Fatty liver evolves when large amounts of lipids accumulate within the liver cells. The risk factors associated with non-alcoholic fatty liver disease (NAFLD), which include obesity, type 2 diabetes mellitus and hyperlipidaemia(Reference Adler and Schaffner1–Reference Sheth, Gordon and Chopra4), are quite similar to the clinical criteria of the metabolic syndrome. Evidence of an aetiological association between NAFLD and the metabolic syndrome has been shown in both obese and non-obese patients(Reference Marchesini, Brizi, Morselli-Labate, Bianchi, Bugianesi, McCullough, Forlani and Melchionda5, Reference Moon, Leem, Bae, Lee, Kim, Chae, Park and Youn6), and this has led to the suggestion that NAFLD is the liver manifestation of the metabolic syndrome(Reference Sanyal7, Reference Marchesini, Bugianesi and Forlani8). NAFLD affects 10–24 % of the general global population, but the prevalence increases significantly to more than 50 % of obese individuals(Reference Silverman, O'Brien, Long, Leggett, Khazanie, Pories, Norris and Caro9, Reference Angulo and Lindor10), and the occurrence and severity of NAFLD increase significantly with increasing obesity(Reference Silverman, O'Brien, Long, Leggett, Khazanie, Pories, Norris and Caro9–Reference Bellentani, Saccoccio, Masutti, Croce, Brandi, Sasso, Cristanini and Tiribelli11).
The primary metabolic cause of lipid accumulation in the liver is not well understood, but it could potentially result from insulin resistance and alterations in the uptake, synthesis, degradation or secretory pathways of lipids(Reference Angulo and Lindor10). It has been demonstrated that impaired mitochondrial β-oxidation in hepatocytes may lead to an abnormal accumulation of fatty acids, impaired ketogenesis and decreased energy supply(Reference Fromenty and Pessayre12). There is no widely accepted therapy for fatty liver, but lifestyle modifications with weight reduction are frequently recommended(Reference McClain, Mokshagundam, Barve, Song, Hill, Chen and Deaciuc13), and one approach has been changes in the diet with special focus on dietary fats. Increasing evidence suggests that not only dietary lipids and fatty acids, but also proteins and amino acids in the diet can affect the lipid metabolism(Reference Wergedahl, Liaset, Gudbrandsen, Lied, Espe, Muna, Mork and Berge14–Reference Gudbrandsen, Wergedahl, Liaset, Espe and Berge17).
Leptin seems to play a key role in pathways that regulate body weight, as elevated levels of leptin signal to the brain that excess energy is being stored, and this signal brings about adaptations of decreased appetite and increased energy expenditure that resist obesity. Human obesity is almost always associated with elevated leptin levels, suggesting that resistance to the effects of leptin, rather than a lack of leptin, is a major feature of this disease process(Reference Friedman18). The genetically obese Zucker fa/fa rats have a null mutation of the leptin receptor gene and have been extensively studied as a model for obesity. These rats also have high plasma TAG levels and abnormally high synthesis of TAG in liver, combined with a low rate of hepatic fatty acid oxidation(Reference Bray19, Reference Triscari, Greenwood and Sullivan20), leading to development of fatty liver at young age(Reference Krief and Bazin21). The obese Zucker fa/fa rats may therefore be useful as an animal model for a deeper understanding of biochemical changes that occurs during the development of fatty liver.
We have previously shown that when obese Zucker rats were fed diets containing proteins with low methionine:glycine and lysine:arginine ratios such as soya protein or a single cell protein (SCP), a hypocholesterolaemic effect was obtained(Reference Wergedahl, Liaset, Gudbrandsen, Lied, Espe, Muna, Mork and Berge14, Reference Gudbrandsen, Wergedahl, Liaset, Espe and Berge17). In addition, we have demonstrated that dietary soya protein, which contains isoflavones, reduced fatty liver in obese Zucker rats(Reference Wergedahl, Liaset, Gudbrandsen, Lied, Espe, Muna, Mork and Berge14, Reference Gudbrandsen, Wergedahl, Mork, Liaset, Espe and Berge22). The aim of the present study was to assess the effect of a dietary protein with low methionine:glycine and lysine:arginine ratios that did not contain isoflavones on the development of fatty liver in obese Zucker rats, and the results were compared with casein-fed rats.
Materials and methods
Animals and diets
Male obese Zucker rats Crl:(ZUC)/FaBR (fa/fa) from Charles River Laboratories (Sulzfeld, Germany), weighing 80–135 g, were divided into two experimental groups of six rats each with comparable mean body weight. The rats were housed individually all through the feeding experiment, the first week of the experiment in metabolism cages, in a room maintained at a 12 h light–dark cycle, a constant temperature of 20 ± 3°C and relative humidity of 65 ± 15 %. The rats were acclimatised under these conditions before the start of the experiment. The rats were fed diets consisting of 20 % (w/w) protein from fermented SCP(Reference Skrede, Berge, Storebakken, Herstad, Aarstad and Sundstøl23) or casein Na salt from bovine milk (C-8654; Sigma-Aldrich Norway AS, Oslo, Norway) for 6 weeks. Commercial soya oil (10 %, w/w) was added to the diets. To all diets were added 1 % of AIN-93VX vitamin mixture (Dyets Inc., Bethlehem, PA, USA), 3 % of AIN-93G mineral mixture (Dyets Inc.) and 0·2 % choline hydrogentartrate (Merck, Darmstadt, Germany). All rats had free access to tap water and food (ad libitum). Table 1 gives an overview of the experimental diets.
