Diabetes mellitus is a global emergency. There are currently 415 million individuals with diagnosed diabetes (DD) or undiagnosed diabetes (UDD) in the world, and this figure is expected to increase to 642 million by the year 2040(1). The ageing population and consequent increase in the prevalence of chronic diseases, along with the increase in the prevalence of obesity(2), are recognised causes of this phenomenon.
Many individuals with diabetes mellitus are undiagnosed and therefore remain without treatment, which increases the risk of complications(Reference Wild, Roglic and Green3). The diagnosis of the disease in individuals older than 50 years of age is often hindered due to the masking of the symptoms resulting from age-related changes(Reference Sue Kirkman, Briscoe and Clark4,Reference Chau and Edelman5) . Moreover, individuals with prediabetes (PD) have a 20-fold greater risk of developing diabetes mellitus(1,6) . Although PD has outcomes of the typical microvascular complications found in diabetes mellitus (nephropathy, retinopathy and neuropathy), this condition has few obvious clinical signs(Reference Haffner7,8) and can last for more than a decade.
The prevalence of PD, UDD and DD can differ substantially between countries depending on socio-demographic characteristics. Low schooling, low income, a sedentary lifestyle and an inadequate diet(Reference Lima-Costa, De Oliveira and MacInko9), together with the difficulty in identifying individuals with diabetes mellitus, are aggravating factors in developing countries(Reference Fisher-Hoch, Vatcheva and Rahbar10). To the best of our knowledge, however, no studies have made comparisons of these aspects between developed and developing countries beyond the determination of different prevalence rates(Reference Fisher-Hoch, Vatcheva and Rahbar10–Reference Kumar, Wong and Ottenbacher14).
We hypothesise that low–middle-income countries may have a higher prevalence of PD, UDD and DD due to adverse health and socio-demographic factors, as well as rapid and intense nutritional transition accompanied by less effective health policies and healthcare system.
Such comparisons are important and can assist in identifying differences among factors associated with diabetes as well as opportunities to minimise such differences. Therefore, the aim of the present study was to analyse differences in the prevalence of PD, UDD and DD and associated factors between English and Brazilian individuals 50 years of age or older.
Methods
Data sources: ELSA and ELSI
Data came from the English Longitudinal Study of Ageing (ELSA) and the Brazilian Longitudinal Study of Aging (ELSI (Estudo Longitudinal de Saúde e Bem-Estar dos Idosos Brasileiros)). They are international harmonised (sister) ageing cohorts that not only provide data for individual countries but also offer the valuable opportunity for cross-national comparisons. ELSA is an ongoing panel study commenced in 2002 involving community-dwelling individuals in England aged 50 years or older. ELSA has a nationally representative sample using a multi-stage stratified probability sampling design(Reference Mindell, Biddulph and Hirani15,Reference Steptoe, Breeze and Banks16) . ELSI is a home-based longitudinal study conducted with a representative sample of individuals aged 50 years or older from 70 municipalities across different geographical regions of Brazil. Initiated in 2015–2016, a comprehensive sampling procedure involved different stages of selection, that is, municipalities, census sectors and households. An inverse sampling process was adopted, with 9412 participants.
For the present analysis, we selected participants from the sixth wave of ELSA (conducted in 2012–2013) and the baseline of ELSI. The eligibility criteria for the current analysis were the determination of serum glycated hemoglobin (HbA1c), information on a medical diagnosis of diabetes mellitus and data on the variables of interest. All participants of ELSA were eligible for laboratory exams. However, 1916 out of the 9169 eligible individuals did not have an HbA1c measurement. A probabilistic subsample of 4000 ELSI participants was selected to have blood samples collected, with HbA1c results in 59 % (2360) of these individuals. Further information on the sampling process of these two studies can be found in previous publications(Reference Steptoe, Breeze and Banks16,Reference Lima-Costa17) .
