Introduction
Frozen–thawed embryo transfer (FET) has been successfully performed world-wide and provides further opportunities for patients to achieve pregnancy in addition to fresh embryo transfers (Ubaldi et al., Reference Ubaldi, Rienzi, Baroni, Ferrero, Iacobelli, Minasi, Sapienza, Martinez, Anniballo, Cobellis, Tesarik and Greco2004). The first successful pregnancy following FET was reported 3 decades ago by Trounson & Mohr (Reference Trounson and Mohr1983). Moreover, this strategy provides a means of reducing the number of embryos transferred per fresh cycle, thus reducing the risk of multiple pregnancy (Tiitinen et al., Reference Tiitinen, Halttunen, Harkki, Vuoristo and Hyden-Granskog2001). Cryopreservation of the entire cohort of a patient's embryos provides additional clinical safety for patients with a high risk of ovarian hyperstimulation syndrome (OHSS; by cancelling fresh embryo transfer and cryopreserving all available embryos). For women who are poor ovarian responders (PORs), a programme of freezing the entire cohort of embryos can accumulate the number of embryos, as well as optimize the timing of embryo transfer. For patients whose endometrium is not receptive for fresh transfer, cryopreservation of the entire cohort of embryos and FET will be chosen to enhance the embryo implantation rate (IR).
Many factors may affect the clinical outcome of FET: controlled ovarian hyperstimulation (COH) protocol, freezing protocol, the selection of embryos for freezing and transfer, and endometrial preparation before embryo transfer, as well as the age of women undergoing FET (Balaban et al., Reference Balaban, Urman, Ata, Isiklar, Larman, Hamilton and Gardner2008; Ghobara & Vandekerckhove, Reference Ghobara and Vandekerckhove2008; Givens et al., Reference Givens, Markun, Ryan, Chenette, Herbert and Schriock2009; Ashrafi et al., Reference Ashrafi, Jahangiri, Hassani, Akhoond and Madani2011; Eftekhar et al., Reference Eftekhar, Aflatoonian, Mohammadian and Tabibnejad2012; Sun et al., Reference Sun, Feng, Zhang, Lu, Niu and Gu2012).
A previous study by Zhu et al. (Reference Zhu, Zhang, Cao, Heng, Huang, Ling, Duan and Tong2011) reported superior clinical outcomes for vitrified–warmed blastocyst transfer (BT) cycles compared with fresh BT, which would imply that vitrification at a later embryonic stage may give better results. In another previous study by Zhang et al. (Reference Zhang, Cui, Ling, Li, Peng, Guo, Heng and Tong2009), it was found that mouse embryos at the 8-cell stage had the best tolerance for vitrification and would yield the highest level of post-vitrification developmental competence amongst early cleavage-stage embryos. Hence, the question is whether cleavage-stage human embryos on day 3 (6–8-cell stage) have a better tolerance for vitrification and hence give better clinical outcome after vitrification–warming, as compared with embryos at the 3–4-cell stage on day 2. To answer this question, the data of patients that had their entire cohort of embryos vitrified, either to prevent ovarian hyperstimulation syndrome (OHSS) or to accumulate embryos in the case of PORs in which up to three oocytes were retrieved (Ferraretti et al., Reference Ferraretti, La Marca, Fauser, Tarlatzis, Nargund and Gianaroli2011) were retrospectively analysed. The clinical outcomes for embryos cryopreserved on day 2 were compared with those cryopreserved on day 3, and significantly different results were observed.
Materials and methods
Patients
This retrospective study included patients who were undergoing day 2 or day 3 vitrified–warmed embryo transfer at the IVF Programme of Shanghai First Maternity and Infant Hospital, affiliated to the Tongji University School of Medicine, from April 2010 to March 2012. Written consent forms were collected for all IVF treatment procedures. The data included 168 patients with 185 oocyte retrieval cycles undergoing 219 vitrified–warmed transfer (VWT) cycles at day 2 embryonic stage (day 2 vitrification group). Among them, 53 POR patients had 69 oocyte retrieval cycles and received 50 VWT cycles (POR day 2 group); the other 115 patients had their entire cohort of embryos vitrified to prevent OHSS, and underwent 116 oocyte retrieval cycles and 169 VWT cycles (OHSS prevention day 2 group). The data also included 128 patients with 142 oocyte retrieval cycles undergoing 161 VWT cycles after vitrification of their entire cohort of embryos on day 3 (day 3 vitrification group). Among them, 35 POR patients had 49 oocyte retrieval cycles and received 34 VWT cycles (POR day 3 group); the other 93 patients had their entire cohort of embryos vitrified to prevent OHSS, and underwent 93 oocyte retrieval cycles and 127 VWT cycles (OHSS prevention day 3 group). The causes of infertility included fallopian tubal factor, male factor, combined female factors, combinations of female and male factors, ovulatory disorder and idiopathic infertility.