Table 1 Composition of the experimental diets (g/kg diet)*
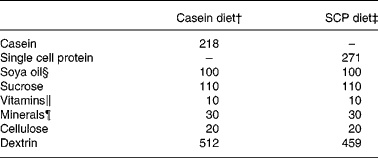
SCP, single cell protein.
* The diets were isoenergetic and isonitrogenous, and contained 200 g crude protein/kg diet.
† The casein diet contained (g/kg diet): fat, 9·8; ash, 31. The casein protein contained 91·9 % crude protein.
‡ The SCP diet contained (g/kg diet): fat, 11·7; ash, 38. The SCP contained 73·9 % crude protein.
§ Fatty acid composition of the soya oil (mean of two measurements, deviation less than 3 %; g/100 g fat): 18 : 2n-6, 55·9; 18 : 1n-9, 21·4; 16 : 0, 11·4; 18 : 3n-3, 5·8; 18 : 0, 3·3; 18 : 1n-7, 1·6.
∥ Vitamin mixture (AIN-93VX; Dyets Inc.).
¶ Mineral mixture (AIN-93G; Dyets Inc.).
At the end of the feeding period, under non-fasting conditions, the rats were anaesthetised with a 1:1 mixture of Hypnorm™ (fentanyl citrate 0·315 mg/ml and fluanisone 10 mg/ml; Janssen Pharmaceutica, Beerse, Belgium) and Dormicum® (midazolam 5 mg/ml; F. Hoffmann-La Roche AG, Basel, Switzerland) injected subcutaneously. Blood was drawn directly from the heart using a syringe containing heparin. The liver was immediately removed and divided into two parts, which were immediately chilled on ice for measurements of β-oxidation activity, or frozen in liquid N2. Epididymal white adipose tissue and skeletal muscle from the thigh were quickly removed. All tissues were stored at − 80°C until analysis. The protocol was approved by the Norwegian State Board of Biological Experiments with Living Animals.
Amino acids in diets and liver
The amino acids in the diets were determined after hydrolysis in 6 m-HCl at 110°C for 22 h and pre-derivatisation with phenylisothiocyanate according to the method of Cohen & Strydom(Reference Cohen and Strydom24). To analyse the amounts of free amino acids in liver, the samples were extracted and deproteinated by the addition of two volumes of 5 % sulfosalisylic acid, kept on ice for 30 min and centrifuged at 5000 g for 15 min. The supernatant fraction was mixed with internal standard (norleucine). The amino acids were quantified using a Biochrom 20 plus Amino Acid Analyser (Amersham Pharmacia Biotech, Uppsala, Sweden) equipped with a lithium column with post-column ninhydrin derivatisation as previously described(Reference Liaset, Julshamn and Espe25).
Scharlach red staining of liver
In order to study neutral fat deposits, 10 μm frozen sections from the livers were cut, stained with a filtered Scharlach red solution and visualised by light microscopy.
Plasma transaminases, alkaline phosphatase and γ-glutamyl transpeptidase
The plasma levels of alanine transaminase, aspartate transaminase, alkaline phosphatase and γ-glutamyl transpeptidase were measured on the Hitachi 917 system (Roche Diagnostics, GmbH, Mannheim, Germany) using the appropriate kits from Roche Diagnostics.
Lipid quantification
TAG in liver, plasma, and TAG-rich lipoproteins were measured enzymically using the TAG kit from Bayer (Tarrytown, NY, USA). Phospholipids in liver were measured using the phospholipid kit from bioMérieux (Lyon, France). The analyses were performed using the Hitachi 917 system (Roche Diagnostics). Liver lipids were extracted by the method of Bligh & Dyer(Reference Bligh and Dyer26), evaporated under N2 and re-dissolved in isopropanol before analysis.
Fatty acid composition
Lipids were extracted from liver and plasma samples using a mixture of chloroform and methanol(Reference Bligh and Dyer26). The lipid classes in liver were separated by TLC on silica gel plates (0·25 mm Silica gel 60; Merck) and developed in hexane–diethyl ether–acetic acid (80 : 20 : 1, by vol.)(Reference Mangold and Stahl27). The spots were identified using Rhodamine G (Fluka Chemie AG, Buchs, Switzerland) and co-migration with known standards. The monoacylglycerol and phospholipid spots were incompletely separated, and were examined as one fraction, referred to as the phospholipid fraction. Heneicosanoic acid (21 : 0) was added to the extracts as internal standard. The extracts were transesterified using boron fluoride–methanol(Reference Morrison and Smith28). To remove neutral sterols and non-saponifiable material, the extracts were heated in 0·5 m-KOH in an ethanol–water solution (9:1). Recovered fatty acids were re-esterified using boron fluoride–methanol. The methyl esters were quantified as previously described(Reference Wergedahl, Liaset, Gudbrandsen, Lied, Espe, Muna, Mork and Berge14). The anti-inflammatory fatty acid index in plasma was calculated as (22 : 5n-3+22 : 6n-3+20 : 3n-6)/20 : 4n-6(Reference Chavali, Zhong, Utsunomiya and Forse29).
