Introduction
Since the late 1960s Amazonian forests have been exposed to unprecedented levels of disturbance, mainly as a result of the intensification of extensive mono-crop agriculture, cattle ranching, logging and the ecological footprints of urban areas (Davidson et al., Reference Davidson, de Araújo, Artaxo, Balch, Brown and Bustamante2012). Predictive models of deforestation estimate that 40% of these forests will be lost by 2050, and c. 25% of mammal species may lose > 40% of their habitat in the Amazon (Soares-Filho et al., Reference Soares-Filho, Nepstad, Curran, Cerqueira, Garcia and Ramos2006).
Habitat loss and fragmentation are considered to be the main direct threat to primates (Estrada et al., Reference Estrada, Garber, Rylands, Roos, Fernandez-Duque and Di Fiore2017). Neotropical primates, which spend all their lives in trees, rarely descending to the forest floor or crossing open areas, often occupy important trophic positions in forest food webs (Terborgh, Reference Terborgh1983). They have an important role as seed dispersers (Hawes & Peres, Reference Hawes and Peres2014) and their demise would cause cascading ecological effects. The black-faced black spider monkey Ateles chamek is an Amazonian primate species categorized as Endangered on the IUCN Red List (Wallace et al., Reference Wallace, Mittermeier, Cornejo and Boubli2008). The forests in the southern portion of its range are among the areas worst affected by deforestation as a result of the expanding Brazilian agricultural frontier, a region referred to as the arc of deforestation.
Species distribution data are required to indicate priority regions for establishing conservation actions (Brooks et al., Reference Brooks, Mittermeier, da Fonseca, Gerlach, Hoffmann and Lamoreux2006), and species distribution models are valuable tools for species and habitat conservation plans, reserve design, and habitat management and restoration (Franklin, Reference Franklin2009). These models evaluate the relationships between occurrences of a species and a set of spatially explicit environmental variables, to estimate the species’ environmental requirements and project them in geographical space. Such models have been widely applied for supporting decision-making to inform conservation actions (Villero et al., Reference Villero, Pla, Camps, Ruiz-Olmo and Brotons2017).
Here we use species distribution modelling to predict the distribution of the black-faced black spider monkey. Our objectives are to quantify the areas with highest habitat suitability that fall within protected areas and Indigenous lands, and to estimate current and future habitat loss for the species, using two scenarios of deforestation across the Amazon Basin (Soares-Filho et al., Reference Soares-Filho, Nepstad, Curran, Cerqueira, Garcia and Ramos2006). We propose priority areas for the conservation of this species, new protected areas and the restoration of connectivity in the forest landscape.
Methods
Species distribution modelling
We used the maximum entropy algorithm, in MAXENT 3.3.3, to map habitat suitability for the species and estimate its potential distribution (Phillips & Dudík, Reference Phillips and Dudík2008). This algorithm seeks non-random relationships between species’ occurrences and environmental variables, building a model that estimates the species’ potential distribution according to relevant variables.
We gathered data on species occurrence from the literature, online datasets and personal observations spanning 1979–2017 (Supplementary Table 1). We inspected all records, excluding those with uncertain species identification, inaccurate geographical location, or from areas already deforested, and retaining pre 1990 records only if they were from areas that still have pristine forests, assuming that the species is still likely to be present. After excluding such records, we randomly removed duplicate records within a 30 km radius to control for sampling bias (Boria et al., Reference Boria, Olson, Goodman and Anderson2014), as records of species in the Amazon are commonly spatially clustered in sites that have been well studied. We obtained a total of 172 occurrence records of A. chamek, of which, after filtering, 99 were used in the model (Supplementary Fig. 1). We then plotted all records in a geographical information system (QGIS 2.14; QGIS Development Team, 2015) and created a polygon layer that included all records. As the ranges of Amazonian primates are usually limited by large rivers (e.g. Boubli et al., Reference Boubli, Ribas, Lynch Alfaro, Alfaro, da Silva, Pinho and Farias2015), we drew the boundaries of this polygon following rivers, encompassing the entire interfluvial region that included species records (Fig. 1). This polygon was defined as the species’ extent of occurrence (sensu IUCN, 2012), and it was used to parameterize the model and to project the species’ distribution.