COH protocol and assessment of embryo quality
Standard long protocol and gonadotropin-releasing hormone (GnRH) antagonist protocol were applied for patients with high risk of OHSS. The GnRH agonist short protocol and mild stimulation protocol were applied for POR patient. Briefly, the standard long protocol was carried out as follows: 0.1 mg subcutaneously (SC) GnRH agonist triptorelin (Decapeptyl; Ipsen-Biotech Inc., Paris, France) was administered in the midluteal phase of the previous cycle, followed by daily administration of 225 IU intramuscular of recombinant human follicle-stimulating hormone (FSH; Gonal-F; Merck-Serono Inc., Geneva, Switzerland) from day 3 onward. For the GnRH agonist short protocol, GnRH agonist and FSH were administered together daily from day 3 onwards. For the GnRH antagonist protocol, FSH was used from day 3 onward and administered 0.125 mg SC GnRH antagonist cetrorelix acetate (Tarceva, Baxter Oncology GmbH, Germany) daily when follicles were ≥14 mm. In a mild stimulated cycle, low-dose human menopausal gonadotrophin (HMG) (Menotrophin for Injection, Livzon Pharmaceutical Group Inc., Guangdong, China) was utilized.
Oocyte retrieval was performed 36 h after human chorionic gonadotrophin (HCG) administration with transvaginal ultrasound-guided aspiration of the follicles. Embryo quality assessment was based on morphology and development rate in culture. Four grades of embryos were defined: grade I, embryos had blastomeres of equal size and cytoplasmic fragmentation was ≤5% of the embryo surface; grade II, embryos had blastomeres of equal or unequal size and cytoplasmic fragmentation was ≤20% of the embryo surface; grade III, embryos had blastomeres of equal or unequal size and 21–49% overall cytoplasmic fragmentation; grade IV, embryos had blastomeres of equal or unequal size and cytoplasmic fragmentation was ≥50% of the embryo surface. Embryos with a normal cleavage rate (3–4 cells on day 2 and 6–8 cells on day 3) of grade I and grade II was vitrified for all the patients of the current study. To minimize the influence of embryo quality variation, grade III and grade IV embryos were excluded from the study.
Embryo vitrification and warming
Embryos of grade I and grade II were vitrified and warmed based on the Cryotop method (Kuwayama, Reference Kuwayama2007). Embryos were vitrified using the Cryotop device and commercially available vitrification solutions (Kitazato, Japan). The first equilibration step was performed in equilibration solution at room temperature for 10–15 min within a 50-μl droplet. Subsequently, the embryos were transferred to vitrification solution for 30–60 s, and then placed on the film strip of the Cryotop within a single small droplet. Excess vitrification solution was removed by aspiration with a flame-pulled pipette to leave just a thin liquid layer around each embryo. The strip was submerged in liquid nitrogen for storage.
Vitrified embryos were warmed to 37°C using a vitrification–warming kit (Kitazato, Japan). During warming, the cap was removed under liquid nitrogen, and the film strip of the Cryotop device was quickly submerged in 1 ml of 37°C warming solution that contained 1.0 M sucrose for 1 min, followed by transfer of the embryos to a diluent solution at room temperature containing 0.5 M sucrose and incubated for 3 min. After two subsequent washes in basic medium at room temperature for 5 min and 1 min respectively, the embryos were transferred into 50 μl droplets of culture medium under mineral oil.