We have further excluded 1952 individuals from ELSA and 413 individuals from ELSI for the lack of information on self-reported doctor-diagnosed diabetes or covariates. The final sample in the present study was composed of 5301 ELSA participants and 1947 ELSI participants. Figure 1 shows the sample selection flowchart.

Fig. 1 Sample selection flowchart at ELSA and ELSI studies
The current study followed the Strengthening the Reporting of Observational Studies in Epidemiology reporting guideline(Reference Von Elm, Altman and Egger18).
Dependent variable
The dependent variable was diabetes mellitus categorised according to self-report and the criteria of the American Diabetes Association for serum HbA1c. The participants were classified as non-diabetic (no self-report of diabetes and HbA1c < 5·7 %), prediabetic (no self-report of diabetes and HbA1c ≥5·7 and <6·5 %), undiagnosed diabetic (no self-report of diabetes and HbA1c ≥ 6·5 %) and diagnosed diabetic (self-reported diabetes independently of HbA1c)(8). Diagnosis tests for serum HbA1c were performed in laboratories using the method certified by the National Glycohemoglobin Standardization Program and standardised to the Diabetes Control and Complications Trial reference assay. The present study adheres to the 2012(19) and 2015(20) editions of the American Diabetes Association for the ELSA and ELSI studies, respectively. Both editions followed the same criteria to diagnose diabetes.
Variables of interest
The variables of interest were those previously defined as being associated with diabetes mellitus, including socio-demographic characteristics, health-related behaviours, anthropometrics, self-reported health conditions and serum cholesterol(Reference Bellou, Belbasis and Tzoulaki21).
Socio-demographic characteristics were age (in years), sex, schooling (0–11, 12–13 or 14 years or more), marital status (married v. single, divorced or widowed), self-declared skin colour (white/non-white) and family income (quintiles).
Heath-related behaviours as alcohol intake (non-drinker, frequent drinker (two to six times per week) or daily drinker) and smoking status (non-smoker, ex-smoker or current smoker) were assessed similarly in both studies. Physically inactive individuals were considered those who performed < 150 min of physical activity per week measured using the Brazilian version of the International Physical Activity Questionnaire in ELSI. In ELSA, physically inactive individuals were those who did not practice any physical activity at least once a week according to the modified version of the Physical Activity and Sedentary Behaviour Assessment Questionnaire(Reference Scholes, Coombs and Pedisic22).
Anthropometrics measurements collected were body mass index (BMI) and waist circumference. The BMI was calculated by dividing weight in kilograms by height in meters squared (kg/m2) and classified as follows: underweight (<18·5 kg/m2), ideal range (18·5 to <25 kg/m2), overweight (25 to <30 kg/m2) or obese (≥30 kg/m2). Waist circumference was measured using a metric tape at the midpoint between the lowest rib and the upper edge of the iliac crest at the end of the expiratory phase(Reference Banks, Breeze and Lessof23). Abdominal obesity was defined based on waist circumference: >102 cm for men and > 88 cm for women(24).
Clinical conditions based on self-reported doctor diagnosis of hypertension, CVD and stroke were obtained. The biochemical measures included were serum high density lipoprotein (HDL) (<1·03 mmol/l for men and <1·29 mmol/l for women) and triacylglycerol (TAG) (≥1·69 mmol/l)(Reference Grundy, Becker and Clark25).
Statistical analysis
The prevalence was calculated with 95 % CI. We performed the direct standardization method having the ELSA sample as the standard population to calculate the standardised prevalence for the ELSI study, adjusting it by age, sex and schooling years(Reference Naing26). Differences in the characteristics of the sample according to diabetes status (non-diabetic, prediabetic, undiagnosed diabetic and diagnosed diabetic) in ELSA were evaluated using the χ 2 test, ANOVA and Tukey’s post-hoc test. These differences in ELSI were evaluated using the χ 2 test with the Rao–Scott correction and the Rao and Scott Wald test, as ELSI has a weighted sample. The multinomial regression model was used to allow the analysis of factors associated with diabetes status, a dependent variable with four categories. This model is a modification of the binary logistic regression recommended for modelling relationships of multinomial responses without imposing any restrictions on the ordinality of the response(Reference Ananth and Kleinbaum27). In interpreting the results calculated as OR, non-diabetic is the reference category (OR = 1·00) to compare the estimates of the prediabetic, undiagnosed and diagnosed diabetic groups. For all analyses, P < 0.05 was used to indicate statistical significance. Stata 14® (StataCorp) was used for the statistical analysis.
