Introduction
Patients with atrial fibrillation (AF) who have suffered a transient ischemic attack (TIA) or ischemic stroke are at high risk for recurrence and require long-term anticoagulation. The risk of recurrent stroke (3–20%) within the first two weeks after stroke/TIA is clinically important.Reference Hart, Palacio and Pearce1,Reference Sage and Van Uitert2 The optimal timing of anticoagulation after an ischemic stroke, however, remains controversial. Previous trials have demonstrated that anticoagulation in acute stroke patients is associated with reduced early stroke recurrence rates, but these benefits are offset by a comparable increase in the rate of symptomatic hemorrhagic transformation (HT).Reference Paciaroni, Agnelli, Micheli and Caso3,Reference Sandercock, Counsell and Kane4 An individual patient-level meta-analysis reported that the hemorrhagic complication rate associated with low-molecular-weight heparin within 14 days of ischemic stroke was 0.8%.Reference Jorth, Trivedi, Rumbaugh and Whiteley5 Similarly, early warfarin use in stroke patients without AF is associated with a significant increase in the rate of HT (relative risk 1.93, 95% confidence interval 1.27–2.94).Reference De Schryver, Algra, Kappelle, van Gijn and Koudstaal6
Dabigatran is a direct oral anticoagulant (DOAC), approved for the prevention of ischemic stroke in patients with AF. Dabigatran is associated with a lower risk of intracranial hemorrhagic complications than warfarin in patients with chronic AF without recent stroke.Reference Connolly, Ezekowitz and Yusuf7 There are limited data related to the use of dabigatran within 14 days of stroke. All safety data related to dabigatran come from trials that excluded patients within 14 days of stroke and 3 days of a TIA. This is precisely the period when stroke patients are at highest risk of recurrent events and most likely to derive benefit from anticoagulation. Based on the limited data, Canadian Stroke Best Practice guidelines recommend basing the timing of anticoagulation on clinical severity and infarct size, but neither are well defined.Reference Wein, Lindsay and Cote8 This recommendation is based on expert consensus originally proposed in the European Heart Rhythm Association guidelines, and is not based on randomized clinical trial advice.Reference Heidbuchel, Verhamme and Alings9 The question of optimal timing of oral anticoagulation after ischemic stroke is frequently encountered in clinical practice. The guidance in this matter has been based largely on expert opinion and clinical experience, along with serial imaging.
Aim
The overall aim of this study was to demonstrate the feasibility and safety of initiating dabigatran therapy within 14 days of TIA or minor ischemic stroke in AF patients. We systematically assessed prospectively collected computed tomography (CT) scan images for evidence of HT.
Methods
The Canadian Pradaxa Acute Stroke Safety study was an investigator-initiated prospective, multicenter, open-label, single-arm phase IV study (clinicaltrials.gov registration NCT02415855). Patients with documented non-valvular AF (newly or previously diagnosed) with acute TIA (defined as acute focal neurological deficits, with complete resolution of symptoms within 24 h of onset) or mild ischemic stroke (National Institutes of Health Stroke Scale (NIHSS) score ≤3) were enrolled. Study investigators approached patients after the treating physician’s decision to treat with dabigatran within 14 days of stroke/TIA, independent of the registry. Informed consent was obtained from the patient or substitute decision maker in all cases prior to enrollment. The research protocol was approved by our local Human Research Ethics Board.
Patients were excluded if they had acute or chronic renal failure (estimated glomerular filtration rate (eGFR) < 30 ml/min), known hypersensitivity to dabigatran or potential medical interactions with dabigatran therapy, and/or significant ongoing systemic bleeding risk.
Procedures
Dabigatran Therapy
The dabigatran dose was determined by the treating physician, based on age and renal function. Patients with an eGFR 30–50 ml/min and/or age ≥80 years received 110 mg twice a day. All other patients received 150 mg twice a day.
