 , Reference Yu, Zhu and Chen 3 ).
Goitre is an important indicator to measure the harm caused by iodine deficiency or excess( Reference Zimmermann 1 ). Palpation and measurement of thyroid volume (T vol) by ultrasound are the key means to detect thyroid goitre. Palpation is a time-honoured method to identify and classify goitre in a field investigation of iodine deficiency disorders. However, palpation has low sensitivity and specificity in areas with mild and moderate iodine deficiency( 4 ). So, thyroid ultrasound measurement, which is more objective and accurate, is preferable and widely used nowadays.
Interpretation of T vol data needs valid reference values of T vol. To date, the T vol reference values in children have been revised on a number of occasions( Reference Delange, Benker and Caron 5 – Reference Zimmermann, Molinari and Spehl 8 ). In 2004, Zimmermann et al. ( Reference Zimmermann, Hess and Molinari 9 ) proposed the latest T vol reference values by measuring the T vol of children from six long-standing iodine-sufficiency countries distributed in five continents. Thanks to the more comprehensive genetic and geographic backgrounds, moreover, the residual effect of iodine deficiency was excluded, and these reference values are proposed to be more representative and accurate and recommended by the WHO in 2007( 4 ).
In China, there is a widespread distribution of areas with excessive iodine in drinking-water in eleven provinces, affecting a population of nearly 40 million( Reference Shen, Liu and Sun 10 ). After iodine deficiency disorders were successfully managed in China, iodine excess caused by drinking-water containing high iodine content has emerged as a prominent public health issue. Hebei is one of these provinces facing this challenge. It has 173 towns with a median water iodine (MWI) above 150 μg/l, comprising a population of about 8 million( Reference Lv, Zhao and Xu 11 ). It has been shown that endemic goitre prevails in areas with a MWI above 300 μg/l in drinking-water in China( Reference Li, Liu and Qu 12 – Reference Zhao, Wang and Shang 14 ).
However, only a few studies have reported goitre prevalence in areas with mildly excessive iodine with a MWI between 150 and 300 μg/l( Reference Lv, Zhao and Xu 11 ). To date, studies on goitre status conducted in iodine-excessive areas in China have been based on the Chinese criteria for measuring T vol. The WHO criteria for measuring T vol have not been used in the evaluation of goitre caused by excess iodine. Therefore, for the first time, we employed the latest WHO criteria in an attempt to evaluate more accurately the prevalence of goitre in children. The aim of the present study was to measure the prevalence of goitre in children and to identify its possible epidemiological characteristics.
Materials and methods
Selection of high-iodine towns and a control town
Hengshui is a small city located in the southern plain of Hebei Province. It has 114 towns in which fifteen towns have been identified as high-iodine towns (HIT) with a MWI of 150–300 μg/l in a survey conducted in 2004( Reference Ma, Zhou and Jia 15 ). The fifteen HIT are located in a small geographical area where a field survey can be conducted easily. The local residents have a similar diet and lifestyle that result in few variations in terms of confounding their iodine nutrition. Therefore, Hengshui City was chosen as the appropriate place to conduct the present study. Using the random sampling method, three towns were randomly selected from the fifteen HIT to measure urinary iodine content and the prevalence of goitre in children. They were Qinghan, Fangzhuang and Miaozhen. A nearby town, Xintun, with a MWI of 105 μg/l was chosen as the control. Its demographic composition, house income, dietary habit and lifestyle are comparable with the three HIT.
Selection of children aged 8–10 years
The sample size was calculated according to the equation for simple random sampling( Reference Li, Ding and Gao 16 ). As the prevalence of goitre in children in the HIT and in the control town was assumed as 50 and 5 %, respectively, and α and δ were both set at 0·05, the minimal sample sizes for the iodine-excessive towns and the control town were 384 and 73, respectively. Only those children who lived in the four towns since birth were included. Children who migrated from other towns were excluded. In each of the four towns chosen, two to five village schools were randomly selected. From each of these schools, all children aged 8–10 years were selected. A total of 452 children aged 8–10 years who lived in twelve villages in the three HIT and 120 children living in two villages in the control town were included for measuring their thyroids in the present survey. All pupils chosen underwent T vol measurements by ultrasound on the spot. Meanwhile, in each school, more than half of the classes to which the selected pupils belong were randomly selected. All the pupils in the selected classes were asked to collect their spot urine samples. For determination of iodine content in the laboratory, a total of 326 spot urine samples were collected from children in the three HIT and sixty urine samples from children in the control town.