Real-time quantitative reverse transcriptase polymerase chain reaction
Total RNA was purified from frozen liver, epididymal white adipose tissue and skeletal muscle using an RNeasy Midi Kit (Qiagen, Hilden, Germany). For isolation of RNA from white adipose tissue, the samples were added to QIAzol Lysis Reagent (Qiagen) and the extraction was performed with chloroform. For isolation of RNA from muscle, the samples were added to Proteinase K (Qiagen). Primers and TaqMan probe for rat carnitine palmitoyltransferase (CPT)-Ia, CPT-II, Δ5 desaturase, Δ6 desaturase, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), PPARα, stearoyl-CoA desaturase (SCD-1) and sterol regulatory element binding protein (SREBP)-1c were designed using Primer Express (Applied Biosystems, CA, USA). Gene expressions were determined using TaqMan probes. The following sequences were used: CPT-Ia forward 5′-CCC AGT GGG AGC GAC TCT T-3′, reverse 5′-TGT GCC TGC TGT CCT TGA TAT G-3′, and probe 5′-AGG AGA CAG ACA CCA TC-3′; CPT-II forward 5′-ATT ATC TGC AGC ACA GCA TCG T-3′, reverse 5′-TGC ATT GAG GTA TCT CTT CAT GGT-3′, and probe 5′-TGC CCA GGC TGC CTA-3′; Δ5 desaturase forward 5′-TGG ATC TTT GGA ACT TCC TTG GT-3′, reverse 5′-CAA AGT CAT GCT GTA GCC AAC CT-3′, and probe 5′-CAG TTC AGG CCC AGG C-3′; Δ6 desaturase forward 5′-CAG CGG GCA CCT CAA TTT-3′, reverse 5′-TGC TTG GCG CAG AGA GAC T-3′, and probe 5′-CAG ATT GAG CAC CAC CTC TTC CCC AC-3′; GAPDH forward 5′-TGC ACC ACC AAC TGC TTA GC-3′, reverse 5′-CAG TCT TCT GAG TGG CAG TGA TG-3′, and probe 5′-TGG AAG GGC TCA TGA CCA CAG TCC A-3′; PPARα forward 5′-TGG AGT CCA CGC ATG TGA AG-3′, reverse 5′-CAG TCT TCT GAG TGG CAG TGA TG-3′, and probe 5′-TGG AAG GGC TCA TGA CCA CAG TCC A-3′; SCD-1 forward 5′-CCT CAT CAT TGC CAA CAC CAT-3′, reverse 5′-CGG CGT GTG TCT CAG AGA AC-3′, and probe 5′-TCC CAG AAC GAT GTA TAT GAA TGG GCC C-3′; SREBP-1c forward 5′-GGA GCC ATG GAT TGC ACA TTT G-3′, reverse 5′-CAA ATA GGC CAG GGA AGT CAC-3′. Adipose differentiation-related protein (ADRP, Rn01472318_m1), arylacetamide deacetylase (AADA; Rn00571934_m1), diacylglycerol acyltransferase (DGAT, subtype DGAT-1, Rn00584870_m1), elongase (subtype rELO-1, Rn00592812_m1), lipoprotein lipase (LPL, Rn00561482_m1), medium-chain acyl-CoA dehydrogenase (Rn00566390_m1), mitochondrial carnitine/acylcarnitine translocase (Rn00588652_m1), PPARγ (Rn00440945_m1), short-chain acyl-CoA dehydrogenase (Rn00574634_m1), very-long-chain acyl-CoA dehydrogenase (Rn00563649_m1) and VLDL-receptor (Rn00565784_m1) are ‘Assay-on Demand’ designed by Applied Biosystems. The primer and TaqMan probe specific for rat TNFα (Fam-MGB 4319411-210003) were obtained from TaqMan Pre-Developed Assay Reagents (Applied Biosystems).
Real-time RT-PCR was carried out in triplicates on an ABI 7900 sequence detection system (Applied Biosystems). A dilution curve from one cDNA source using dilutions of 1:2, 1:4, 1:8 and a no-template control was run for each gene. The gene expression was determined by relative quantification using the standard curve method. For each sample, results were normalised to GAPDH mRNA and 18S rRNA (RT-CKFT-18S; MedProbe, Oslo, Norway). Only results normalised to 18S rRNA are shown, as they were similar to the results normalised to GAPDH mRNA.
Preparation of hepatic subcellular fractions
Homogenisation and subcellular fractionation of the livers were performed as previously described(Reference Berge, Flatmark and Osmundsen30). The procedure was performed at 0–4°C, and the fractions were stored at − 80°C. Protein was assayed with the BioRad protein assay kit (BioRad, Richmond, CA, USA) using bovine serum albumin as the standard.
Enzyme assays
Palmitoyl-CoA and palmitoyl-l-carnitine oxidation were measured in the mitochondrial fraction as acid-soluble products(Reference Willumsen, Hexeberg, Skorve, Lundquist and Berge31). Fatty acyl-CoA oxidase was measured in the peroxisomal fraction(Reference Small, Burdett and Connock32). Acetyl-CoA carboxylase activity was measured in mitochondrial and cytosolic fractions by measuring the amount of NaH14CO3 incorporated into malonyl-CoA(Reference Tanabe, Nakanishi, Hashimoto, Ogiwara, Nikawa and Numa33). Fatty acid synthase activity was measured in the cytosolic fraction as described by Roncari(Reference Roncari34), modified according to Skorve et al. (Reference Skorve, al-Shurbaji, Asiedu, Bjorkhem, Berglund and Berge35). Glycerol-3-phosphate acyltransferase activity was measured in mitochondrial and microsomal fractions as described by Bates & Saggerson(Reference Bates and Saggerson36). DGAT activity was measured in the microsomal fraction(Reference Coleman and Bell37).
