Essential fatty acids (EFA) and their longer-chain polyunsaturated derivatives (LCPUFA) are indispensable for human development and optimum health. DHA (22: 6n-3) and arachidonic acid (ARA, 20 : 4n-6) are considered the most important functional LCPUFA. Although they can be synthesised in the body from α-linolenic acid and linoleic acid, respectively, this synthesis capacity is restricted by the low activity of the rate-limiting enzyme Δ6-desaturase. Endogenous formation of DHA and ARA is thought to be insufficient under certain circumstancesReference Hornstra1 and additional dietary intake is needed to cover metabolic requirements. This applies particularly under conditions of increased EFA and LCPUFA requirements, such as pregnancy, lactation and early human development.
DHA and ARA are found in high concentrations in structural lipids of the central nervous system and have been shown to be important for brain development and functionReference Innis2–Reference Clandinin, Chappell, Leong, Heim, Swyer and Chance4. Adequate accretion of both DHA and ARA in the brain and retina is particularly important during the rapid brain growth, which takes place in the perinatal periodReference Clandinin, Chappell, Leong, Heim, Swyer and Chance4. It is now generally accepted that postnatal DHA administration promotes, at least temporarily, cognitive and visual development of infants born pretermReference SanGiovanni, Parra-Cabrera, Colditz, Berkey and Dwyer5–Reference Carlson, Werkman and Tolley9. Developmental benefit of postnatal DHA supplementation has been observed in term infants also, but the results are less consistentReference SanGiovanni, Berkey, Dwyer and Colditz10, Reference Simmer11.
Additionally, lactating mothers may benefit from postnatal LCPUFA supplementation themselves, since a low maternal DHA status has been suggested to increase the risk of postnatal depressionReference Hibbeln12. This hypothesis is supported by recent findings that a higher DHA status at deliveryReference De Vriese, Christophe and Maes13 and a better recovery of the maternal DHA status after deliveryReference Otto, de Groot and Hornstra14 are associated with fewer depressive symptoms during the postnatal period. In a longitudinal study it appeared that postnatal depression is often preceded by depression around the thirty-second week of pregnancyReference Evans, Heron, Francomb, Oke and Golding15, which may also be DHA-related. Therefore, a higher DHA status during early pregnancy may be beneficial in the prevention of pre- and postnatal depression.
Dietary supplementation with DHA not only improves the DHA status, but also reduces γ-linolenic acid (GLA, 18 : 3n-6), dihomo-GLA (DGLA, 20 : 3n-6) and ARA concentrations in plasma and erythrocytesReference Buckley, Shewring, Turner, Yaqoob and Minihane16–Reference Theobald, Chowienczyk, Whittall, Humphries and Sanders23. ARA is the second-most abundant LCPUFA in the brain, and ARA-derived eicosanoids are important functional mediatorsReference Youdim, Martin and Joseph24. ARA is involved in cell signallingReference Khan, Blobe and Hannun25 and is one of the major fatty acid moieties of anandamides, which are ligands of the cannabinoid receptor in the brainReference Di Marzo26. However, whereas DHA has been investigated in several prospective trials, the possible relevance of ARA for development and function has yet to be confirmed in clinical settings. There is limited evidence for a beneficial effect of ARA on brain development and function, when it is given in combination with DHA to term infantsReference Birch, Garfield, Hoffman, Uauy and Birch27. But cerebral ARA may be involved in neuromental disorders like schizophreniaReference Peet and Horrobin28. Whether or not the ARA reduction associated with DHA supplementation interferes with DHA-induced functional benefits is not known. Because of this risk, the supplementation of mothers or infants with the aim to improve the DHA status should not compromise maternal and neonatal ARA status.