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Hebei Provincial Bureau of Science and Technology. Since ultrasound examination and urine sample collection are non-invasive, oral consents for thyroid measurements and urinary sample collection were obtained from the headmasters of the schools investigated. Verbal consent was witnessed and formally recorded. The survey was conducted in June 2011.
Anthropometric measurements
The exact age of each child was calculated based on the date of his/her birth and the date of survey. Height and weight were measured with standard anthropometric techniques( 17 ). Before measuring, children were asked to remove their shoes, empty their pockets and wear light indoor clothes. Height was recorded to the nearest millimetre and weight was recorded to the nearest 100 g.
Measurements of thyroid volume by ultrasound
T vol was measured by using an Aloka SSD-500 echocamera (Aloka) equipped with 7·5 MHz linear transducers. The measurement was performed while the child lay on a bed with the neck being fully exposed. For each thyroid lobe, the maximum perpendicular anteroposterior and mediolateral dimensions were measured on a transverse image of the largest diameter, without including the isthmus. The maximum craniocaudal diameter of each lobe was then measured on a longitudinal image. The thyroid capsule was not included. The ultrasound measurements were done by one experienced examiner who had specialised in thyroid measurement by ultrasound for 10 years.
T vol was calculated by using the equation of Brunn et al. ( Reference Brunn, Block and Ruf 18 ), in which the volume of each lobe (ml) is equal to anteroposterior diameter (cm) × mediolateral diameter (cm) × craniocaudal diameter (cm) × 0·479, and the lobe volumes are summed. According to the WHO criteria for thyroid measurement( 4 ), if the T vol of a child exceeds the 97th percentiles for boys or girls by age-specific or body surface area (BSA)-adjusted T vol, then the child (he/she) is judged as goitrous.
Collection of drinking-water and edible salt samples
Drinking-water and edible salt samples were collected to measure iodine content in children from the households of the fourteen villages where those investigated children had lived since birth. Because drinking-water supply was centralised (tap water) in all of these villages, two water samples were randomly collected from two households in each village. For salt sample collection, a systematic sampling method was employed according to the east, west, north, south or central region of the village. In each location, four households were randomly selected to collect edible salt samples, leading to twenty salt samples in each village.
Biological and environmental sample measurements and analyses
The analyses of iodine content in biological and environmental samples were conducted in the laboratory of Hengshui Municipal Center for Disease Control and Prevention. Iodine content in urine samples was measured by the method of ammonium persulfate oxidation( 19 ). According to the WHO, median urinary iodine concentrations (UIC) of 300 μg/l and above define a population as having excess iodine( 4 ).
Iodine content in salt samples was determined quantitatively by the titration method( 20 ). According to the Chinese national plan for iodine deficiency disorders surveillance, edible salt with iodine content less than 5 mg/kg is classified as non-iodised salt. Iodine content in drinking-water was determined by the method of arsenic–cerium oxidation–reduction spectrophotometry( 21 ).
Data processing and statistical analyses
Data processing and statistical analyses were performed using the statistical software packages Epi-InfoTM 2002 (Centers for Disease Control and Prevention) and SPSS version 13.0 (SPSS, Inc.). Since the distributions of iodine in edible salt, drinking-water and children's urine samples were not normal, the median was employed to describe their central tendency. Differences in median UIC across the three towns, sex and age group were tested by the Mann–Whitney or Kruskal–Wallis test. Prevalence was employed to indicate the magnitudes of goitre in the present study. Comparisons in the prevalence of goitre in children across age group and sex were performed using a χ2 test. A linear trend in proportion in BSA-adjusted T vol was analysed using EPI6 (Centers for Disease Control and Prevention).
Results
Iodine content in drinking-water and edible salt samples
In the three HIT, the overall MWI was found to be 246 (interquartile range (IQR) 194–308) μg/l. The coverage rates of iodised salt (the proportion of iodised salt in the whole salt samples) in the twelve villages ranged from 60 to 100 %, with an overall coverage rate for 194 of the 240 iodised salt samples being 80·8 %. The overall median salt iodine concentration was 24·1 (IQR 15·1–29·3) mg/kg. The details of MWI and iodised salt supply in the twelve villages are listed in Table 1.