Endometrium preparation for vitrified–warmed embryo transfer
For endometrium preparation, hormone replacement was applied, in which estradiol valerate (Progynova, Germany) was administered orally at a dose of 3 mg twice daily from day 2 to day 10 of the menstrual cycle (Wright et al., Reference Wright, Guibert, Weitzen, Davy, Fauque and Olivennes2006). When the endometrium thickness exceeded 8 mm, 60 mg of progesterone injection was administered daily for 3 days before vitrified–warmed embryo transfer. The original dosage of estradiol valerate and progesterone were maintained until pregnancy testing was carried out, 14 days after vitrified–warmed embryo transfer.
Embryo transfer
Vitrified–warmed embryos were transferred transcervically to the middle of the uterine cavity under ultrasound guidance, 3 h after warming. According to the IVF-embryo transfer (ET) regulations in China, no more than two embryos were transferred in patients under 35 years old for their first transfer cycle and up to three embryos were transferred in older patients (more than 35 years old) or patients with history of failed IVF treatment.
Luteal-phase support was performed with intramuscular injections of 60 mg of progesterone daily for 14 days until pregnancy testing was performed. Pregnant patients received daily administration of 90 mg sustained-release vaginal progesterone gel (Crinone 8%, Merck-Serono, England) until 7 weeks of gestation, when clinical pregnancy was observed (i.e. gestational sac and fetal heartbeat was detected by transvaginal ultrasound scan). The number of sacs was taken as the number of implantations. The miscarriage rate included both biochemical and clinical miscarriages, calculated as a proportion of positive β-hCG test data. None of the patients suffered ectopic pregnancy in the current study.
Statistical analysis
Statistical analysis was performed with either Student's t-test for comparison of mean values or chi-squared test for comparison of percentages using the Statistical Package for Social Science, version 17.0 (SPSS Inc., Chicago, IL, USA). A two-sided P-value <0.05 was considered to be statistically significant.
Results
Comparison of clinical outcomes of POR groups
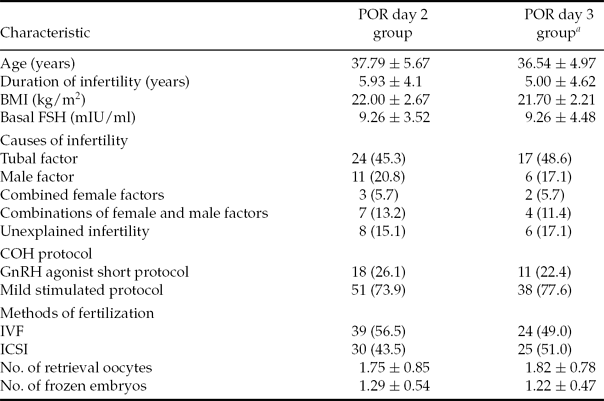
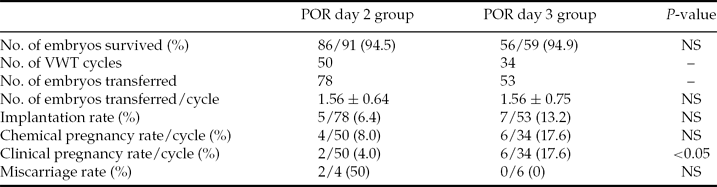
The various patient parameters were not significantly different between the day 2 and day 3 vitrification groups for POR patients (Table 1). For POR patients, the day 2 group included 69 oocyte retrieval cycles. However, nine patients cancelled VWT because no embryo was available (did not survive: <50% blastomeres were intact) and 20 embryos were combined for 10 VWT cycles. In total, 78 day 2 vitrified embryos were transferred in 50 VWT cycles (mean number of embryos transferred per cycle was 1.56 ± 0.64). The IR was 6.4% (5 of 78), with four patients having increasing serum β-hCG concentrations and two patients achieving clinical pregnancy (4.0% per transfer cycle). The miscarriage rate was 50% (two out of four). The day 3 POR group included 49 oocyte retrieval cycles: five patients cancelled VWT because no embryo was available (did not survive: <50% blastomeres were intact) and 20 combined their embryos together into nine VWT cycles. Out of 34 VWT cycles, 53 day 3 vitrified embryos were transferred (mean number of embryos transferred per cycle was 1.56 ± 0.75). The IR was 13.2% (seven out of 53), with six patients testing positive for pregnancy and all of them achieving clinical pregnancy (17.6% per transfer cycle) with no miscarriages. Therefore, the day 3 group for POR patients yielded better clinical outcomes than the day 2 group. A significant difference was observed in the clinical pregnancy rate (CPR) (17.6% vs. 4.0%, P = 0.036) (Table 2). Additionally, the percentages of grade I and grade II embryos for the day 2 and day 3 groups were not significantly different and the various different COH protocols did not affect the outcomes (data not shown).