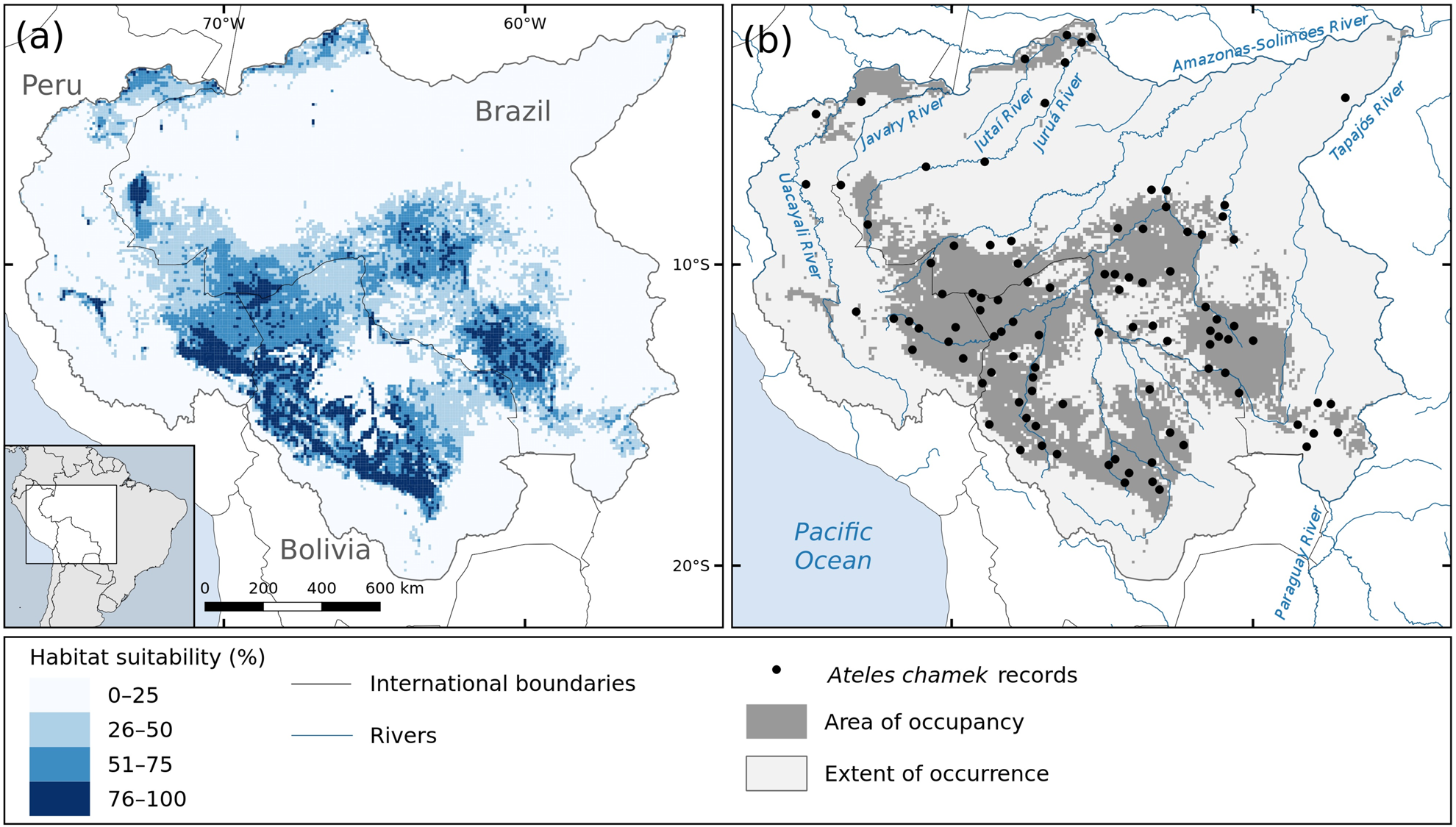
Fig. 1 (a) Habitat suitability and (b) predicted area of occupancy (i.e. areas with habitat suitability > 35.75%) for the black-faced black spider monkey Ateles chamek in the Amazon and Upper-Paraguay Basins; the area of occupancy comprises 28% of the species’ extent of occurrence.