Clinical Assessments
All study participants were followed for 30 days after dabigatran initiation. An NIHSS score was assessed, by certified study personnel at baseline, 7 and 30 days after enrollment. Functional outcome was assessed with a modified Rankin Scale (mRS) at baseline, 7, and 30 days. Montreal Cognitive Assessments were performed at baseline, day 7, and day 30, and quality of life was assessed with the EuroQol-5 Dimension (EQ-5D) and Visual Analog Scale. The Charlson Co-morbidity Index was calculated at baseline.
Imaging Procedures and Analysis
All patients had a non-contrast CT scan at baseline (within 24 h from study recruitment) and at 7 ± 2 days after enrollment using a non-contrast CT scan. In the event of clinical deterioration, CT scans were repeated.
Anonymized dicom CT data were analyzed centrally. Baseline and recurrent infarct volumes were measured using planimetric techniques (Analyze 11.0, Biomedical Imaging Resource, Biodynamics Research Unit, Mayo Foundation, Rochester, MN, USA).Reference Robb, Hanson, Karwoski, Larson, Workman and Stacy10 Any HT seen at baseline and day 7 was graded using European Cooperative Acute Stroke Study criteria: hemorrhagic infarction type 1 (HI1; small petechiae along the margins of the infarct), hemorrhagic infarction type 2 (HI2; confluent petechiae within the infarcted area but no space-occupying effect), parenchymal hemorrhage type 1 (PH1; blood clots in 30% or less of the infarcted area with some slight space-occupying effect), or parenchymal hemorrhage type2 (PH2; blood clot in more than 30% of the infarcted area with substantial space-occupying effect).Reference Fiorelli, Bastianello and von Kummer11
Endpoints
The primary endpoint was symptomatic HT, defined as PH2 associated with a ≥ four-point increase in NIHSS score within 30 days of initiating dabigatran therapy. Secondary outcomes included any HT at day 7, systemic hemorrhagic complications, and recurrent ischemic stroke within 30 days of enrolment. Serious adverse events (SAEs) within the study period were recorded using standardized event, resolution and association codes, and reported to the local Human Research Ethics Boards and Health Canada.
Sample Size and Statistical Analysis
The study was an open-label registry not powered to demonstrate safety or efficacy. A sample of 100 patients was planned in order to obtain initial estimates of the frequency of symptomatic and asymptomatic HT. Given the lack of published data with respect to dabigatran in this population, the maximum acceptable rate of symptomatic HT was considered 2%. This was based on a meta-analysis of low-molecular-weight heparin treatment in acute stroke, indicating the absolute symptomatic HT rate ranged from 2.4% to 2.9%.Reference Bath, Iddenden and Bath12 An a priori stopping rule therefore governed study continuation; enrollment would be halted immediately and permanently if ≥2 symptomatic HT events occurred.
All statistical analyses were performed using the Statistical Package for Social Sciences version 23.0.0 (IBM SPSS Statistics Inc., 2015, Armonk, NY, USA). Differences between groups were assessed using independent t-tests for parametric data and Mann–Whitney U-tests for non-parametric data. Pearson’s correlation coefficients were used to estimate the relationship between time to dabigatran initiation and infarct volume. The five-digit health state in EQ-5D was calculated using preference weights, as described previously.Reference Shaw, Johnson and Coons13
Role of the Funding Source
The trial was an Investigator-Initiated Study, funded by Boehringer-Ingelheim. The study sponsor was “The Governors of the University of Alberta.” The protocol was written by the Principal Investigator (KB) and reviewed by the Boehringer-Ingelheim global medical team prior to funding. Boehringer-Ingelheim had no role in study design, data collection, analysis, interpretation, or manuscript preparation. Additional support was provided by the Canada Research Chairs Program and the Heart and Stroke Foundation of Alberta, Northwest Territories and Nunavut. The authors had full access to all data in the study and had final responsibility for the presentation of results.
Results
Baseline Characteristics
Between December 2013 and February 2018, a total of 101 patients (65% male) were enrolled at three Canadian stroke centers (Figure 1). Patient characteristics are summarized in Table 1. At the time of treatment initiation, median (interquartile range (IQR)) NIHSS 1 (0–2) and the median (IQR) infarct volume was 0 (0–7.43) ml.