Table 1 Median water iodine (MWI) and iodised salt supply in the fourteen villages investigated in Hengshui City of Hebei Province of China in June 2011 (Number of salt samples (total n 20) and percentages)
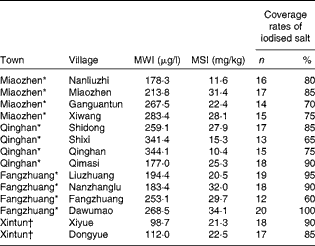
MSI, median salt iodine.
* High-iodine towns.
† Control town.
In the control town, four drinking-water samples were collected from two villages, with an overall MWI being 105 (IQR 89–122) μg/l. A total of forty edible salt samples were collected from the two villages, with the coverage rate for thirty-five iodised salt samples being 87·5 % and an overall median salt iodine concentration being 20·2 (IQR 17·9–24·2) mg/kg. The details of MWI and iodised salt supply in the two villages are listed in Table 1.
Urinary iodine content in children aged 8-10 years
In the three HIT, a total of 326 urine samples of children aged 8–10 years were collected and determined for iodine content, with an overall median UIC being 511 (IQR 340–720) μg/l. The median UIC of children in these three towns (Qinghan, Fangzhuang and Miaozhen) was 534 (IQR 325–721), 504 (IQR 349–672) and 511 (IQR 332–789) μg/l, respectively, with no significant difference being found (Kruskal–Wallis test, P= 0·641). In the control town, the median UIC of sixty urine samples from children aged 8–10 years was 401 (IQR 213–544) μg/l. The overall median UIC in the three HIT was significantly higher than that in the control town (Mann–Whitney, P= 0·017).
Of the 326 urine samples in the three HIT, 167 were from boys and 159 from girls. The median UIC in boys and girls were 526 (IQR 338–713) and 506 (IQR 312–698) μg/l, respectively, with no statistically significant difference being found (Mann–Whitney, P= 0·664). The number of urine samples collected from children aged 8, 9 and 10 years was 93, 105 and 128, and their median iodine content was 511 (IQR 349–711), 497 (IQR 315–697) and 522 (IQR 345–732) μg/l, respectively, with no statistically significant difference among them (Kruskal–Wallis test, P= 0·883).
Children's goitre by age-specific thyroid volume
A total of 452 children aged 8–10 years were examined by ultrasound in the three HIT. According to the WHO criteria for age-specific T vol, 111 of the 452 children aged 8–10 years were identified as having goitre, with its prevalence being 24·6 %. In the control town, seventeen of the 120 children aged 8–10 years were judged as goitrous, with its overall prevalence being 14·0 %, which was significantly lower than that in the three HIT (P= 0·015).
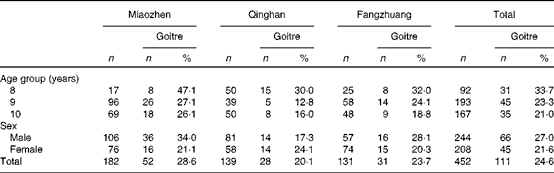
The prevalence of goitre in each of the three HIT was 28·6 % (52/182), 20·1 % (28/139), 23·7 % (31/131), respectively, but there were no significant differences among them (P= 0·078). Among all the 452 children aged 8, 9 and 10 years, the prevalences of goitre were 33·7 % (31/92), 23·3 % (45/193) and 21·0 % (35/167), respectively, with no significant difference being found (P= 0·065). The prevalences of goitre in boys and girls were 27·0 % (66/244) and 21·6 % (45/208), respectively, with no significant difference being found (P= 0·183). The details of goitre status in children across age group and sex in the three HIT are presented in Table 2.
Table 2 Children's goitre status across age group and sex by age-specific thyroid volume in the three high-iodine towns in Hengshui City of Hebei Province of China in June 2011 (Number of children and percentages)
Children's goitre by body surface area-adjusted thyroid volume
The overall prevalence of goitre by BSA-adjusted T vol in 149 of the 452 children aged 8–10 years in the three HIT was 33·0 %. In the control town, the overall prevalence of goitre in twenty-one of the 120 children was 17·5 %, which was significantly lower than that in the three HIT (P= 0·001).