We chose 19 available environmental variables that we would expect to influence the species’ distribution (Supplementary Table 2). These variables consisted of climatic (10), topographic (4), edaphic (2) and vegetation (3) layers at 10 km resolution. We used the species’ extent of occurrence polygon to crop the environmental variables and then performed a pair-wise correlation test (Supplementary Table 3). We removed all highly correlated variables (r > |0.8|) to avoid collinearity (Carvalho et al., Reference Carvalho, Sousa-Neves, Cerqueira, Gonsioroski, Silva, Silva and Santos2017). We used nine predictor variables in the model: temperature seasonality; minimum temperature of coldest month; annual precipitation; precipitation seasonality; annual potential evapotranspiration; flooded areas; compound topographic index, which is a measure of soil wetness; height above nearest drainage; and net primary productivity.
We used 5,000 random background records and divided the occurrences into 10 subsets (one for training, nine for testing), using the cross-validation technique to validate the model (Phillips & Dudík, Reference Phillips and Dudík2008; see Supplementary Material 1 for further details of the modelling procedure). We converted the continuous prediction of habitat suitability into a binary prediction by setting a threshold of habitat suitability, above which we considered that habitat was occupied (i.e. the species’ area of occupancy, sensu IUCN, 2012). We accomplished this by choosing the threshold with equal sensitivity (rate of true presences) and specificity (rate of true absences). We evaluated model accuracy with the True Skill Statistic (TSS), an effective and well-accepted measure of accuracy for binary predictions (Allouche et al., Reference Allouche, Tsoar and Kadmon2006). All procedures were performed in R v.3.3.3 (R Development Core Team, 2015).
Threat assessment and priority regions for conservation
We used QGIS to overlay the prediction of species distribution, the protected areas and Indigenous lands (UNEP-WCMC, 2016), and the current and future scenarios of forest cover modelled by Soares-Filho et al. (Reference Soares-Filho, Nepstad, Curran, Cerqueira, Garcia and Ramos2006). These authors modelled the future patterns of deforestation across the Amazon Basin (2002–2050) for two scenarios: (1) a business-as-usual scenario, which considers that current deforestation trends will continue, highways scheduled for paving will be paved, compliance with legislation requiring forest reserves on private land will remain low, and new protected areas will not be implemented; and (2) a governance scenario, which also considers the current deforestation trends but assumes a 50% limit imposed for deforested land within each basin's sub-region, and that existing and proposed protected areas play a determinant role in hindering deforestation. We calculated the extent of areas currently covered by protected areas and Indigenous lands, as well as current and future forested, deforested and non-forest areas within the species’ area of occupancy.
We used these overlaid layers to indicate priority regions for the conservation of the species. We indicate regions for expanding the protected area network as those (1) with > 40% habitat suitability for the species; (2) with forest cover > 75%; (3) expected to lose > 50% of forested area by 2050 because they are not under any protection; and (4) that are adjacent to existing protected areas/Indigenous lands. We also indicated human-modified regions (1) with > 40% of habitat suitable for the species, (2) that have already been deforested but are predicted to lose > 70% of forest area by 2050, and (3) that are adjacent to existing protected areas/Indigenous lands, as potential areas for landscape planning, management and/or restoration.
Results
The habitat suitability and predicted species distribution are shown in Fig. 1. According to our model, the species has a considerably reduced area of occupancy within its extent of occurrence, being expected to occupy an area of 927,754 km2 (only 28% of the extent of occurrence). We found that the species is more likely to occur in the central-southern region of its range, where the habitat suitability is higher (Fig. 1). We also found suitable habitats for the species in the north-west of its range, in the Amazonas–Javary interfluviual region, in Peru, and along the Lower-Jutaí and Juruá rivers, in Brazil.