Figure 1: Consort diagram of enrollment, allocation, follow-up, and outcomes. The intention-to-treat population included all the patients who were enrolled in this study. All study participants were followed for 30 days after dabigatran initiation. Day 7 follow-up included clinical assessment and CT scan, while day 30 included clinical assessment. *One patient was in a rehabilitation hospital. †Three patients were discharged to rural communities, without access to CT. ‡One patient died within 7 days of enrollment as a consequence of recurrent ischemic stroke and concomitant systemic emboli, CT scan was repeated and included in the analysis. §Four patients whom clinical assessment and CT scan were not completed at day 7 follow-up, returned for their day 30 follow- up.
Table 1: Characteristics of patients with/without baseline HT

CHA2DS2-VASc Score, congestive heart failure, hypertension, age, diabetes mellitus, stroke (doubled), vascular disease, age, and sex category (female); HAS-BLED Score, hypertension, abnormal renal/ liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs/alcohol concomitantly; MoCA, Montreal Cognitive Assessment.
The median (IQR) time from onset to first dabigatran dose was 2 (1–5) days for all patients (Figure 2). The majority of patients (64%) received dabigatran 150 mg twice a day, and the remainder received 110 mg twice a day. The number of days between symptom onset and dabigatran initiation was directly correlated with the infarct volume (r = 0.49, p < 0.0001). An acute infarct was visible on CT in 48 (48%) of patients, and the most common location was cortical (35%). Evidence of previous infarction was seen on baseline CT in 30 (30%) patients.

Figure 2: Time from ischemic stroke/TIA onset to dabigatran initiation in all patients. Patients initiated on day 0 received their first dose on the day of the index event. The median time to dabigatran initiation was 2 days.
Dabigatran Compliance and Follow-up
During the follow-up period, two patients withdrew consent prior to the follow-up CT scan at day 7. Two patients withdrew consent after the day 7 assessment. One patient died within 7 days of enrollment as a consequence of recurrent ischemic stroke and concomitant systemic emboli. A CT scan was not obtained in four patients at day 7. A follow-up CT scan was obtained in 95 patients at a median (IQR) of 7 (5–8.5) days after dabigatran initiation. One patient was non-compliant on dabigatran therapy at day 7 and developed a recurrent ischemic event. The remaining patients were compliant at day 7 and 30.
Baseline HT
On baseline CT, HT was present in seven patients (7%). Of these, four patients had HI1 and three patients had HI2 (Table 1). In three of these seven patients, the petechial hemorrhage completely resolved by day 7, despite treatment with dabigatran (Figure 3). In three patients, the HT grade did not change at day 7. A single patient developed asymptomatic progression from HI1 to HI2 (Figure 3).
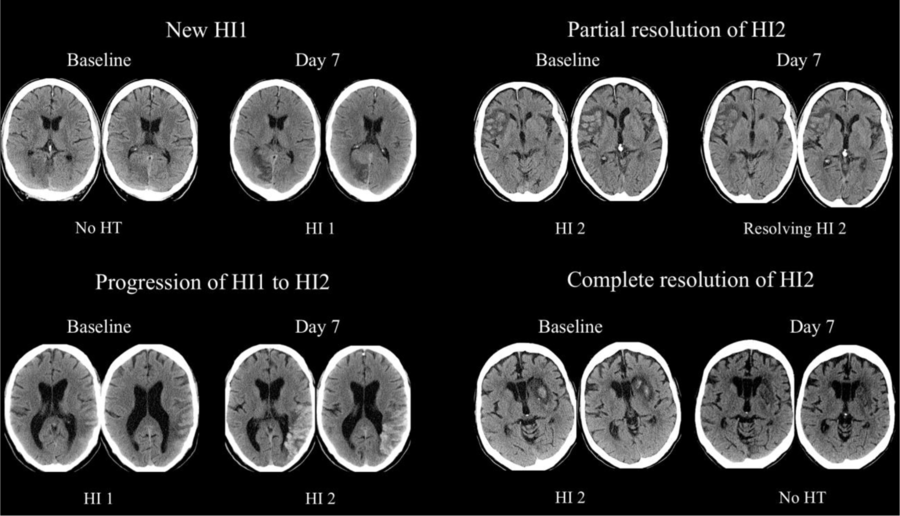
Figure 3: Examples of asymptomatic incident (new/progressive) and resolving HT observed after dabigatran initiation. HI1 was defined as small petechiae along the margins of the infarct, and hemorrhagic infarction type 2 (HI2) as confluent petechiae within the infarcted area but no space-occupying effect.