A lower maternal GLA and DGLA status following DHA supplementation during pregnancy can be expected to decrease the neonatal status of these fatty acids, as the maternal and neonatal EFA–LCPUFA status is positively correlatedReference Al, van Houwelingen and Hornstra29. A low intrauterine availability of (D)GLA could predispose individuals to increased risk of obesity, insulin resistance and hypertriacylglycerolaemia in later life, since these conditions at 7 years of age have been shown to be inversely related to the (D)GLA status at birthReference Rump, Popp-Snijders, Heine and Hornstra30. In addition, a significant, positive relationship has been observed between the DGLA status of the neonate and its birth weightReference Rump, Mensink, Kester and Hornstra31. A limited availability of nutrients early in life (resulting in reduced birth weight) could lead to the development of certain chronic diseases in later life according to the foetal origins of adult diseases hypothesisReference Barker32, Reference Godfrey and Barker33. In addition by activating PPAR GLA may be essential in the transcription of genes involved in glucose and lipid homeostasisReference Xu, Lambert and Montana34, Reference Gervois, Torra, Fruchart and Staels35 and GLA is thought to reduce the risk of atopyReference Manku, Horrobin, Morse, Kyte, Jenkins, Wright and Burton36, Reference Melnik and Plewig37. Therefore, any supplementation of mothers or infants intended to improve their DHA status should not compromise, but rather improve maternal and neonatal GLA and DGLA status.
A simple way to avoid the decrease of maternal and neonatal GLA, DGLA and ARA levels with DHA supplementation may be the addition of GLA to the supplement. Previous studies observed increased plasma concentrations of DGLA as well as (in most studies) ARA and GLA levels after GLA supplementationReference Yaqoob, Pala, Cortina-Borja, Newsholme and Calder38–Reference Thies, Nebe-von-Caron, Powell, Yaqoob, Newsholme and Calder43. The objective of this study was to investigate the effects of a fish oil (FSO)/evening primrose oil (EPO) blend on plasma fatty acid composition and its tolerance and safety in healthy, non-pregnant women. We hypothesised that the co-supplementation of DHA and GLA would increase plasma DHA, GLA, and DGLA levels without impairing the ARA status.
Subjects and methods
Subjects and study design
Forty apparently healthy women aged from 19 to 36 years were recruited in the Munich area via posters displayed on the university campus, and through personal contacts. Inclusion criteria were female gender, age between 18 and 40 years and BMI between 18 and 25 kg/m2. Exclusion criteria were hypertension (defined as a systolic blood pressure >140 mm Hg or a diastolic blood pressure >90 mm Hg44), diagnosed metabolic, cardiovascular, renal or neurological diseases, long-term use of medication (contraceptives excluded), use of EFA or LCPUFA supplements, drug abuse, smoking of >5 cigarettes per day, consumption of >7 glasses alcohol per week, consumption of fish more than twice weekly, pregnancy or lactation, vegetarian lifestyle during the last 3 months, and participation in any other study. Vegetarians were excluded because they have a different background diet with low intakes of ARA, EPA and DHA and also often have different life-styles as compared with the majority of the population. These differences would likely have an impact on the EFA status and, consequently, increase the variability of the results. Ethical approval for the study was obtained from the Bavarian Board of Physicians. Subjects gave their written informed consent and received financial compensation for their participation.
The study was conducted between June and August 2005 as a randomised, double-blind, placebo-controlled intervention study with two parallel groups. All volunteers completed a health and lifestyle questionnaire before entering the study. The subjects consumed 3·4 g daily of either a FSO/EPO blend (providing 456 mg DHA, 73 mg EPA, 14 mg ARA and 353 mg GLA as TAG per day) or the same amount of a placebo (blend of palm oil, rapeseed oil, and sunflower seed oil) for 8 weeks. The doses of DHA and GLA were chosen so as to meet the recommended minimum intake of 200 mg DHA/d for pregnant and lactating womenReference Koletzko, Cetin and Brenna45, to lead to measurable increases of the corresponding plasma levels, the highest GLA amount possible (for optimum efficiency to increase the (D)GLA contents and to prevent a FSO-induced ARA reduction), and to keep the total number of capsules of available preparations (mixtures of EPO and FSO) to be taken within practicable limits (six capsules per day).
The subjects were randomly assigned to one of the intervention groups. There were no significant differences in subject characteristics between the two intervention groups at study entry (Table 1).
Table 1 Baseline characteristics of the subjects
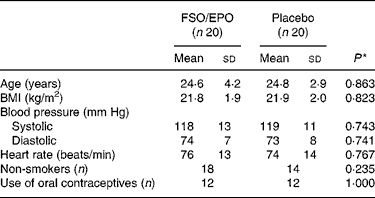
FSO/EPO, fish oil and evening primrose oil blend.
* The between-group differences at baseline were analysed using Student's unpaired t test or X 2 test as appropriate.