The most important variables in the model were temperature seasonality, net primary productivity and potential evapotranspiration, which jointly contributed 57% to the model gain in all iterations (24, 21 and 12%, respectively; Supplementary Table 4). According to our model, higher forest productivity and higher temperature variation are associated with higher habitat suitability for the species. The higher the potential evapotranspiration (i.e. drier environments), the lower the habitat suitability for the species (Supplementary Fig. 2). The mean threshold of equal sensitivity–specificity was 35.75%, above which we consider that the species is present. The model-averaged TSS score was 0.56 ± SD 0.05, and therefore we are confident of the accuracy of our prediction (Supplementary Material 1).
We found that only 297,603 km2 (32% of the species’ area of occupancy) lies within protected areas (231,009 km2; 24%) and Indigenous lands (81,489 km2; 8%). Based on the deforestation estimates (Soares-Filho et al., Reference Soares-Filho, Nepstad, Curran, Cerqueira, Garcia and Ramos2006), by 2002 the species had already lost 15% (c. 127,306 km2) of the forest cover within its predicted area of occupancy (Fig. 2). Most of the forest loss occurred in Rondônia state, in Brazil. According to the future scenarios of deforestation, the species may lose 31% (273,287 km2) of its highly suitable habitat in the governance scenario, and up to 40% (377,951 km2) in the business-as-usual scenario (Fig. 2).