Median (IQR) infarct volume in patients with baseline HT was 31.2 (27.8–47.5) ml, which was larger than patients without HT 0 (0–5.1) ml (p < 0.0001). Dabigatran initiation in patients with baseline HT was delayed to 5 (4.5–9) days, which was significantly longer than in patients without baseline HT (2 (1) days, p < 0.0001; Table 1). The relationship between infarct volume, baseline and incident HT, recurrent ischemic events, and time to dabigatran initiation is illustrated in Figure 4.

Figure 4: Relationship between baseline infarct volume and time from symptom onset to first dabigatran dose. Blue bars: No HT. Red bars: Baseline HT. Yellow bars: Incident HT at day 7. Green bars: Recurrent ischemic events. *Patient with baseline HI1 that progressed to HI2 at day 7.
Incident HT
No patients developed symptomatic HT or asymptomatic PH at any point. The total number of patients with incident HT by day 7 was 6 (6%). Clinically silent, but new HI1 was seen in four patients, and a single patient developed asymptomatic progression from HI1 to HI2. Another patient developed incident asymptomatic HI2 (Figure 3). Patients with incident HT had larger median (IQR) baseline infarct volume 27.3 (13–48.5) ml, than those without (HT 0 (0–5.4) ml, p < 0.0001; Table 1). The only predictor of incident HT was infarct volume (odds ratio = 1.063 [1.020–1.107], p < 0.003).
Recurrent Ischemic Events
Recurrent ischemic events occurred within 30 days in four patients (Table 1). One of these patients also developed fatal systemic emboli 3 days after dabigatran initiation. All ischemic events occurred within 10 days of dabigatran initiation. There were no predictors of recurrent ischemic stroke.
Clinical Outcomes
All six patients with incident HT were functionally independent (mRS = 0–2) at 30 days, which was similar to the good outcome rates seen in those without HT (90%, p = 0.422). Conversely, two of the four patients with recurrent ischemic events were dead or severely disabled (mRS = 5) by day 30. There were no systemic bleeding complications or other SAEs were reported within the study period.
Discussion
These data support the safety of initiating dabigatran in AF patients early after TIA or minor ischemic stroke. Even in cases where HT was present prior to anticoagulation, dabigatran did not result in symptomatic HT. In contrast to HT, which was always clinically silent, early recurrent ischemic events were always clinically evident and resulted in clinical disability/death in half the cases. Recurrent ischemic events occurred within 10 days of the index event, suggesting there is a cost to delaying anticoagulant therapy.
There are limited published data related to the safety of early anticoagulation with dabigatran after cardioembolic stroke or TIA. There are several retrospective analyses of AF management in acute stroke that describe early anticoagulation, primarily with heparin, and/or warfarin.Reference Paciaroni, Agnelli, Micheli and Caso3–Reference De Schryver, Algra, Kappelle, van Gijn and Koudstaal6 A meta-analysis of heparin/low-molecular-weight heparin/heparinoids suggested that early anticoagulation with these agents in cardioembolic stroke is harmful, due to increased rates of symptomatic HT.Reference Paciaroni, Agnelli, Micheli and Caso3 Conversely, a more recent meta-analysis suggested that recurrent ischemic stroke is more common than symptomatic HT within 90 days and that early antithrombotic therapy (antiplatelets and anticoagulants were combined) may be beneficial.Reference Abdul-Rahim, Fulton and Frank14
The DOACs have all been shown to safer than warfarin with respect to intracranial hemorrhage in patients with chronic AF.Reference Lopez-Lopez, Sterne and Thom15 The DOACs specifically target single steps in the coagulation cascade, whereas warfarin impairs production of multiple pro-factors. This may theoretically make DOACs safer in the acute stroke setting but given the multifactorial and incompletely understood pathogenesis of HT, this is highly speculative.