Fasted blood samples were collected at weeks 0 (baseline), 4, 6 and 8. Body weight, blood pressure, and heart rate were measured at study entry and after 8 weeks of intervention; height was measured at baseline only. During the intervention, the subjects noted side effects, signs of illness, intake of medication and the number of capsules they had failed to take. Compliance was assessed by counting the returned capsules and expressing the number as a percentage of the prescribed number of capsules. To check the success of blinding, the subjects were asked at the end of the study to guess their group assignment.
Study oils
Each FSO/EPO capsule (mean filling weight 572·2 mg) consisted of 76·5 % EPO (EFAMOL Ltd, Brackenholme, Selby, North Yorkshire, UK) and 22·5 % FSO (Incromega DHA 500 TG SR, Croda Chemicals Europe Ltd, Cowick Hall, East Yorkshire, UK). Matching placebo capsules (mean filling weight 567·2 mg) contained a blend of palm oil (59·4 %), rapeseed oil (19·8 %), and sunflower seed oil (19·8 %) with a fatty acid composition comparable to the habitual fatty acid composition of a standard Western diet. Because of the non-translucent encapsulation, FSO/EPO and placebo capsules were visually indistinguishable. All capsules contained 1 % of synthetic vitamin E acetate for stabilisation of the oils. Table 2 gives the fatty acid composition of the study oils as determined in our laboratory. The volunteers were asked to take 2 × 3 capsules or 3 × 2 capsules daily together with the meals (a total of six capsules per day). The capsules were stored either refrigerated or at room temperature in a dry, dark place.
Table 2 Major fatty acids of FSO/EPO and placebo oils (g/100 g total fatty acids)
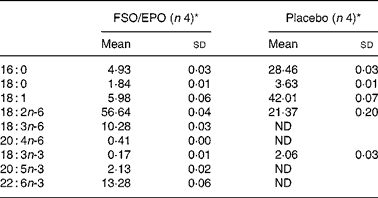
FSO/EPO, fish oil and evening primrose oil blend; ND, not detected.
* As determined in our laboratory.
Measurements, blood sampling and storage
All anthropometric measurements followed standardised procedures. Height without shoes was measured to the nearest 5 mm and body weight without shoes and outdoor clothing to the nearest 100 g using a digital scale. A lump sum of 1 kg was subtracted for the weight of the remaining clothes. Blood pressure and heart rate were determined in sitting position using a digital sphygmomanometer.
Venous blood samples were collected from the antecubital vein into an EDTA-containing tube (Sarstedt, Nümbrecht, Germany) from sitting or lying subjects. The blood was centrifuged at 4°C (1500 g for 5 min) within 2 h. Aliquots of the plasma were transferred into two plastic storage vials, which were tightly closed under a stream of N2, and stored at − 80°C for 4 months maximally before the analysis of total lipid (TL), phospholipid (PL), TAG and cholesterol ester (CE) fatty acids. At baseline and after 8 weeks of supplementation, two additional blood samples were collected into a lithium heparin tube and into an EDTA tube (both Sarstedt, Nümbrecht, Germany) for the measurement of liver enzyme activities and a full blood cell count, respectively. All samples of a particular subject (weeks 0, 4, 6, and 8) were analysed in the same analytical run. Full blood count and liver enzyme assays were done on the day of blood drawing using the routine methods of the clinical chemistry laboratories of the University of Munich hospital.
Analytical methods
Fatty acid methyl esters (FAME) from 30 μl study oil TAG were obtained by reacting the oils with 2 ml 1·5 m methanolic HCl and 0·5 ml hexane at 90°C for 60 min in closed glass tubes. After adding 2 ml distilled water and 2 ml hexane with butylated hydroxytoluene (2 g/l), the samples were vortexed and centrifuged. The upper hexane phase containing the FAME was analysed by capillary GLC (Hewlett-Packard 5890 Series II gas chromatograph, equipped with a 60 m × 0·32 mm BPX-70 column; SGE, Weiterstadt, Germany). Flame ionisation detector signals were evaluated with the software EZ-Chrom Elite version 2.61 (Scientific Software, Pleasanton, USA). FAME peaks were identified by comparison with commercial standards (Nu-Check-Prep, Elysian, MN, USA). The instrument was calibrated regularly by using a quantitative standards mixture (GLC-85, Nu-Chek, Elysian, MN, USA) to update the response factors. Area percentages of all fatty acids determined (14–24 C-atoms) corrected with response factors were calculated.