Table 1 Comparison of patient characteristics between day 2 vitrification group and day 3 vitrification group for POR patients

Note: Values are presented as n, n (%) or mean ± standard deviation (SD). BMI, body mass index, weight (kg)/height (m)2; COH, controlled ovarian hyperstimulation; FSH, follicle stimulating hormone; GnRH, gonadotropin releasing hormone; ICSI, intracytoplasmic sperm injection; IVF, in vitro fertilization; POR, poor ovarian responders.
aP = not statistically significant (NS) (POR day 2 group vs. day 3 group for all values).
Statistical analysis: Student's t-test for comparison of mean values and a chi-squared test for comparison of percentages.
Table 2 Clinical outcomes, POR day 2 group versus POR day 3 group

Note: Values are presented as n, n (%), or mean ± standard deviation (SD). NS, not statistically significant.
Statistical analysis: Student's t-test for comparison of mean values and a chi-squared test for comparison of percentages.
POR, poor ovarian responders; VWT, vitrified–warmed transfer.
Comparison of clinical outcomes of OHSS prevention groups
The various patient parameters were not significantly different between day 2 and day 3 OHSS prevention groups (Table 3), making comparison between the two data sets meaningful.
Table 3 Comparison of patient characteristics between day 2 vitrification group and day 3 vitrification group for OHSS prevention patients

Note: Values are presented as n, n (%), or mean ± standard deviation (SD). BMI, body mass index weight (kg)/height (m)2; COH, controlled ovarian hyperstimulation; FSH, follicle stimulating hormone; ICSI, intracytoplasmic sperm injection; IVF, in vitro fertilization; OHSS, ovarian hyperstimulation syndrome.
aP = not statistically significant (NS) (OHSS prevention day 2 group vs. day 3 group for all values).
Statistical analysis: Student's t-test for comparison of mean values and a chi-squared test for comparison of percentages.
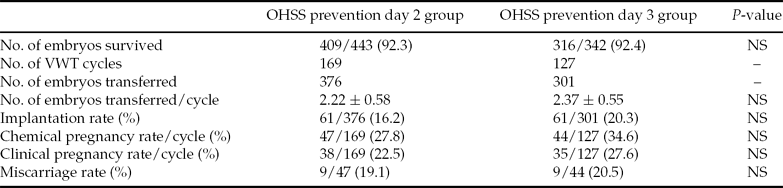
In total, 376 day 2 vitrified embryos were transferred in 169 cycles (mean number of embryos transferred per cycle was 2.22 ± 0.58) for the OHSS prevention day 2 group. The IR was 16.2% (61 of 376), with 47 patients having an increasing serum β-hCG concentration, and the CPR was 18.3% per transfer cycle (38 of 169) while the miscarriage rate was 19.1% (9 of 47), including one patient with a hydatidiform mole. In the day 3 group, a total of 301 embryos were transferred in 127 VWT cycles and the mean number of embryos transferred per cycle was 2.37 ± 0.55). The IR was 20.3% (61 of 301): 44 patients tested positive for pregnancy and 35 of these achieved clinical pregnancy (27.6% per transfer cycle), while the miscarriage rate was 20.5% (9 of 44). The implantation and CPR of the OHSS prevention day 3 group were higher than those of the OHSS prevention day 2 group, although the differences were not statistically significant (Table 4). The percentages of grade I and grade II embryos for the day 2 and day 3 groups were not significantly different and different COH protocols did not influence the outcomes (data not shown).
Table 4 Clinical outcomes, OHSS prevention day 2 group versus day 3 group

Note: Values are presented as n, n (%), or mean ± standard deviation (SD). NS, not statistically significant.