Four prospective observational studies of early anticoagulation after stroke, included some patients initiated on an DOAC. The Stroke Acute Management with Urgent Risk-factor Assessment and Improvement study was a prospective observational study of anticoagulant decision-making in patients admitted to hospital with stroke/TIA and AF.Reference Toyoda, Arihiro and Todo16 A total of 1116 patients were started on warfarin (n = 650, median 3 days) or an DOAC (n = 466, median 4 days) after stroke/TIA onset. Although there were no reports of intracerebral hemorrhage prior to hospital discharge, systematic neuroimaging after anticoagulation initiation was not performed. At 90 days, the recurrent stroke/systemic embolism rate was 2.84% and the rate of major bleeding was 1.1% in the DOAC treated patients.Reference Arihiro, Todo and Koga17 The early Recurrence and cerebral bleeding in patients with Acute ischemic stroke and atrial Fibrillation (RAF) study was a similar prospective observational study performed in 1092 patients that included 93 patients treated with an DOAC.Reference Paciaroni, Agnelli and Falocci18 Timing of anticoagulation was at the discretion of the treating physician, varying from 1 to 90 days, and 24% of patients were never anticoagulated. The recurrent ischemic stroke rate was 7.6% risk, and symptomatic cerebral hemorrhage occurred in 3.6% of patients by day 90. Analysis of data from the Clinical Relevance Of Microbleeds In Stroke-2 study assessed the effect of oral anticoagulant timing in patients with AF and stroke.Reference Wilson, Ambler and Banerjee19 The time to treatment was determined by the investigators and retrospectively dichotomized into early (0–4 days) and late (≥ 5 days or never started) periods. Of 1355 patients prescribed an oral anticoagulant, 358 patients (26%) were started early and 997 (74%) were started late. Recurrent ischemic stroke and intracranial hemorrhage rates were similar between the two groups. The majority of patients (65%) were treated with warfarin, rather than an DOAC. Finally, one single-center registry of anticoagulation practice patterns in cardioembolic stroke indicated that 65% of 155 patients prescribed an DOAC after stroke was initiated within 7 days of onset and that this was not associated with increased risk of symptomatic HT or recurrent stroke.Reference Seiffge, Traenka and Polymeris20 Once again, however, systematic imaging was not part of this registry.
Previous assessments specific to the safety of DOAC initiation in acute cardioembolic stroke have largely been limited to retrospective and small prospective studies. One single-center study reported no symptomatic HT or recurrent stroke in 41 patients treated with DOACs at a median of 2 (IQR 5) days after onset.Reference Shibazaki, Kimura, Aoki, Saji and Sakai21 Our group previously reported a prospective assessment of the safety of rivaroxaban initiation at a median of 3 (IQR 5) days after cardioembolic stroke symptom onset.Reference Gioia, Kate and Sivakumar22 Using serial magnetic resonance imaging (MRI) pre- and post-treatment, we determined asymptomatic petechial HT was common at baseline (25/60) and remained clinically silent despite immediate treatment with rivaroxaban.
One randomized evaluation of rivaroxaban versus warfarin within 5 days of cardioembolic stroke has been published (Triple AXEL; Acute Stroke With Xarelto to Reduce Intracranial Hemorrhage, recurrent Embolic Stroke, and hospitaLstay).Reference Hong, Kwon and Lee23 Rivaroxaban (n = 101) and warfarin (n = 94) were associated with similar recurrent ischemic stroke and intracranial hemorrhage rates. Asymptomatic HT on MRI performed at 4 weeks was seen in 49.5% and 54.5% of patients receiving rivaroxaban and warfarin, respectively. The higher rates of HT than seen in our study were most certainly related to the higher sensitivity of MRI for petechial bleeding, rather than differences between anticoagulants. As in our study, most patients had mild stroke symptoms (a median NIHSS score of 2 in both groups).