For the quantification of fatty acids in plasma TL and lipid fractions, defined concentrations of dipentadecanoyl phosphatidylcholine, pentadecanoic acid, tripentadecanoin, and cholesteryl pentadecanoic acid (Sigma, Deisenhofen, Germany) were used as internal standards. Plasma lipids were extracted according to a modified Folch methodReference Folch, Lees and Stanley46 using chloroform–methanol (2 : 1, v/v, containing 50 mg butylated hydroxytoluene/l). TLC with heptane–diisopropyl ether–acetic acid (60 : 40 : 3, by vol.) as mobile phaseReference Carnielli, Pederzini, Vittorangeli, Luijendijk, Boomaars, Pedrotti and Sauer47 was used for separating PL, free cholesterol, NEFA, TAG and CE. For the analysis of plasma TL, TL extracts were directly transferred into glass tubes and taken to dryness under N2.
FAME from individual fractions and from TL were obtained by reaction with 3m methanolic HCl at 85°C for 45 min. Derivatives were extracted into hexane, dried under N2 and stored in 50 μl hexane (2 g/l butylated hydroxytoluene) until analysis at − 80°C. Analysis of FAME was performed by capillary GLC as described for the study oils. Absolute fatty acid concentration (mg/l) of all identified fatty acids with 14–24 C atoms was determined via comparison with the peak area of the internal standard (15 : 0) and correction with the accordant response factor. Additionally, fatty acid weight percentages (wt% (g/100 g total fatty acids)) were calculated. The method used for fatty acid analysis in plasma TL and lipid fractions showed a good reproducibility: intra-assay CV (n 8) of all presented fatty acids were below 3 % and inter-assay CV below 5 % (n 10 or 11).
Full blood cell counts were performed in EDTA blood on an automatic analyser (Coulter Micro Diff II, Beckmann Coulter, Krefeld, Germany). γ-Glutamyl transpeptidase, alanine aminotransferase, aspartate aminotransferase, and cholinesterase were measured from lithium heparin plasma with an automated sample processor (Roche/Hitachi 912, Roche Diagnostics GmbH, Mannheim, Germany) with the appropriate reagent systems.
Statistical analyses
Statistical evaluation was carried out with SPSS 12·0 for Windows (SPSS Inc., Chicago, USA). Data were checked for normality by visual inspection and by Kolmogorov–Smirnov test (with Lilliefors correction).
For volunteer characteristics and safety parameters at baseline, as well as for the impact of treatment on their absolute changes (week 8 minus week 0), differences between groups were evaluated using either Student's unpaired t test (for normally distributed variables) or the Mann–Whitney U test (for variables not normally distributed). Exact significances were calculated for all nonparametric tests.
Fatty acid concentrations were transformed for statistical analyses in case of non-normality to obtain a normal distribution. The overall effect of treatment on fatty acid composition was determined using the General Linear Model for repeated measures corrected for fatty acid concentrations at baseline (week 0). The between-subjects factor had two levels (‘placebo’ v. ‘FSO/EPO’), whereas the within-subjects factor ‘time’ had three levels (weeks 4, 6, and 8). Levene's test was used to check equality of variances of studentised residuals. In case transformation did not result in normally distributed data, the General Linear Model procedure was still adopted, if studentised residuals showed a normal distribution.
The effects of treatment were further described by comparing data from weeks 4, 6, and 8 with baseline data within each group using Student's paired t test or Wilcoxon nonparametric test both with the Bonferroni–Holm correctionReference Holm48 for multiple comparisons. Additionally, relative fatty acid changes from baseline values after 8 weeks (%) were compared between treatment groups by Student's unpaired t test for normally distributed variables or the Mann–Whitney U test for variables not normally distributed.
Correlations between parameters were estimated by computing the Spearman ρ correlation coefficient. For bivariate tabular analysis the χ2 test (exact calculation) was used. In cases of expected values smaller than 5, a Fisher exact test was used instead. Unless otherwise noted, results are given as mean values with corresponding standard errors. Values of P < 0·05 were considered significant unless otherwise stated.
Results
One subject from the FSO/EPO group dropped out during the first 4 weeks of the intervention period because she contracted abdominal influenza and could not take the capsules for more than 7 d. Thus, thirty-nine subjects (97·5 %) were included in the statistical analysis.