Results
Mean age was 67 years in the English sample and 62 years in the Brazilian sample. The characteristics of the samples are presented in Table 1. Nearly half of the English sample was prediabetic, and 9·6 % had a diagnosis of diabetes, whereas the prevalence of UDD was low (3 %). The standardised prevalence showed that a total of 33 % of the Brazilian sample had PD, and 20·0 % had DD, whereas 6 % (twice the proportion as that in the English sample) had UDD (Table 2).
Table 1 Characteristics of participants of English Longitudinal Study of Ageing (ELSA, 2012–2013) and Brazilian Longitudinal Study of Aging (ELSI, 2015–2016)

All data presented as percentages, except where indicated: mean, sd. Self-reported doctor diagnosis of hypertension, stroke and CVD (angina, heart attack, congestive heart failure, vesicular murmur and arrhythmia).
Table 2 Prevalence of diabetes in ELSA study (2012–2013) and ELSI study (2015–2016)
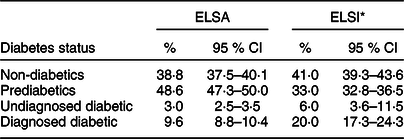
The prevalence was calculated with 95 % CI. ELSA study is the population standard.
* Standardised prevalence in ELSI study adjusted by age, sex and schooling.
In both samples, diagnosed diabetics were older than non-diabetics and prediabetics. English undiagnosed diabetics predominantly had less schooling and, along with diagnosed diabetics, had a lower frequency of daily drinkers, were more physically inactive, had higher frequencies of hypertension, stroke and obesity, a larger waist circumference, lower levels of HDL and higher levels of TAG than non-diabetics and prediabetics. Moreover, diagnosed diabetics had a greater frequency of hypertension and higher HbA1c levels than undiagnosed diabetics.
Brazilian prediabetics had higher levels of HbA1c and a greater frequency of high TAG levels than non-diabetics. Undiagnosed diabetics had greater frequencies of obesity and abdominal obesity than non-diabetics and prediabetics. Undiagnosed diabetics and diagnosed diabetics had higher HbA1c and a lower frequency of arterial hypertension compared with non-diabetics and prediabetics. Moreover, diagnosed diabetics had higher levels of TAG than non-diabetics (see online supplementary material, Supplementary Tables 1 and 2).
In comparison between the included and excluded individuals of the analytical sample, those excluded from ELSI were older. Those excluded from ELSA were older, more frequently non-white, had lower schooling and income, less frequently had a conjugal life, consumed less alcohol and were more physically inactive, had higher HbA1c levels and a higher prevalence of hypertension, stroke and CVD, higher TAG levels, lower HDL levels, a higher BMI and waist circumference than the included individuals (see online supplementary material, Supplementary Table 3).
Table 3 displays the results of the multinomial regression models for factors associated with diabetes status in the English and Brazilian samples. The increase in age, non-white skin colour, smoking, obesity (BMI ≥ 30 kg/m2) and abdominal obesity were associated with PD, UDD and DD in the English samples, with stronger associations found in those with UDD. These associations differed from those found in the Brazilian sample, in which the increase in age was associated with UDD and DD, non-white skin colour and smoking were associated only with UDD and abdominal obesity and hypertriglyceridaemia were associated with the three diabetic conditions.