In the present study, we observed that the infarct volume was the only predictor of asymptomatic HT after anticoagulation. In addition, clinicians delayed dabigatran initiation in patients with larger infarcts, irrespective of the presence or absence of HT. A similar pattern was observed in the RAF study,Reference Paciaroni, Agnelli and Falocci18 reflecting common clinical practice patterns and expert recommendations.Reference Diener, Aisenberg and Ansell24 Symptomatic HT after anticoagulation is an infrequent event, making it difficult to predict, but thrombolysis related HT as previously been associated with infarct volume.Reference Butcher, Christensen and Parsons25 The practice of delaying anticoagulation in patients with large lesions due to concerns that they are more prone to symptomatic HT appears reasonable at present. The absolute risk of HT and the optimal timing in individual patients remain unknown however.
Ultimately, the risk/benefit ratio of early versus delayed anticoagulation in AF patients with stroke will remain an area of clinical equipoise until randomized trials are completed. The only randomized trial of early dabigatran versus aspirin published to date was completed in patients without AF.Reference Butcher, Ng and Sheridan26 Several trial protocols have been published or registered.Reference Seiffge, Werring and Paciaroni27 Patients in these trials are randomized to an DOAC, initiated as early as 48 h and up to 4 days after onset, or delayed to 5–10 days. The primary outcome is the composite of recurrent ischemic stroke and symptomatic HT. These include the TIMING (Timing of Oral Anticoagulant Therapy in Acute Ischemic Stroke With Atrial Fibrillation: a Prospective Multicenter Registry-based Non-Inferiority Randomized Controlled Clinical Trial, NCT02961348),Reference Asberg, Hijazi, Norrving, Terent, Ohagen and Oldgren28 ELAN (Early Versus Late Initiation of Direct Oral Anticoagulants in Post-ischaemic Stroke Patients With Atrial fibrillation, NCT03148457), START (Optimal Delay Time to Initiate Anticoagulation After Ischemic Stroke in Atrial Fibrillation, NCT03031928) trials, and OPTIMAS (OPtimal TIming of Anticoagulation After Acute Ischemic Stroke, NCT03759938).
Limitations
This non-randomized study does not provide definitive evidence for the safety of early anticoagulation after cardioembolic stroke or TIA. Cardioembolic stroke is often associated with moderate to severe deficits and larger infarct volumes. Based on our study design, these patients were systematically excluded. Caution with respect to early dabigatran use in these patients is therefore still warranted. Low rates of the events, both recurrent ischemic stroke and HT, seen as a limitation.
Conclusions
Using prospective clinical and CT scans data, early anticoagulation with dabigatran after TIA and minor cardioembolic stroke is safe and is not associated with symptomatic hemorrhage. Asymptomatic HT is associated with larger baseline infarct volumes. Evidence of baseline petechial or confluent HT (HI1 or HI2) on CT scan at the time of dabigatran initiation does not appear to increase the risk of symptomatic HT after minor stroke/TIA. Early recurrent ischemic events may be clinically more important. This observation provides reassurance that current practice patterns are safe, but conclusive evidence will require a larger sample size.
Funding
This study was funded by an Investigator-Initiated Study Grant from Boehringer Ingelheim.
Conflicts of Interest
Dr. MSharma reports grants from Boehringer Ingelheim, during the conduct of the study; grants and personal fees from Bayer, grants and personal fees from Bristol Myers Squibb, personal fees from Portola, outside the submitted work; Dr. KB reports grants and personal fees from Boehringer Ingleheim, during the conduct of the study, grants and personal fees from BMS-Pfizer Alliance, grants and personal fees from Bayer, grants and personal fees from Servier Canada, outside the submitted work. The other authors have no conflicts of interest to declare.
Statement of authorship
AA completed the image and clinical data analyses and drafted the initial manuscript. KN, DD, BB, GS, ST, MS, HK, and AS recruited patients into the study and made critical revisions of the manuscript. LS oversaw centralized image and clinical data collection, assisted with analysis and made critical revisions of the manuscript. MSharma and KB wrote the initial protocol, obtained funding from the sponsor, recruited patients into the study, and made critical revisions of the manuscript.