Tumour necrosis factor-α in plasma
Plasma levels of TNFα were analysed in duplicate using the Bioplex Protein Array system (BioRad). Circulating TNFα were quantified on the Bio-Plex 200 System from Bio-Rad using Luminex xMAP Technology, using beads specific for TNFα, according to the manufacturer's instructions.
Isolation of TAG-rich lipoproteins
Plasma from two rats was pooled to obtain a volume of 3 ml. The plasma TAG-rich lipoprotein fraction was prepared as previously described(Reference Muna, Gudbrandsen, Wergedahl, Bohov, Skorve and Berge38).
Statistical analysis
All data in tables and figures are presented as mean values with standard deviations for six rats per group. The data were evaluated by unpaired Student's t test with the level of statistical significance set at P < 0·05. Rats fed casein-based diet served as controls.
Results
Growth and feed intake
The experimental diets were well tolerated by the rats. Both the growth and the feed intake were similar between the experimental groups (Table 2).
Table 2 Growth and feed intake in obese Zucker rats fed a diet containing casein or single cell protein (SCP)*
(Mean values and standard deviations for six rats per group)
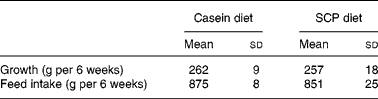
* No significant differences were detected between the groups.
Dietary and hepatic amino acids
The amino acid compositions of the diets are shown in Table 3. Differences in the amino acid content in the SCP diet when compared with the casein diet were observed for alanine, arginine, aspartate, glycine, threonine and tryptophan (more than 20 % higher in the SCP diet), and for glutamate, methionine, proline and serine (more than 20 % lower in the SCP diet). The SCP diet is characterised by low ratios of methionine:glycine and of lysine:arginine when compared with the casein diet.
Table 3 Amino acids in the experimental diets (%, w/w) shown as mean values of two measurements*
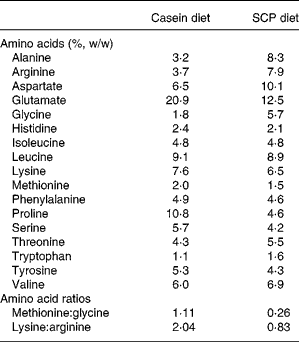
SCP, single cell protein.
* The difference between the two measurements was < 4 %.
As could be expected, feeding different proteins affected the levels of free amino acids in the liver of the rats (Table 4). However, the differences in the amino acid composition of the diets were not directly reflected in the liver. Of the nineteen amino acids investigated, the levels of seven amino acids were increased (citrulline, glycine, leucine, methionine, proline, threonine and tyrosine), and the levels of two amino acids were decreased (glutamate and taurine) in liver after SCP feeding (Table 4). Of profound interest is the increased hepatic level of citrulline, a co-product of NO synthase.
Table 4 Free amino acids in liver (μmol/g liver) from obese Zucker rats fed a diet containing casein or single cell protein (SCP)
(Mean values and standard deviations for six rats per group)
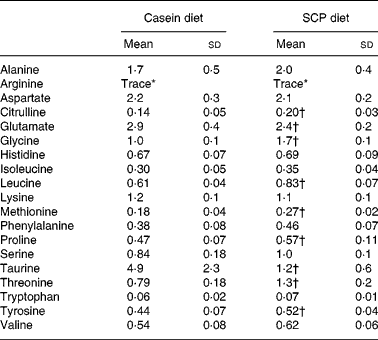
* Trace ( < 0·03 μmol/g liver).
† Mean value was significantly different from that of the control (casein) group (P < 0·05).
Hepatic lipids and fatty acid composition, and plasma transaminases
The SCP diet reduced the level of neutral lipids in liver, as visualised by Scharlach red staining (Fig. 1). Quantification of the liver lipids revealed that SCP feeding reduced the levels of TAG whereas the content of phospholipids was unchanged (Table 5). The reduced fatty liver was accompanied by decreased plasma levels of alanine transaminase and aspartate transaminase (Table 5), whereas no changes were observed in the plasma levels of alkaline phosphatase and γ-glutamyl transpeptidase (data not shown). However, the hepatic mRNA level of ADRP, which is highly expressed in the liver of animals with hepatic steatosis(Reference Motomura, Inoue, Ohtake, Takahashi, Nagamine, Tanno, Kohgo and Okumura39), showed only a weak tendency to decrease after SCP feeding (P < 0·18; Table 5).

Fig. 1 Fat-stained microphotographs from livers of obese Zucker rats fed a diet containing (a) casein or (b) single cell protein, showing one representative example from each feeding group (Scharlach red, original magnifications × 50).
Table 5 Hepatic lipids, plasma transaminases and hepatic mRNA level of adipose differentiation-related protein (ADRP) in obese Zucker rats fed a diet containing casein or single cell protein (SCP)
(Mean values and standard deviations for six rats per group)
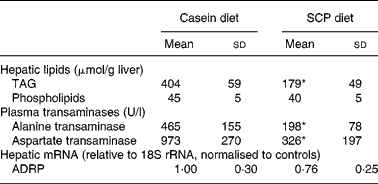
* Mean value was significantly different from that of the control (casein) group (P < 0·05).
We have recently shown that TNFα, an immunomodulating cytokine, induced steatosis and reduced fatty acid β-oxidation in mice(Reference Glosli, Gudbrandsen, Mullen, Halvorsen, Rost, Wergedahl, Prydz, Aukrust and Berge40). Thus, pro-inflammatory processes may play a role in the pathogenesis of fatty liver disease. It was of interest to observe that SCP feeding decreased the plasma TNFα level (Fig. 2 (a)) and showed a weak tendency to decrease the mRNA level of TNFα in liver (P < 0·15; Fig. 2 (b)).