Compliance, side effects and success of blinding
Compliance as judged by capsule count (median) was 99 % (interquartile range 98–100 %) for the FSO/EPO group and 99 % (interquartile range 97–99 %) for the placebo group with no significant between-group difference. In both the active as well as the placebo group, three subjects reported mild adverse effects including gastrointestinal upsets (indigestion, belching), minor skin reactions (pimples) and slightly increased bleeding tendency. Side effects were evenly distributed between FSO/EPO and placebo groups (P>0·05). Group assignment was guessed correctly in the FSO/EPO group by 63 % and in the placebo group by 50 % of the subjects with no significant group difference (P = 0·523).
BMI, blood pressure and heart rate
Body weight, BMI, blood pressure and heart rate did not differ between FSO/EPO and placebo groups at week 0 and absolute changes from baseline after 8 weeks of intervention were not significantly different between the two groups (data not shown).
Full blood cell count and liver enzymes
All observed changes in haematology and liver enzymes were minor and within the normal ranges (data not shown). Incidence of liver enzymes and haematological parameters beyond the laboratory's reference ranges were comparable between FSO/EPO and placebo groups, both at baseline as well as at week 8. Basal values and supplementation effects of plasma fatty acids were not different between subjects whose laboratory parameters were within or outside the reference range, respectively (data not shown).
Fatty acid composition of plasma total lipids and lipid fractions
For plasma TL and for all three plasma lipid fractions, the General Linear Model for repeated measures (weeks 4, 6, and 8) corrected for fatty acid percentages at baseline (week 0) demonstrated significant increasing effects of FSO/EPO treatment compared to placebo on GLA, DGLA, ARA (in plasma TAG only), and DHA. No significant effects of treatment were observed on ARA levels in plasma TL, PL, and CE (Table 3456).
Table 3 Fatty acid composition of plasma total lipids (g/100 g total fatty acids)
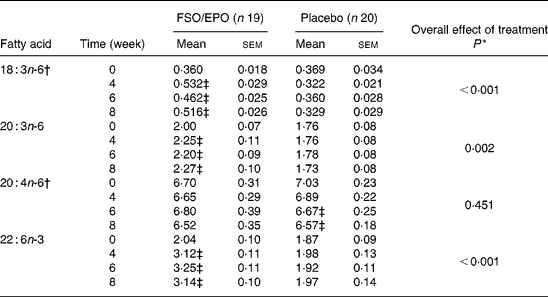
FSO/EPO, fish oil and evening primrose oil blend.
* The overall effect of treatment on fatty acid composition was determined using the General Linear Model for repeated measures corrected for fatty acid concentrations at baseline.
† Data were transformed before statistical analyses.
‡ Significantly different from baseline values (Bonferroni-Holm adjusted significancesReference Holm48).
Table 4 Fatty acid composition of plasma cholesterol esters (g/100 g total fatty acids)
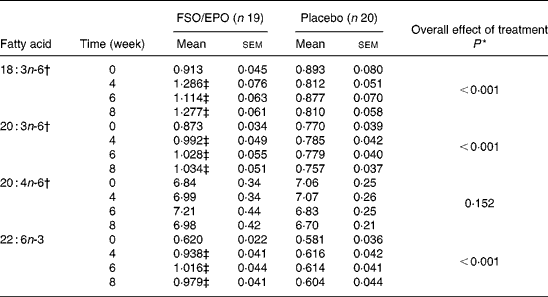
FSO/EPO, fish oil and evening primrose oil blend.
* The overall effect of treatment on fatty acid composition was determined using the General Linear Model for repeated measures corrected for fatty acid concentrations at baseline.
† Data were transformed before statistical analyses.
‡ Significantly different from baseline values (Bonferroni-Holm adjusted significancesReference Holm48).
Table 5 Fatty acid composition of plasma phospholipids (g/100 g total fatty acids)
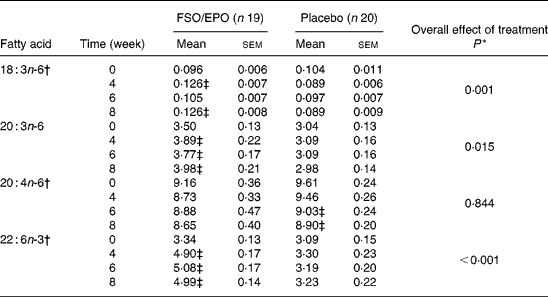
FSO/EPO, fish oil and evening primrose oil blend.