Statistical analysis: Student's t-test for comparison of mean values and a chi-squared test for comparison of percentages.
OHSS, ovarian hyperstimulation syndrome; VWT, vitrified–warmed transfer.
Comparison of clinical pregnancy rates of day 2 and day 3 vitrified embryos with one or two VWT cycles
Of those patients who had only one embryo vitrified on day 2, none became pregnant (44 oocyte retrieval cycles), either VWT with the single embryo alone (24 oocyte retrieval cycles) or combined with other embryos. However, four patients became pregnant among those who had only one embryo vitrified on day 3 (35 oocyte retrieval cycles). Two of these patients had VWT with the one single embryo (19 oocyte retrieval cycles), while the other two patients became pregnant by transfer in combination with other embryos. Vitrification of one embryo on day 3 is recommended for these patients, and accumulating more embryos for transfer is suggested as a better clinical strategy.
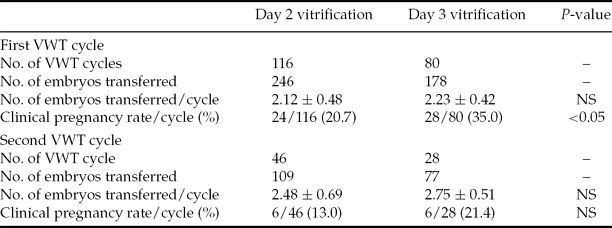
For patients who had more than three embryos vitrified on day 2, 24 patients achieved clinical pregnancy after their first VWT out of 116 cycles (20.7% per transfer cycle). The pregnancy rate was 35.0% (28 of 80) per transfer cycle after the first VWT for patients who had more than three embryos vitrified on day 3, which was significantly higher than that for day 2 vitrified embryos (35.0 vs. 20.7%, P = 0.026). Forty-six patients who did not become pregnant after their first VWT underwent a second VWT with day 2 vitrified embryos and six patients achieved clinical pregnancy (13.0%). Twenty-eight patients underwent a second VWT with day 3 vitrified embryos, and six of these patients also achieved clinical pregnancy (21.4%) (Table 5). To date, none of the 46 patients with enough day 2 vitrified embryos or 24 patients with enough day 3 vitrified embryos has undergone a second VWT, so the cumulative pregnancy rate cannot be calculated.
Table 5 Clinical pregnancy rate of day 2 vitrified embryos and day 3 vitrified embryos with one or two VWT cycles

Note: Values are presented as n, n (%), or mean ± standard deviation (SD). NS, not statistically significant.
Statistical analysis: Student's t-test for comparison of mean values and a chi-squared test for comparison of percentages.
VWT, vitrified–warmed transfer.
Discussion
It was observed that delaying fresh ET from day 2 to day 3 could yield increased pregnancy rates through better selection of good-quality embryos (Oatway et al., Reference Oatway, Gunby and Daya2004), although a previous study showed that the implantation and pregnancy rates were comparable for day 2 and day 3 ET (Laverge et al., Reference Laverge, De Sutter, Van der Elst and Dhont2001). Prolonging the duration of culture in vitro before freezing could increase the possibility of obtaining more high quality embryos for cryopreservation, with better developmental potential. The results of the current retrospective study demonstrated that day 3 vitrification did not affect embryo survival rate, but yielded better clinical outcomes compared to day 2 vitrification. This correlates with the results of Sifer and colleagues, but the cryopreservation method is different, and in their study all the day 2 frozen embryos were transferred after culturing overnight (Sifer et al., Reference Sifer, Sellami, Poncelet, Martin-Pont, Porcher, Hugues and Wolf2006).
When embryo quality is optimal, the synchronization of embryo development with endometrial growth becomes an important factor for successful pregnancy. In a previous study, vitrified–warmed BT cycles had superior clinical outcomes compared with fresh blastocyst transfer, due to better embryo-endometrial synchronization (Zhu et al., Reference Zhu, Zhang, Cao, Heng, Huang, Ling, Duan and Tong2011). In the current study, it was found that day 3 vitrification resulted in a significantly higher CPR (17.6 vs. 4.0%, P = 0.036) for women who were PORs than day 2 vitrification. Additionally, it has also been shown that day 3 vitrification resulted in better clinical outcomes with higher implantation and CPR than day 2 vitrification for women who had their entire cohort of embryos vitrified to prevent OHSS, although the differences were not statistically significant. For patients who had more than three embryos vitrified, the CPR after the first VWT cycle with day 3 vitrified embryos was significantly higher than that with day 2 vitrified embryos (35.0 vs. 20.7%, P = 0.026). All of the current findings imply that vitrification at a later embryonic stage yields better results, particularly for patients who are PORs.