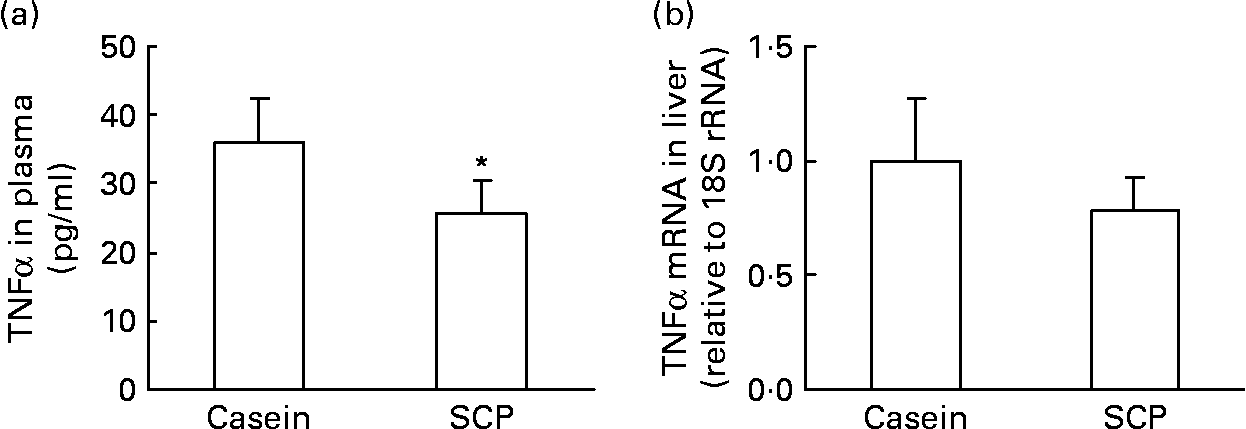
Fig. 2 The plasma level of TNFα (a) and the mRNA level of TNFα in liver (b) from obese Zucker rats fed a diet containing casein or single cell protein (SCP). Values are means for six rats per group, with standard deviations represented by vertical bars. * Mean value was significantly different from that of the control (casein) group (P < 0·05).
The fatty acid compositions of hepatic TAG, 1,2-diacylglycerol and phospholipids, but not of NEFA, were affected by the SCP diet when compared with the casein diet (Table 6). The level of 16 : 0 was not affected in the liver TAG, 1,2-diacylglycerol or phospholipids. The amount of 18 : 0 esterified as TAG or 1,2-diacylglycerol was increased, whereas the level of 18 : 1n-9 was decreased in these lipids after SCP feeding (Table 6), accompanied by a decreased gene expression of SCD-1 (Fig. 3 (a)) and a tendency to reduced gene expression of elongase (P < 0·1; Fig. 3 (b)). The mRNA levels of Δ6 desaturase (Fig. 3 (c)) and Δ5 desaturase (Fig. 3 (d)) in liver were reduced by SCP feeding, reflecting a decreased ratio of 20 : 4n-6:18 : 2n-6 in the phospholipid fraction (calculated from Table 6). SCP feeding increased the amount of 18 : 3n-3, 20 : 5n-3 and 22 : 5n-3 esterified as phospholipids in the liver, and in addition the levels of 18 : 3n-3 and 20 : 5n-3 were increased in TAG and 1,2-diacylglycerols (Table 6).
Table 6 Selected fatty acids (g/100 g fatty acids) in hepatic lipids from obese Zucker rats fed a diet containing casein or single cell protein (SCP)
(Mean values and standard deviations for six rats per group)

ND, not detected
* Mean value was significantly different from that of the control (casein) group (P < 0·05).

Fig. 3 mRNA levels (relative to 18S rRNA and normalised to controls) of desaturases and elongase in the liver of obese Zucker rats fed a diet containing casein or single cell protein (SCP). mRNA levels of stearoyl-CoA desaturase (SCD-1) (a), elongase (b), Δ6 desaturase (c) and Δ5 desaturase (d). Values are means for six rats per group, with standard deviations represented by vertical bars. * Mean value was significantly different from that of the control (casein) group (P < 0·05).
Fatty acid β-oxidation, lipogenesis and lipid biosynthesis
The mitochondrial fatty acid β-oxidation in liver was increased after SCP feeding, as measured using palmitoyl-CoA (Fig. 4 (a)) or palmitoyl-l-carnitine (Fig. 4 (b)) as substrates. The increased activity of acyl-CoA oxidase (Fig. 4 (c)) after SCP feeding implied an increased peroxisomal β-oxidation activity in the liver of these rats. The mRNA levels of CPT-I, CPT-II, mitochondrial carnitine/acylcarnitine translocase, short-chain acyl-CoA dehydrogenase, medium-chain acyl-CoA dehydrogenase, very-long-chain acyl-CoA dehydrogenase or PPARα were, however, not significantly increased in liver after SCP feeding (data not shown).

Fig. 4 Activities of enzymes involved in mitochondrial and peroxisomal fatty acid β-oxidation measured in the liver of obese Zucker rats fed a diet containing casein or single cell protein (SCP). (a) β-Oxidation in the mitochondrial fraction using palmitoyl-CoA as substrate; (b) β-oxidation in the mitochondrial fraction using palmitoyl-l-carnitine as substrate; (c) the activity of acyl-CoA oxidase in the peroxisomal fraction. Values are means for six rats per group, with standard deviations represented by vertical bars. * Mean value was significantly different from that of the control (casein) group (P < 0·05).