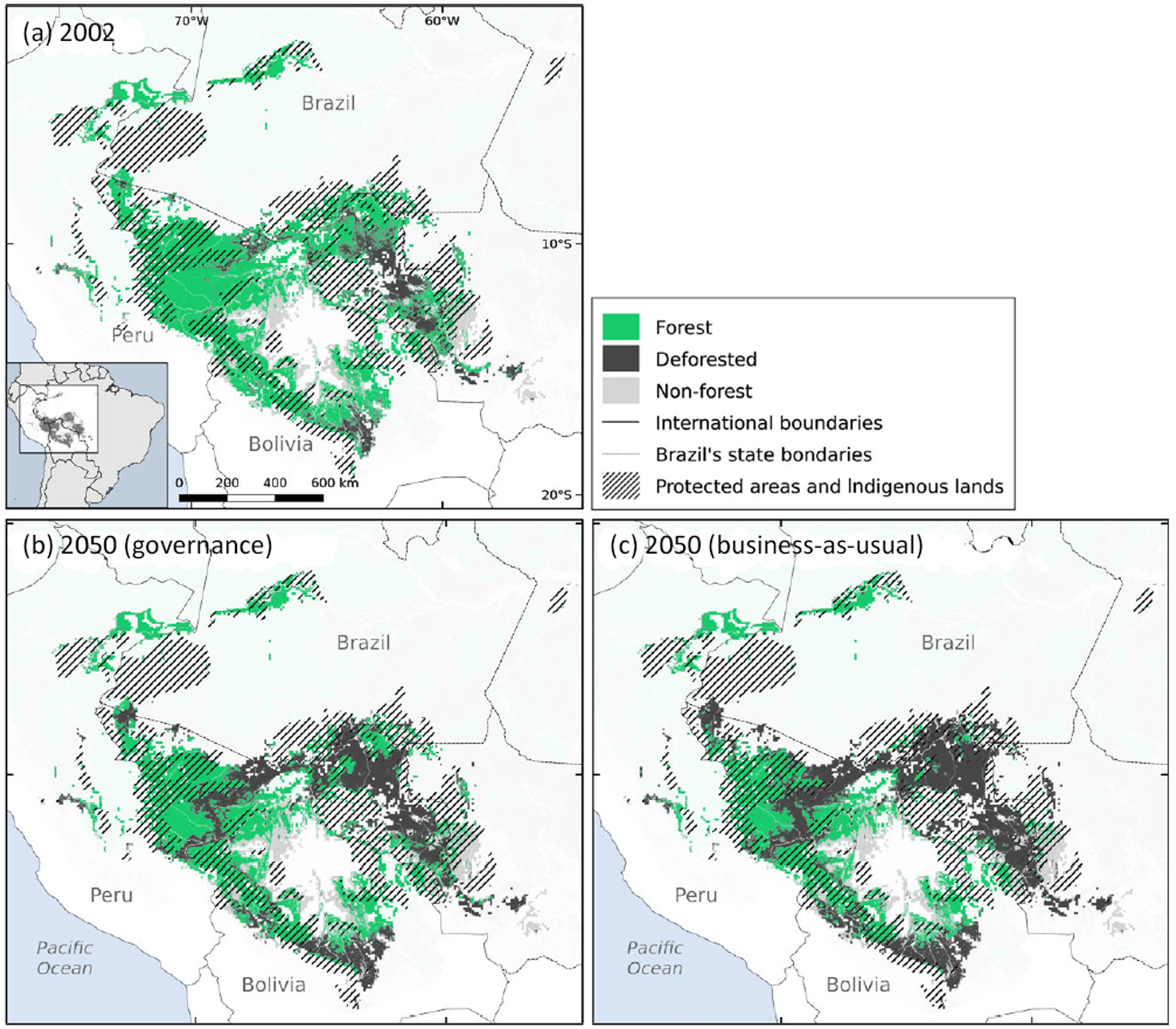
Fig. 2 Habitat loss and protected area network cover within the predicted distribution of A. chamek in Amazonia by 2050, according to the governance (b) and business-as-usual (c) scenarios of deforestation, relative to the situation in 2002 (a). Data on deforestation scenarios are from Soares-Filho et al. (Reference Soares-Filho, Nepstad, Curran, Cerqueira, Garcia and Ramos2006).
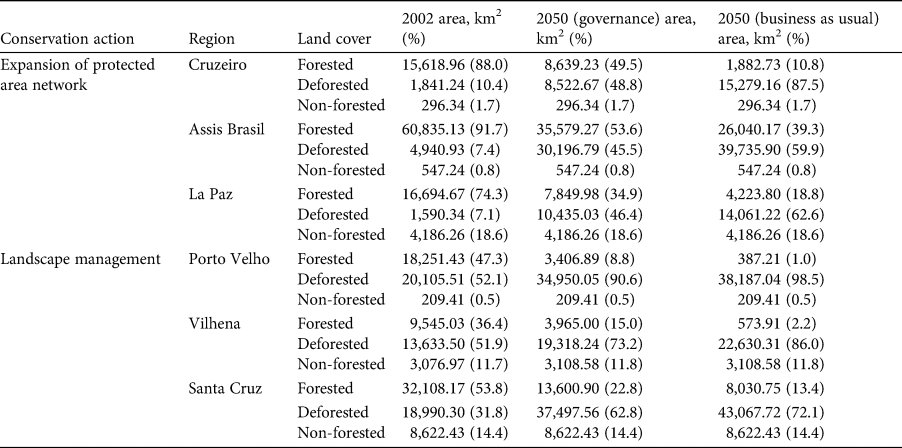
We indicate six priority regions for the conservation of the species (Supplementary Fig. 3; Table 1). Three of these priority regions are proposed for the designation of new protected areas (Supplementary Fig. 3a–c). One of these regions encompasses three countries: Peru, Bolivia and Brazil (Supplementary Fig. 3a), and the other two lie entirely in Brazil (Supplementary Fig. 3b) and Bolivia (Supplementary Fig. 3c). Three other human-modified regions are indicated as areas for landscape planning, involving protection of forest remnants, as well as landscape management (Supplementary Fig. 3d–f). Two of these are in Rondônia state in Brazil (Supplementary Fig. 3d, e), and one in Santa Cruz department in Bolivia (Supplementary 3f).
Table 1 Forest cover in the priority regions for the conservation of the black-faced black spider monkey Ateles chamek, based on two future scenarios of deforestation (governance, governance; business-as-usual) in the Amazon Basin (Soares-Filho et al., Reference Soares-Filho, Nepstad, Curran, Cerqueira, Garcia and Ramos2006).