* The overall effect of treatment on fatty acid composition was determined using the General Linear Model for repeated measures corrected for fatty acid concentrations at baseline.
† Data were transformed before statistical analyses.
‡ Significantly different from baseline values (Bonferroni-Holm adjusted significancesReference Holm48).
Table 6 Fatty acid composition of plasma TAG (g/100 g total fatty acids)
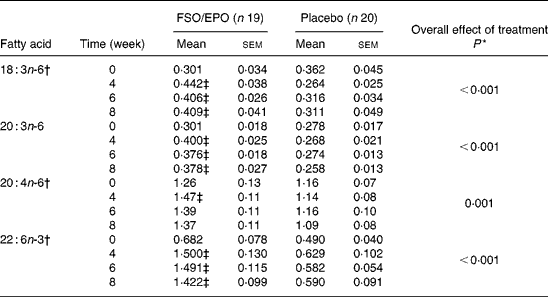
FSO/EPO, fish oil and evening primrose oil blend.
* The overall effect of treatment on fatty acid composition was determined using the General Linear Model for repeated measures corrected for fatty acid concentrations at baseline.
† Data were transformed before statistical analyses.
‡ Significantly different from baseline values (Bonferroni-Holm adjusted significancesReference Holm48).
Furthermore, compared to placebo, the General Linear Model procedure revealed significant increasing effects of FSO/EPO treatment on EPA levels and significant decreasing effects on concentrations of Mead acid, n-6 and n-3 docosapentaenoic acids, α-linolenic acid, and the sum of MUFA in some or all investigated lipid domains (data not shown).
Relative changes in fatty acid composition after eight weeks of intervention
After 8 weeks of intervention, relative increases (as a percentage of baseline values) were significantly greater with FSO/EPO than with placebo treatment for GLA, DGLA and DHA in plasma TL and lipid fractions (Fig. 1). There were no between-group differences in relative ARA changes for plasma TL, PL and CE, but a significantly greater ARA increase occurred in plasma TAG with FSO/EPO treatment compared to placebo. In the FSO/EPO group, mean GLA change from baseline was +49·9 % in plasma TL and +42·8 %,+47·2 % and +47·3 % in plasma PL, CE and TAG, respectively. DGLA levels increased by +13·8 % in plasma TL and by +13·5 %,+18·0 % and +27·5 % in the respective lipid fractions. Relative ARA changes from baseline with FSO/EPO treatment accounted for − 2·2 % in plasma TL, − 5·4 % in PL, +2·2 % in CE and +13·7 % in TAG. DHA changes from baseline values in plasma TL, PL, CE, and TAG were +59·6 %,+53·7 %,+60·7 % and +145·0 %, respectively.

Fig. 1 Percentage fatty acid changes from baseline values after 8 weeks of intervention. Values are means with standard deviations indicated by vertical bars. Percentage changes from baseline values were significantly different between FSO/EPO (n 19, ♦) and placebo (n 20, ●) treatments with *P < 0·05; †P < 0·01; ‡P < 0·001. ARA, arachidonic acid; CE, cholesterol esters; DGLA, dihomo-γ-linolenic acid; GLA, γ-linolenic acid; PL, phospholipids; TL, total lipids.
Correlations between relative fatty acid concentrations
Spearman ρ correlation coefficients were computed between plasma TL and lipid fraction fatty acid levels (wt%) for GLA, DGLA, ARA, and DHA in all subjects at respective time points (n 39–40). Because of multiple comparisons, values of P < 0·001 are considered significant, while values of P < 0·01 are considered a strong trend. Consistently significant positive correlations or a strong positive trend (P = 0·002 between ARA in TL and TAG at week 4) were observed between plasma TL and lipid fraction fatty acid levels. Correlation coefficients between TL and CE were >0·85 for all presented fatty acids. Furthermore, correlations between TL and PL were also >0·73 for all presented fatty acids. Correlation coefficients between TL and TAG fatty acids were >0·66 for GLA, DGLA, and DHA and >0·47 for ARA levels (data not shown).