SCP feeding seemed to have no effect on lipogenesis and TAG biosynthesis, as no change was seen in the hepatic activities of acetyl-CoA carboxylase, fatty acid synthase, glycerol-3-phosphate acyltransferase or DGAT (data not shown). In addition, the hepatic mRNA levels of SREBP-1c, PPARγ and DGAT-1 were not altered by SCP feeding (data not shown).
Genes involved in uptake and secretion of lipoproteins
The clearance of VLDL by the liver may be reduced after SCP feeding, as the hepatic mRNA level of the VLDL-receptor was markedly down-regulated in these rats (Fig. 5 (a)). Takahashi et al. (Reference Takahashi, Suzuki, Kohno, Oida, Tamai, Miyabo, Yamamoto and Nakai41) have demonstrated the important role for LPL in the binding of lipoproteins to the VLDL-receptor, and, indeed, the hepatic mRNA level of LPL was also strongly reduced in SCP-fed rats (Fig. 5 (b)).
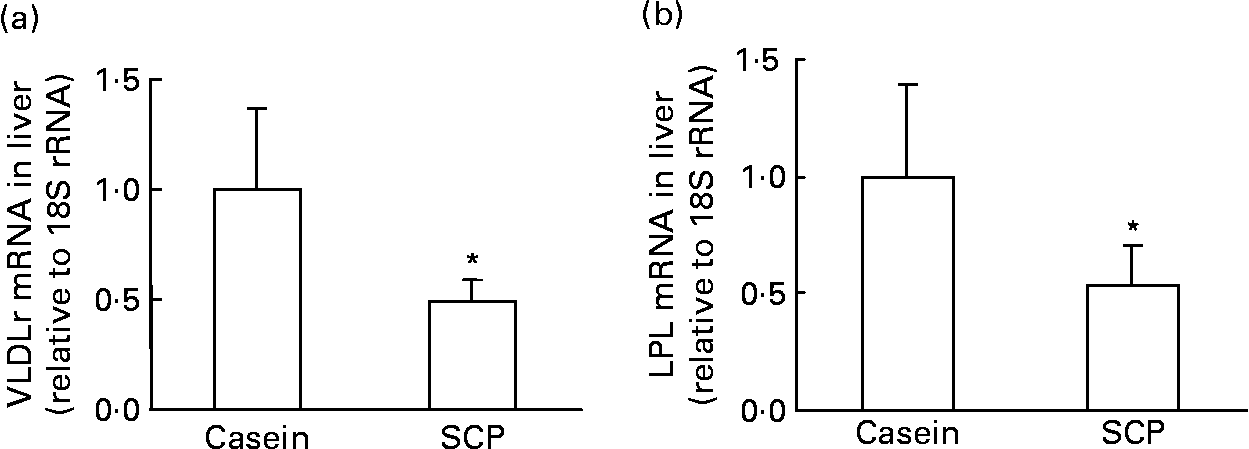
Fig. 5 mRNA levels (relative to 18S rRNA and normalised to controls) of VLDL-receptor (VLDLr) (a) and lipoprotein lipase (LPL) (b) in the liver of obese Zucker rats fed a diet containing casein or single cell protein (SCP). Values are means for six rats per group, with standard deviations represented by vertical bars. * Mean value was significantly different from that of the control (casein) group (P < 0·05).
The clearance of VLDL by white adipose tissue and skeletal muscle seems to be unaffected by SCP feeding, as no changes were seen in the mRNA levels of VLDL-receptor and LPL in these tissues (data not shown).
In order to get an impression of the VLDL secretion rate by the liver, we measured the hepatic mRNA levels of AADA, a lipase involved in the mobilisation of TAG from hepatocytes to the VLDL particle(Reference Trickett, Patel, Knight, Saggerson, Gibbons and Pease42). There was no change of the gene expression of AADA after SCP feeding (data not shown), suggesting that the secretion of VLDL from the liver was not affected.
Lipids, lipoproteins and fatty acids in plasma
Administration of SCP to obese Zucker rats resulted in an increased plasma TAG level when compared with controls, and this was accompanied by an increased TAG level in TAG-rich lipoproteins (Table 7).
Table 7 Levels of TAG in plasma and TAG-rich lipoproteins (TRL) from obese Zucker rats fed a diet containing casein or single cell protein (SCP)
(Mean values and standard deviations for six rats per group (plasma) or three pooled samples of two rats each (TRL))

* Mean value was significantly different from that of the control (casein) group (P < 0·05).
The fatty acid composition in plasma was affected by SCP feeding, as shown in Table 8. Significantly increased levels of SFA and MUFA were observed, mainly due to increased amounts of 16 : 0 and 18 : 1n-9 (Table 8). The plasma level of n-6 PUFA was significantly decreased in the SCP-fed rats, mainly due to a markedly reduced level of 20 : 4n-6 (Table 8). SCP feeding increased the plasma levels of 18 : 3n-3, 20 : 5n-3 and 22 : 5n-3, and reduced the level of 22 : 6n-3, but no change was seen in the total level of n-3 PUFA, and thus the ratio of n-3:n-6 PUFA was increased in these rats (Table 8). The anti-inflammatory fatty acid index, calculated as (22 : 5n-3+22 : 6n-3+20 : 3n-6)/20 : 4n-6(Reference Chavali, Zhong, Utsunomiya and Forse29), was increased in plasma after SCP feeding (Table 8).