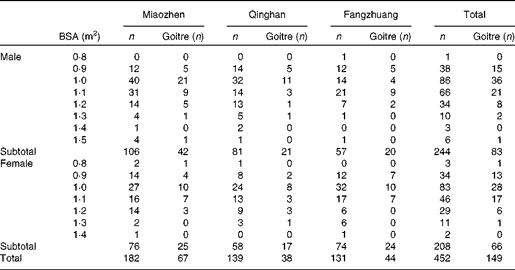
The prevalences of goitre in each of the three HIT were 36·8 % (67/182), 27·3 % (38/139) and 33·6 % (44/131), respectively, but there were no significant differences among them (P= 0·192). Among all the 467 children, the prevalences of goitre in boys and girls were 34·0 % (83/244) and 31·7 % (66/208), respectively, with no significant difference being found (P= 0·692). The details of goitre status in children across sex in the three HIT are presented in Table 3.
Table 3 Children's goitre status across sex by body surface area (BSA)-adjusted thyroid volume in the three high-iodine towns in Hengshui City of Hebei Province of China in June 2011
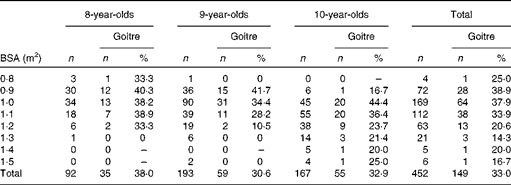
Among all the 452 children aged 8, 9 and 10 years, the prevalences of goitre were 38·0 % (35/92), 30·6 % (59/193) and 32·9 % (55/167), respectively, with no significant difference being found (P= 0·461). The prevalences of goitre in children with a BSA from 0·9 to 1·3 declined from 38·9 to 14·3 %, with a significant descending trend (P= 0·002). The details of goitre status in children across age group by BSA-adjusted T vol in the three HIT are presented in Table 4.
Table 4 Children's goitre status across age group by body surface area (BSA)-adjusted thyroid volume in the three high-iodine towns of Hengshui City of Hebei Province of China in June 2011 (Number of subjects and percentages)
Discussion
A number of studies have demonstrated that increased T vol is a frequently encountered thyroid dysfunction in response to excess iodine( Reference Li, Liu and Qu 12 , Reference Zhao, Chen and Maberly 13 , Reference Pearce, Gerber and Gootnick 22 , Reference Khan, Li and Gootnick 23 ). The cut-off value for excess iodine intakes to induce goitre remains to be unclear. Zimmermann et al. ( Reference Zimmermann, Ito and Hess 24 ) argued that a UIC ≥ 500 μg/l was associated with increasing T vol in a study on children ingesting excess amounts of iodine from coastal Hokkaido. In the present study, the median UIC of children in the three HIT was 511 μg/l, exceeding the aforementioned cut-off value, indicating that the high prevalence of goitre probably resulted from excess iodine. The prevalence of goitre in children in the three HIT was significantly higher than that in the control town, which also supported the above-mentioned argument. However, goitre is also a common manifestation of thyroiditis, which is usually diagnosed through thyroid function and anti-thyroid antibody tests( Reference Hans 2 ). A limitation of the present study is the fact that thyroid function and anti-thyroid antibody tests were not evaluated, so that the reason as to why goitre is present cannot be fully understood.
The MWI of children from the twelve villages in the three HIT reached 246 μg/l, exceeding greatly the suggested iodine content in drinking-water( 25 ). Given that the daily water consumption of local children is 1·5 litres, they can ingest 369 μg iodine from drinking-water every day, which is over threefold more than the daily iodine intake of 120 μg/l recommended by the WHO for children aged 6–12 years( 4 ). Assuming that the average salt uptake of rural residents is 8·4 g( Reference Wu, Li and Chang 26 ), the daily salt intake of a child could be assumed to be 5 g. Calculating from the overall median salt iodine concentration obtained in the present survey (i.e. 24·1 mg/kg), iodine intake of a child from edible salt would be 96 μg (after deducting 20 % loss during cooking and serving( 27 )). So, the iodine intake of a child from drinking-water was much higher than that estimated from iodised salt. Excess iodine could be mainly attributed to the high content of iodine in drinking-water.
There were no significant differences found in the prevalence of goitre in children among the three HIT. This is consistent with the results that the three HIT had a similar MWI and children in the three HIT had a similar median UIC. However, in the present study, the overall prevalence of goitre by age-specific T vol in children aged 8–10 years was 24·6 %. This estimate was higher than those reported by many other similar studies conducted in areas with a MWI between 150 and 300 μg/l in China, for instance 8·9 % by palpation in Henan Province( Reference Wang, Zheng and Wang 28 ), 13·1 % by ultrasound in Shanxi Province( Reference Zhang, Jia and Wang 29 ) and 12·7 % by ultrasound in Inner Mongolia( Reference Zhang, Fan and Guo 30 ).