Table 3 Multinomial regression model according to prediabetes, undiagnosed diabetes and diagnosed diabetes individuals aged 50 years or older: ELSA study, 2012–2013 and ELSI study, 2015–2016
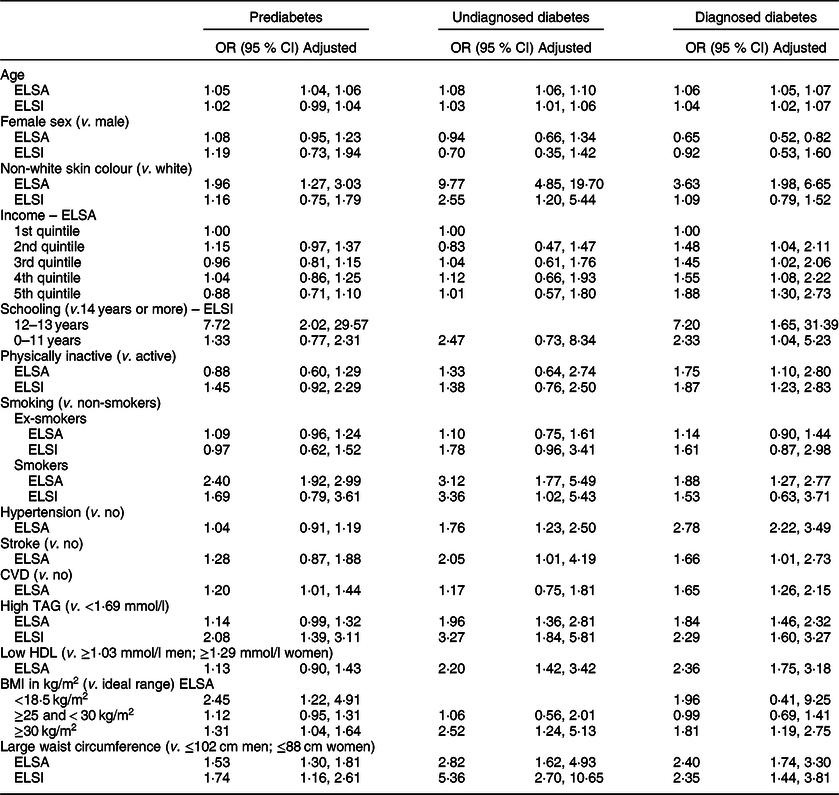
Dash (–) denotes the absence of category in group. ELSA model adjusted by sex, age, income, skin colour, level of physical activity and smoking. ELSI model adjusted by sex, age, schooling, skin colour, level of physical activity, smoking, TAG and waist circumference. In ELSI, CI calculated considering sample weight.
Hypertriglyceridaemia, low HDL levels, hypertension and stroke were associated with both UDD and DD in the English sample. CVD in the English sample and low schooling in the Brazilian sample were associated with PD and DD. BMI < 18·5 kg/m2 was associated with PD and lower income was associated with DD in the English sample. Being physically inactive was associated with DD in both samples. The female sex had a protective role regarding DD only in the English sample.
Discussion
The main findings of the current study were the differences in prevalence and associated factors between the two countries, with the rates of PD, UDD and DD, respectively, 48·6, 3 and 9·6 % in England and 33, 6 and 20 % in Brazil. The common associated factors in the two samples were abdominal obesity and hypertriglyceridaemia among the prediabetics, undiagnosed diabetics and diagnosed diabetics; the increase in age among the undiagnosed diabetics and diagnosed diabetics; non-white skin colour and smoking among the undiagnosed diabetics and a sedentary lifestyle among the diagnosed diabetics.
The prevalence of diabetes was similar to rates described in epidemiological studies conducted in the two countries. Using data from the surveillance system of risk and protection factors for chronic diseases in Brazil, Mendes and collaborators(Reference Mendes, Goldbaum and Segri28) found that the prevalence of diabetes among individuals older than 65 years of age was 18·6 %, which is a little higher than the rate found in the present study involving individuals aged 50 years or older. In a representative sample of 18 399 adults in England with a mean age of 51 years, Moody and collaborators(Reference Moody, Cowley and Fat29) found that the prevalence of UDD and DD was 2 and 6 %, respectively, which are similar to the rates found in the English sample of the present study.