Correlations between relative fatty acid changes from baseline
Spearman ρ correlation coefficients were computed between plasma TL and lipid fraction fatty acid changes (% of basal value) for GLA, DGLA, ARA, and DHA in all subjects at respective time points (n 39). Because of multiple comparisons, values of P < 0·001 are considered significant, while values of P < 0·01 are considered a strong trend. Significant positive correlations (P < 0·001, r>0·70) were observed between TL and CE as well as between TL and PL fatty acid changes (Table 7). Correlations between TL and TAG fatty acid changes were significant and positive for GLA, DGLA, and DHA (r>0·58). Strong positive trends (P < 0·01) were observed for ARA changes after 4 and 8 weeks between TL and TAG (r>0·43).
Table 7 Spearman ρ correlations between plasma total lipid and lipid fraction percentage fatty acid changes (% of basal values, n 39)
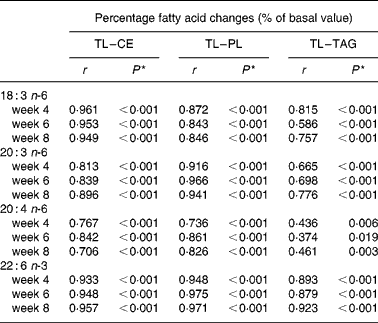
CE, cholesterol ester; PL, phospholipids; TL, total lipids.
* Because of multiple comparisons values of P < 0·001 are considered significant; Values of P < 0·01 are considered a strong trend.
Discussion
The objective of this study was to investigate the effects of a FSO/EPO blend on plasma fatty acid composition in healthy, non-pregnant women. Previous studies had shown that supplementation with DHA alone increased plasma lipid concentrations of DHA and (in most studies) EPA, but decreased GLA, DGLA, and ARA concentrationsReference Buckley, Shewring, Turner, Yaqoob and Minihane16–Reference Theobald, Chowienczyk, Whittall, Humphries and Sanders23. Supplementation with GLA on the other hand increased plasma concentrations of DGLA as well as (in most studies) ARA and GLA levels, but did not affect DHA levelsReference Yaqoob, Pala, Cortina-Borja, Newsholme and Calder38–Reference Thies, Nebe-von-Caron, Powell, Yaqoob, Newsholme and Calder43. We hypothesised that the tested n-3 LCPUFA–GLA mixture would result in an increase of plasma DHA, GLA, and DGLA levels without impairing ARA status.
This study showed that 8 weeks of supplementation with FSO/EPO (456 mg DHA, 72 mg EPA, and 353 mg GLA/d, n-3 LCPUFA:GLA ratio 1 : 0·67) increased the proportions of GLA and its elongation product DGLA in plasma TL and in all measured plasma lipid fractions (CE, PL, and TAG) compared to baseline, suggesting that at least some of the GLA is elongated before incorporation into PL, CE or TAG. These observations are consistent with those of Laidlaw & HolubReference Laidlaw and Holub49, who found significant increases from baseline in the proportions of GLA and DGLA (measured in plasma PL) in healthy women given 4 g EPA + DHA plus 2 g or 4 g GLA, respectively, for 4 weeks; no GLA and DGLA changes could be observed in this study when the ratio of n-3 LCPUFA to GLA was 4 g : 1 g. Miles et al. Reference Miles, Banerjee and Calder40 observed increases in the proportion of GLA in plasma TAG and CE, but not in PL, after supplementation with 1·1 g EPA, 0·5 g DHA, and 1 g GLA/d (n-3 LCPUFA:GLA ratio1 : 0·625). Other studies supplementing n-3 LCPUFA and GLA (in a ratio from 1 : 0·33 to 1 : 1·14, absolute GLA amounts ranged between 320 mg and 3 g /d) could not detect any changes in absolute GLA and DGLA amounts in plasma TL or in relative concentrations of plasma PLReference Barham, Edens, Fonteh, Johnson, Easter and Chilton41, Reference Khan, Elherik, Bolton-Smith, Barr, Hill, Murrie and Belch42, Reference Haglund, Wallin, Wretling, Hultberg and Saldeen50, Reference van Papendorp, Coetzer and Kruger51. The inconsistent effects of combined n-3 LCPUFA and GLA supplementation on plasma GLA and DGLA might be caused by the fact that different absolute GLA amounts or different n-3 LCPUFA:GLA ratios were studied: Laidlaw & HolubReference Laidlaw and Holub49 observed increasing DGLA values with increasing amounts of GLA and constant EPA + DHA dosages. Concerning the changes in GLA levels, an additional reason for the variable findings in the literature may be the lipid fraction studied; as demonstrated by several investigatorsReference Miles, Banerjee and Calder40, Reference van Houwelingen, Kester, Kromhout and Hornstra52, GLA is mainly found in CE and TAG, but hardly in PL ( < 0·1 wt%). Thus, GLA changes in plasma PL might be more difficult to detect, because reproducibility and accuracy of the method might be worse for small analyte concentrations. The power of a study to detect an existent difference depends also on the dimension of the difference, the variation of the parameter in the tested population as well as on the sample size.