The discrepancy in the prevalences of goitre between the present study and others can be accounted for by four reasons. First, the employed criteria for measuring T vol in these studies were different. The upper limits of T vol by the WHO employed in the present study were much lower than those applied in other studies in China( 31 ). So, the WHO criteria could identify more goitre cases and result in a higher prevalence of goitre. Second, palpation is less sensitive than ultrasound in detecting goitre, which could lead to a lower prevalence of goitre than that detected by ultrasound. Third, goitrogens present in the food of local residents may play some role in aggravating the prevalence of goitre in children. Goitre can be aggravated by goitrogens, such as glucosinolates, cyanogenic glucosides and flavonoids, present in some foods( 4 ). These goitrogens exist in the staple food consumed by the local residents, which could aggravate the prevalence of goitre in children. Fourth, the WHO criteria for measuring T vol may not be suitable for Chinese children. The study that applied the WHO criteria contained a broad genetic background, including children from five continents. In that study, although Japanese girls had UIC distributions that were comparable with American girls, they had much higher age-adjusted and BSA-adjusted T vol. The authors have suggested that these differences may be due to genetic differences in the growth and development of children( Reference Zimmermann, Hess and Molinari 9 ). Chinese and Japanese children are of Asian ethnicity, and they may have a similar genetic background. So, the WHO criteria for measuring T vol may be too low for Chinese children. In the present study, the overall prevalence of goitre in children in the control town reached 14·0 % by age-specific T vol and 17·5 % by BSA-adjusted T vol. Given that the median UIC in children in the control town was lower than 500 μg/l, the prevalence of goitre in these children was not supposed to exceed 5 %, which signals the epidemic of goitre in a population. So, the high prevalence of goitre found in the control town suggested that the reference values of T vol recommended by the WHO could be too low for Chinese children. However, considering the relatively small size and the high median UIC (401·3 μg/l) in the control town, further research is needed to confirm this.
No significant differences were found in the prevalence of goitre across sex and age group by both age-specific and BSA-adjusted T vol. This is in accordance with previous findings( Reference Henjum, Barikmo and Gjerlaug 32 , Reference Rossi, Tomimori and Camargo 33 ). In the present study, the prevalence of goitre by BSA-adjusted T vol across age group and sex was higher than that by age-specific T vol. Similar findings have also been reported in other studies( Reference Rossi, Tomimori and Camargo 33 , Reference Xu, Sullivan and Houston 34 ).
There was a descending trend identified in the prevalence of goitre with the BSA of children. No similar finding has been reported in other studies on goitre induced by excess iodine. The cause underlying this trend warrants further research in the future.
Conclusion
Goitre prevalence is an important indicator that describes the adverse effect on thyroid function caused by iodine deficiency or excess. It has been proved that goitre is prevalent in children living in areas with a MWI above 300 μg/l in drinking-water. However, goitre status in children remains to be unclear in areas with a MWI between 150 and 300 μg/l. Using the reference values of T vol recommended by the WHO, the present study revealed that goitre was prevalent in areas with mildly excessive iodine in drinking-water, i.e. 150–300 μg/l. No significant differences were found in the prevalence of goitre across sex and age group. Goitre could mainly be attributed to excess amounts of iodine in drinking-water. The present study is the first to measure the magnitude of goitre in children living in such kind of areas by using an internationally acknowledged reference value. It expanded the application of this reference value from iodine deficiency to iodine excess and explored its feasibility in evaluating goitre induced by excess iodine. The reference values of T vol recommended by the WHO could be too low for Chinese children.
Acknowledgements
The present study was supported by the Hebei Provincial Bureau of Science and Technology (grant no. 11276103D-3). The study did not receive any other specific grant from any commercial or not-for-profit sector. The authors thank the staff in the Endemic Control Department of CDC of Jingxian and Gucheng County for their assistance in the field investigation. S. L. was responsible for the study design, data analysis, writing of the manuscript and field investigation. D. X. performed the thyroid measurement by ultrasound and data entering. Y. W. was in charge of the coordination of the field investigation and laboratory detection of iodine. J. Z. and J. M. performed the quality control of laboratory detection. Z. C. and Y. D. took part in the field investigation. The authors declare that there are no conflicts of interest.