For women with few available embryos or poor ovarian response, some previous research has suggested that day 2 fresh ET is preferable for better clinical outcomes (Bahceci et al., Reference Bahceci, Ulug, Ciray, Akman and Erden2006; Shen et al., Reference Shen, Rosen, Dobson, Fujimoto, McCulloch and Cedars2006). However, Dayal and colleagues found that, for PORs, day 3 fresh ET yielded higher pregnancy rates (13 vs. 16%) and higher live birth rates (10 vs. 16%), although the differences were not statistically significant (Dayal et al., Reference Dayal, Frankfurter, Athanasiadis, Peak, Dubey and Gindoff2011). To our knowledge, there is no reported data on clinical outcomes of vitrified embryos for PORs prior to the current study. If embryo vitrification is required for PORs, the current study can be a reference although the data are limited. Nevertheless, the current results would suggest day 3 embryo vitrification.
It was reported that the observed frequency of blastomere loss resulted in a reduction of approximately 30% in the implantation potential of a population of embryos following cryopreservation (Edgar et al., Reference Edgar, Bourne, Speirs and McBain2000). Joshi and colleagues found that FETs of embryos cultured overnight yielded a higher pregnancy rate than embryos not cultured overnight (24.3 vs. 20.3%, not significantly different), because there were more blastomeres within the transferred embryos after culturing overnight (Joshi et al., Reference Joshi, Banker, Patel and Shah2010). It should be noted that the freezing method for the above study was programmed slow freezing, which is different from the vitrification protocol used in the present study. Some studies have also shown that vitrification is associated with higher survival rates than programmed slow freezing (Balaban et al., Reference Balaban, Urman, Ata, Isiklar, Larman, Hamilton and Gardner2008; Loutradi et al., Reference Loutradi, Kolibianakis, Venetis, Papanikolaou, Pados, Bontis and Tarlatzis2008). Spatio-temporal expression of developmentally important transcriptional factors (Oct4, Stk40, Cdx2, etc.) is of the utmost importance for early survival of mammalian embryos. Vitrification performed one day earlier probably impaired the transcriptional activation network, resulting in early embryo growth retardation and therefore lower CPR.
It is also possible that day 3 vitrified embryos may have some interactive ‘cross-talk’ with the endometrium, whereas day 2 vitrified embryos may be too immature to participate in this process. The possibility that day 2 vitrification could have impaired the implantation potential of day 2 embryos cannot be excluded. Indeed, embryonic genomic activation is supposed to occur between the 4- and 8-cell stages and extended exposure to in vitro conditions may be deleterious to embryonic development (Braude et al., Reference Braude, Bolton and Moore1988; Jurisicova & Acton, Reference Jurisicova and Acton2004). However, it was recently reported that transfer of blastocysts derived from frozen–thawed cleavage-stage embryos or vitrified–warmed blastocysts can improve ongoing pregnancy, and the most important reason was better embryo-endometrial synchronization (Zhu et al., Reference Zhu, Zhang, Cao, Heng, Huang, Ling, Duan and Tong2011; Eftekhar et al., Reference Eftekhar, Aflatoonian, Mohammadian and Tabibnejad2012; Zhu et al., Reference Zhu, Zhang, Cao, Heng, Huang, Ling, Duan and Tong2011).
In conclusion, day 3 embryo vitrification resulted in better clinical outcome than day 2 embryo vitrification for patients who had their entire cohort of embryos cryopreserved. Therefore, it is highly recommended that cleavage-stage embryos should be vitrified on day 3 but not on day 2, particularly for poor ovarian responder patients.
Acknowledgements
The study was supported by the National Natural Science Foundation of China (Grant Numbers 81070494 and 81170571).