Discussion
We have compiled the largest published dataset of A. chamek occurrence records to date, and found that the species occurs beyond the extent proposed by IUCN (Wallace et al., Reference Wallace, Mittermeier, Cornejo and Boubli2008; Supplementary Fig. 1), as recorded by Palminteri et al. (Reference Palminteri, Powell and Peres2011), Rabelo et al. (Reference Rabelo, Silva, Vieira, Ferreira-Ferreira, Paim and Dutra2014) and Santos-Filho et al. (Reference Santos-Filho, Bernardo, Van der Laan Barbosa, Gusmão, Jerusalinsky and Canale2017). However, according to our model, the species occupies only c. 28% of its extent of occurrence; this is empirically based information that should be taken into account for a species of conservation concern.
Following the definitions of Soberón & Nakamura (Reference Soberón and Nakamura2009), which have been adopted for the IUCN criteria (IUCN, 2012), a species’ extent of occurrence is the area that is/has been accessible to the species, given its dispersal ability, during a given time frame. However, it is not expected that a species occupies its entire extent of occurrence uniformly, because of biotic or environmental limitations. In this context, having set the threshold of habitat suitability above which we expect the species to be present, we may assume that (1) the areas within the extent of occurrence with habitat suitability > 35.75% correspond to the species’ area of occupancy (Fig. 1b); (2) species records located within the area of occupancy correspond to populations occurring in highly suitable habitats; and (3) records located outside the area of occupancy represent populations occurring in habitat with environmental constraints.
It could be argued that our model did not predict suitable areas for the species in the central-north and east of the species’ extent of occurrence because of the absence of records in these regions. However, there have been exhaustive and long-term primate surveys in those regions that did not record the species, or recorded it at very low densities (Peres, Reference Peres1997; Haugaasen & Peres, Reference Haugaasen and Peres2005; Kasecker, Reference Kasecker2006; Bastos, Reference Bastos2012; J.R. Gonçalves et al., unpubl. data). Therefore, we do not believe that our model was biased by the absence of records in these regions. We believe that these regions were predicted to be unoccupied because of unsuitable (or less suitable) environmental conditions.
According to our model, temperature variation and net primary productivity were the most important variables for predicting the species’ distribution (Supplementary Table 4). Trees in seasonal and highly diverse forests tends to produce fruits asynchronously (van Schaik et al., Reference van Schaik, Terborgh and Wright1993), and forests with high primary productivity generally have high fruit production (Clark et al., Reference Clark, Brown, Kicklighter, Chambers, Thomlinson and Ni2001). Spider monkeys are primary consumers and have been consistently identified to be among the most frugivorous Neotropical primates (Di Fiore et al., Reference Di Fiore, Link, Dew and Campbell2008). Thus, we would expect that areas with higher temperature seasonality and primary productivity would have high habitat suitability for the species.
We found that 68% of the species’ area of occupancy is outside protected areas. As habitat loss advances in tropical forests, protected areas become essential refuges for wildlife. Protected areas harbour higher species richness and abundance than unprotected areas, emphasizing their importance for conservation of viable populations in natural ecosystems (Gray et al., Reference Gray, Hill, Newbold, Hudson, Börger and Contu2016). As studies have consistently shown the role of protected areas and Indigenous lands in safeguarding Amazonian forests (Nepstad et al., Reference Nepstad, Schwartzman, Bamberger, Santilli, Ray and Schlesinger2006; Dobrovolski et al., Reference Dobrovolski, Diniz-Filho, Loyola and De Marco2011; Blackman et al., Reference Blackman, Corral, Lima and Asner2017), we believe that they are still an effective tool for the conservation of spider monkeys and many other species.