Table 8 Selected fatty acids (g/100 g fatty acids) and the anti-inflammatory fatty acid index (AIFAI) in plasma from obese Zucker rats fed a diet containing casein or single cell protein (SCP)
(Mean values and standard deviations for six rats per group)
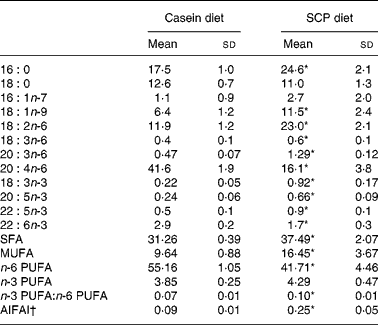
* Mean value was significantly different from that of the control (casein) group (P < 0·05).
† AIFAI was calculated as (22 : 5n-3+22 : 6n-3+20 : 3n-6)/20 : 4n-6(Reference Chavali, Zhong, Utsunomiya and Forse29).
Discussion
It has been reported that the prevalence of NAFLD in Western countries is increasing(Reference Marchesini, Marzocchi, Agostini and Bugianesi43), and that the prevalence and severity of NAFLD increase significantly with increasing obesity(Reference Silverman, O'Brien, Long, Leggett, Khazanie, Pories, Norris and Caro9–Reference Bellentani, Saccoccio, Masutti, Croce, Brandi, Sasso, Cristanini and Tiribelli11). In the present study we have shown that SCP feeding reduced the fatty liver as compared with casein feeding in obese Zucker rats.
SCP feeding increased the hepatic mitochondrial β-oxidation, and this could very well contribute to the reduced fatty liver. However, the increased mitochondrial β-oxidation of palmitoyl-CoA and palmitoyl-l-carnitine was not accompanied with increased mRNA levels of neither PPARα nor the classical PPARα target genes involved in fatty acid oxidation. Whether the increased mitochondrial β-oxidation was determined mostly by post-translational mechanisms should be considered. Moreover, the activity of acyl-CoA oxidase in the peroxisomal fraction was increased after SCP feeding, suggesting that the peroxisomal fatty acid β-oxidation was increased and thus may be important for the reduced fatty liver seen in these rats. It should be emphasised that mitochondria are by far the quantitatively dominating organelle in liver cells, proposing that an increase in mitochondrial oxidation might have the largest impact on the total β-oxidation and thus on the reduced hepatic TAG level.
The diets in the present experiment were similar except for the amino acid compositions of the proteins. Since both dietary and endogenously produced amino acids may affect lipid metabolism in liver, it was of interest to have knowledge about the amino acids of both dietary sources and in the liver. NO is produced from arginine by NO synthase in most mammalian cells(Reference Wu and Morris44), and is an important regulator of lipid metabolism since endogenous NO stimulates the oxidation of fatty acids in liver(Reference Khedara, Kawai, Kayashita and Kato45). In addition, physiological levels of NO mediate blood flow, therefore increasing the supply of metabolic substrates and O2 to mitochondria. The increased level of citrulline, a co-product of NO synthase, in the liver from SCP-fed rats suggested an increased systemic NO production in response to the higher arginine content in the SCP diet when compared with the casein diet, as others have demonstrated after dietary arginine supplementation to Zucker diabetic rats(Reference Fu, Haynes, Kohli, Hu, Shi, Spencer, Carroll, Meininger and Wu46). The reduced taurine level observed in the liver of SCP-fed rats could also contribute to a lower NO level, since taurine is shown to block NO production in hepatocytes(Reference Redmond, Wang and Bouchier-Hayes47). The reduced plasma level of the oxidant homocysteine previously observed after SCP feeding(Reference Gudbrandsen, Wergedahl, Liaset, Espe and Berge17) may also contribute to an increased NO production and bioavailability(Reference Wu and Meininger48). Although we have no direct measurements of the NO level or of the synthesis thereof, our findings suggest that the hepatic β-oxidation in SCP-fed rats could be up-regulated by PPARα-independent mechanisms through an increased NO production.
Several mechanisms of action may be involved in the progress of fatty liver. Studies in mice have provided evidence that SCD-1 could play an important role in the development of hepatic steatosis, since it is an enzyme necessary for the synthesis of TAG(Reference Dobrzyn and Ntambi49). In line with this, we found that the reduced fatty liver after SCP feeding was accompanied by a decreased mRNA level of SCD-1 and reduced level of 18 : 1n-9 in TAG and 1,2-diacylglycerol in liver, and in addition we have previously shown that SCP-fed rats had a low level of 18 : 1n-9 also in hepatic cholesteryl esters(Reference Gudbrandsen, Wergedahl, Liaset, Espe and Berge17). This suggests that also the activity of SCD-1 was lower in SCP-fed rats. The low content of neutral lipids in the liver of SCP-fed rats may, at least partially, be a consequence of the low SCD-1 activity, as 18 : 1n-9 is the preferred substrate for DGAT and acyl-CoA:cholesterol acyltransferase(Reference Miyazaki, Kim, Gray-Keller, Attie and Ntambi50), the rate-determining enzymes for the biosynthesis of TAG and cholesteryl esters, respectively.