The present results show several differences and some similarities regarding factors associated with diabetes status (prediabetes, undiagnosed and diagnosed), which may be explained by the process of nutritional transition, access to healthcare services, the use of such services and the consequent differences in mortality rates due to diabetes in the two populations studied. Countries undergo the nutritional transition process at different rates. This process is characterised by an increase in the consumption of SFA, trans fatty acids, sugar and sweetened beverages, along with a reduction in the consumption of complex carbohydrates, fruits, vegetables and legumes(Reference Sartorelli and Franco30–Reference Batista Filho and Rissin33). Moreover, changes in work structure and technological advances favour a sedentary lifestyle and the replacement of foods in natura for high-processed products, which contributes to obesity(Reference Sartorelli and Franco30). A sedentary lifestyle, smoking, obesity and metabolic disorders are key elements to the emergence of noncommunicable diseases, and all these factors were associated with diabetes status in both countries. Similar findings are described in previous studies(Reference Leahy, O’ Halloran and O’ Leary11,Reference Kumar, Wong and Ottenbacher14,Reference Mendes, Goldbaum and Segri28,Reference Brito, Lopes and Araújo34) .
The greater number of individuals with the diabetes (diagnosed or undiagnosed) in Brazil may be the product of the rapid, intense nutritional transition occurring in developing countries, in which lower income and education levels lead to the greater consumption of high-energy foods(Reference Sartorelli and Franco30). Moreover, the difficulty on the part of the Brazilian healthcare system (particularly primary care) in dealing with the double burden of diseases in the population exerts a direct effect on the inadequate establishment of disease prevention and health promotion measures for type 2 diabetes, hindering early diagnosis and efficient treatment(Reference Costa, Flor and Campos35,Reference Lebrão36) .
In contrast, although the nutritional transition in England is mainly the result of dietary practices guided by practicality and convenience, characterised by the consumption of highly processed foods(Reference Popkin and Gordon-Larsen32), the country has a more effective healthcare system(Reference Lima-Costa, De Oliveira and MacInko9) and certainly performs more timely screening of older adults(Reference Pierce, Zaninotto and Steel37). The threshold condition in the English sample may be explained by better health care that avoids to progression to diabetes per se.
Although both England and Brazil have universal healthcare systems with accessible medical care and strong primary care not found in other countries, expenditures on health per capita in Brazil correspond to only one-fourth of those in England, the density of physicians is much smaller and Brazilians have a poorer perception of their health in comparison to the English population(Reference Lima-Costa, De Oliveira and MacInko9).
Diabetes often occurs concomitantly with lipid disorders, hypertension(Reference Flor and Campos38), cardiovascular events(Reference Kumar, Wong and Ottenbacher14,Reference Pérez, Soto-Salgado and Suárez39) and obesity(Reference Flor and Campos38), which is in agreement with the present findings. In the English sample, a poor lipid profile, hypertension and stroke were characteristics of individuals with diabetes (diagnosed or undiagnosed). Moreover, general and/or abdominal obesity was found in all three diabetes statuses. In Brazil, abdominal obesity and hypertriglyceridaemia occurred in all three diabetes statuses, with a stronger association with UDD.
Obesity can trigger insulin resistance and both conditions exert a negative impact on the metabolism of lipids, favouring the occurrence of CVD. Moreover, ethnic-racial differences may explain the occurrence of outcomes such as hypertension, heart disease and stroke in the English sample, the majority of which was white, compared with the Brazilian sample, in which such associations were not found, given the fact that blacks have a less atherogenic lipid profile(Reference Bentley and Rotimi40).