Although expanding the protected area network would help to inhibit habitat loss, this may not be sufficient for the conservation of the black-faced black spider monkey. Even under the most conservative scenario of deforestation (the governance scenario), the deforested area within the species’ distribution in 2050 is expected to be twice as large as it was in 2002. The governance scenario assumes effective implementation of environmental legislation across the Amazon Basin through the enforcement of mandatory forest reserves on private properties, agro-ecological zoning of land use and the expansion of the protected area network (Nepstad et al., Reference Nepstad, McGrath, Alencar, Barros, Carvalho, Santilli and Vera Diaz2002), requiring international and national conservation efforts. However, it is unlikely that frontier governance will be refined in the way required, especially following the controversial changes in Brazilian legislation regulating land use on private properties (Soares-Filho et al., Reference Soares-Filho, Rajão, Macedo, Carneiro, Costa and Coe2014). Therefore, the business-as-usual scenario is more realistic: it will result in a loss of 40% of the forested area within the range of the black-faced black spider monkey.
We identify six priority regions for the conservation of the black-faced black spider monkey, all of them currently outside protected areas. Three of these priority regions still have a considerable amount of forest cover (Supplementary Fig. 3; Table 1) but according to the deforestation scenarios these areas will become severely deforested because they are not under protection. Spider monkeys are known for their long daily journeys (460–5,690 m) and large home ranges (153–340 ha), which overlap little with the home ranges of other groups, and often show a preference for using tall forest types and may avoid edge habitats (Wallace, Reference Wallace and Campbell2008). Additionally, they are particularly vulnerable to habitat loss and fragmentation, which means that large areas of continuous forest (or at least large forest patches) are more likely to sustain viable populations of the species (Ramos-Fernández & Wallace, Reference Ramos-Fernández, Wallace and Campbell2008). Therefore, we suggest these three regions as priority areas for implementation of new protected areas to conserve these large forest tracts.
We also propose three priority regions where conservation measures for regulating land use and management of private lands would help to protect forest remnants and conserve the species outside reserves. Given the scale of our analysis (10 km resolution), we are targeting wide-scale human-modified regions, where infrastructure development and large-scale farming have caused severe habitat loss and fragmentation. Forest-dwelling species, such as spider monkeys, may potentially persist in human-modified regions if forest connectivity is managed at the landscape scale (Pardini et al., Reference Pardini, Bueno, Gardner, Prado and Metzger2010), particularly in this case as we found high habitat suitability for the species in these regions. The restoration of forest connectivity could be planned by the implementation of environmental legislation that regulates vegetation loss and restoration within private properties, especially in riparian zones. However, further fine-scale studies must be conducted to consolidate landscape management within these regions.
This study provides key information for the conservation of the threatened black-faced black spider monkey, as required by IUCN and the Brazilian National Action Plans: an update of the species’ extent of occurrence, the predicted area of occupancy of the species, the protection status of suitable areas for the species, and a recommendation of priority regions for establishing protected areas and for enhancing habitat connectivity (e.g. Jerusalinksy et al., Reference Jerusalinksy, Talebi and Melo2011). The black-faced black spider monkey is only one of thousands of species that are being threatened by land-cover changes in the arc of deforestation in the Amazon. This species could be endorsed by conservation organizations as a flagship species to motivate public support for conservation actions (e.g. Home et al., Reference Home, Keller, Nagel, Bauer and Hunziker2009). Our findings will be of value in assessing the conservation status of the species, and establishing goals in National Action Plans for the conservation of the species. We are passing our recommendations to relevant stakeholders to facilitate this process.
Acknowledgements
This paper was written during a visiting internship by RMR to the University of Salford, funded by the Student Conference on Conservation Science Miriam Rothschild Travel Bursary Programme. RMR received research fellowships from the Brazilian National Research Council (CNPq, 313108/2016-1, 142352/2017-9). DGR received a scholarship from CAPES/Doutorado Pleno no Exterior (88881.128140). We thank Parque Nacional dos Campos Amazônicos and Parque Nacional Mapinguari for logistical support in obtaining new species records, and Cazuza Junior, Fernando Figueiredo and Marcelo Santos Jr for their contribution of ideas for this research.
Author contributions
Study design, data collection, analysis and writing: RMR; data collection, interpretation of results, and assistance with writing: JRG, FES and DGR; study design, interpretation of results, and assistance with writing: GRC, CSSB and JPB.
Conflicts of interest
None.
Ethical standards
This research complied with the Oryx Code of Conduct.