Since SCP feeding did not significantly affect the activities of acetyl-CoA carboxylase or fatty acid synthase, or the mRNA levels of PPARγ or SREBP-1c, this argues against the contention that decreased lipogenesis is of major importance to improve hepatic steatosis. In addition, as no change was seen in the activities of glycerol-3-phosphate acyltransferase and DGAT or in the mRNA level of DGAT-1, SCP feeding seemed not to affect the TAG synthetic rate. However, the hepatic SCD-1 activity determines the metabolic fate of endogenous lipids by driving newly synthesised fatty acids preferentially towards TAG esterification and VLDL assembly and secretion rather than mitochondrial influx and β-oxidation(Reference Ntambi, Miyazaki, Stoehr, Lan, Kendziorski, Yandell, Song, Cohen, Friedman and Attie51). Thus, the reduced SCD-1 mRNA level in the liver of SCP-fed rats could be an important mechanism to reduce the hepatic TAG content. No direct examination of VLDL secretion was undertaken, but the gene expressions of AADA, a lipase involved in the mobilisation of TAG from hepatocytes to the VLDL particle(Reference Trickett, Patel, Knight, Saggerson, Gibbons and Pease42), was not changed by SCP feeding. Thus, the increased TAG level in plasma and TAG-rich lipoproteins is probably not associated with an increase in the synthesis and secretion of newly synthesised TAG.
The hepatic gene expressions of Δ6 desaturase and Δ5 desaturase were decreased after SCP feeding, suggesting that the biosynthesis of 20 : 4n-6 from 18 : 2n-6 and of 20 : 5n-3 from 18 : 3 n-3 were restrained. In line with these findings, the level of 18 : 2n-6 was increased in hepatic phospholipids, TAG and 1,2-diacylglycerol, and in plasma, whereas the level of 20 : 4n-6 was decreased in hepatic phospholipids and especially in plasma. Interestingly, the level of 20 : 5n-3, the synthesis of which is catalysed by the same enzymes as 20 : 4n-6, was increased in all liver lipids and in plasma after SCP feeding. Upon cell activation, 20 : 4n-6 released from membrane phospholipids can be converted into pro-inflammatory eicosanoids, whereas anti-inflammatory eicosanoids can be derived from 20 : 5n-3. Thus, since feeding SCP reduced the content of 20 : 4n-6 and increased the content of 20 : 5n-3 in phospholipids, SCP may prevent the development of inflammatory diseases such as fatty liver, as patients with NAFLD can be characterised by a low-grade systemic inflammation(Reference Haukeland, Damås, Konopski, Løberg, Haaland, Goverud, Torjesen, Birkeland, Bjoro and Aukrust52). It was therefore of interest that SCP feeding increased the anti-inflammatory fatty acid index in plasma almost three-fold, implying that SCP may improve both the hepatic steatosis and the systemic inflammation in these rats. This was supported by a reduced plasma level of TNFα and a propensity to decrease the gene expression of TNFα in liver (P < 0·15) after SCP feeding. ADRP may play an important role in lipid droplet formation in liver(Reference Steiner, Wahl, Mangold, Robison, Raymackers, Meheus, Anderson and Cordier53), and the gene expression of ADRP is up-regulated in liver steatosis in humans and mice(Reference Motomura, Inoue, Ohtake, Takahashi, Nagamine, Tanno, Kohgo and Okumura39); it has therefore been suggested that ADRP may be induced by inflammation(Reference Corsini, Viviani, Zancanella, Lucchi, Visioli, Serrero, Bartesaghi, Galli and Marinovich54). However, we found that SCP showed only a weak tendency to decrease the gene expression of ADRP in liver (P < 0·18). Overall, the changes in the fatty acid composition in liver and plasma, the reduced amount of TNFα in plasma together with the tendency to decrease the hepatic mRNA levels of TNFα and ADRP after SCP feeding suggested that this dietary protein could improve the inflammatory status in these rats.
The gene expressions of VLDL-receptor and LPL in liver were down-regulated by approximately 50 % after feeding SCP, suggesting that the hepatic uptake of TAG-rich lipoproteins was decreased. Normally, the VLDL-receptor and LPL are expressed in adipose tissue and skeletal muscle, and only in very low levels in the liver(Reference Oka, Ishimura-Oka, Chu, Sullivan, Krushkal, Li and Chan55). However, no changes were seen in the expressions of VLDL-receptor and LPL in adipose tissue after SCP feeding, suggesting that the mechanism of reduced fatty liver by SCP did not involve an increased capacity to store lipids in epididymal adipose tissue. Similarly, the uptake of lipids and fatty acids to skeletal muscle seemed not to be affected by SCP feeding, as no changes were seen in the mRNA levels of these genes in the muscle. A reduced clearance of VLDL and chylomicrons by the liver could, at least partially, explain both the increased plasma TAG level and the reduced fatty liver in SCP-fed rats.
In summary, SCP feeding of obese Zucker rats reduced fatty liver, and the mechanisms involved seem to include increased fatty acid oxidation combined with changed fatty acid composition, reduced SCD-1 mRNA level and possibly reduced clearance of TAG-rich lipoproteins by the liver.
Acknowledgements
The research was supported by the Norwegian Research Council.
We declare that there are no conflicts of interest regarding this paper.
O. A. G., H. W., B. L., M. E. and R. K. B. designed the study. O. A. G., H. W. and B. L. performed the animal experiment, and analysed and interpreted the data except from the Scharlach red staining of the livers (performed by S. M). M. E. and R. K. B. supervised the experiment, obtained funding and provided administrative, technical, and material support. O. A. G. wrote the draft of the manuscript; all authors critically reviewed the manuscript.