In agreement with the present findings, studies conducted in England have demonstrated the increase in age, non-white skin colour and low income to be associated with diabetes(Reference Moody, Cowley and Fat29). Brazilian studies also list these same factors(Reference Mendes, Goldbaum and Segri28,Reference Ferreira and Ferreira41) . However, the lack of knowledge regarding diabetes control measures and reasons for not seeking healthcare services among Brazilian seniors has been associated with low schooling and income, resulting in poor disease control, with a significant impact on the occurrence of complications(Reference Mendes, Goldbaum and Segri28). These issues may contribute to the high mortality rate due to diabetes in Brazil, which is twice the rate as that found in the English population(Reference Lima-Costa, De Oliveira and MacInko9). Moreover, there is evidence that diabetes is underreported as a cause of death in Brazil, since people with diabetics die due to chronic complications of these diseases, which are listed as the main cause of death(Reference Mendes, Goldbaum and Segri28). Therefore, one may put forth the hypothesis that, together with lower schooling and non-white skin colour, individuals who have little or no access to healthcare services may be unable to control diabetes and therefore die prematurely, resulting in such associations being found less in Brazil.
The present findings are particularly important, as PD is a high-risk state for the development of diabetes mellitus and because UDD increases the risk of complications due to the non-control of blood glucose levels. Thus, the three diabetes statuses pose risks of serious complications to the health of individuals aged 50 years or older. It is therefore essential to implement health policies and perform screening to minimise or avoid the harm caused by this condition.
The strong points of the current study are the use of large national representative samples of individuals aged 50 years or older and the use of HbA1c to confirm the cases. The current study also has limitations that should be considered. First, the cross-sectional design does not enable establishing causality because it not possible to establish the temporal sequence between outcome and exposure. Second, the lack of information on the time since the diagnosis of diabetes, and the characteristics of the excluded individuals such as worse socio-demographic, behavioural and health characteristics may have influenced the results. However, the findings agreed with the data reported in the literature. Finally, the use of self-reported clinical conditions could be a potential source of information bias. Perhaps due to limited contact with the medical system, individuals in different countries or across different socio-economic status groups may be unaware that they have a particular disease or may think they are cured when the disease is only under control. However, the participants were asked whether a doctor had diagnosed their condition, and this could have minimised inaccuracy and recall bias. Information on comorbidities given by the patients has been described to be reliable especially for chronic conditions such as diabetes or heart disease(Reference Lucke, Herrera and Wacker42). Further studies should be conducted on the incidence of PD, UDD and DD, and their determinants.
Conclusion
The prevalence of diabetes (diagnosed or undiagnosed) was higher in the Brazilian sample than the English sample. Different associated factors were found in the two samples, which may be related to differences in nutritional transition, access to healthcare services and the use of such services.
Acknowledgements
Acknowledgements: None. Financial support: This work was supported by the Fundação de Amparo á Pesquisa do Estado de São Paulo – FAPESP (The São Paulo Research Foundation) (2017/22820-0); Economic and Social Research Council (ESRC) and National Institute on Aging USA (5R01AG017644-16 and 5R01AG017644-18 to ELSA study); Brazilian Health Ministry and the Science and Technology Ministry (to ELSI study) and Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq (National Council of Scientific and Technological Development) (303981/2017-2 to Tiago da Silva Alexandre). Conflict of interest: The authors declare that there is no conflict of interest. Authorship: Study concept and design: T.S.A., E.S.M.d.S. and R.O.M. Acquisition of data: T.S.A., M.F.L.-C. and C.d.O. Data analysis and interpretation: T.S.A., E.S.M.d.S., R.O.M., M.F.L.-C. and C.d.O. Preparation of manuscript: All authors. Revising and final approval: All authors. Ethics of human subject participation: The current study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving research study participants were approved by the The National Research Ethics Service (London Multicentre Research Ethics Committee (MREC/01/2/91) to ELSA study, and Human Research Ethics Committee of the René Rachou Research Centre of the Oswaldo Cruz Foundation (state of Minas Gerais, Brazil) (certificate number: 886·754) to ELSI study. Written informed consent was obtained from all subjects/patients.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980020003201