In the present study, the proportion of ARA increased in plasma TAG, whereas ARA levels did not change significantly in plasma TL, CE, and PL. Other investigators reported decreasedReference Laidlaw and Holub49, Reference Haglund, Wallin, Wretling, Hultberg and Saldeen50 or unchangedReference Miles, Banerjee and Calder40, Reference Barham, Edens, Fonteh, Johnson, Easter and Chilton41, Reference van Papendorp, Coetzer and Kruger51 plasma ARA levels in TL or PL with n-3 LCPUFA–GLA supplementation compared to baseline. The inconsistent effects on ARA levels may relate on the one hand to the lipid fraction studied, on the other hand to differences in the absolute GLA, ARA, and EPA contents or to the EPA:GLA ratios of the supplements used. EPA and DGLA compete with ARA for esterification into phospholipids and furthermore, the (n-3) fatty acid product of the Δ5-desaturase reaction, EPA, attenuates the conversion of (GLA-derived) DGLA to ARAReference Barham, Edens, Fonteh, Johnson, Easter and Chilton41, Reference Rubin and Laposata53. In the current study, the GLA amount of the supplement appeared to be adequate (n-3 LCPUFA:GLA 1 : 0·67; EPA:GLA 1 : 4·8) to avoid a decrease of ARA levels in plasma, which is often observed with n-3 LCPUFA supplementation.
FSO/EPO supplementation increased the proportions of DHA in all investigated plasma lipid domains. Consistent with our results, other studies supplementing n-3 LCPUFA combined with GLA also observed increased DHA levels in plasma TL and PLReference Miles, Banerjee and Calder40–Reference Khan, Elherik, Bolton-Smith, Barr, Hill, Murrie and Belch42, Reference Laidlaw and Holub49–Reference van Papendorp, Coetzer and Kruger51.
The present study also serves to compare the intervention effect on plasma fatty acid composition and the variation of fatty acid changes between the different lipid domains. This information is valuable for the planning of future intervention studies with plasma fatty acid changes as outcome variables. The maximal effects on GLA, DGLA, and DHA content were already reached after 4 – 6 weeks of FSO/EPO supplementation in all studied plasma lipids; therefore, the intervention period of 8 weeks seems to be adequate and could possibly be reduced to 6 weeks in future studies. Regarding all subjects at study entry (n 40), highest GLA contents were found in CE, TAG, and TL (medians: 0·85, 0·31 and 0·37 wt% respectively), but GLA percentages were very low in phospholipids ( < 0·1 wt%) as already demonstrated by several investigatorsReference Miles, Banerjee and Calder40, Reference van Houwelingen, Kester, Kromhout and Hornstra52. DGLA, ARA, and DHA concentrations, on the other hand, were highest in plasma PL (medians: 3·43, 9·34 and 3·09 wt%, respectively) and considerably lower in CE and TAG. As relative fatty acid changes (in % of basal values) for GLA, DGLA, ARA, and DHA were significantly and positively correlated between plasma TL and lipid fractions or showed strong positive trends (exception ARA levels between TL and TAG at week 6), fatty acid analyses might be limited to plasma TL, provided that the subjects are healthy (no dyslipidaemia), non-pregnant and fasted (because these latter conditions cause general lipidaemia or NEFA increase, respectively). In our subjects, the ratio of PL, CE or TAG to plasma TL fatty acids (C14 – C24, mg/l:mg/l) changed only negligibly between weeks 0, 4, 6, and 8 (PL:TL 44·7–45·0 %, CE:TL 26·0–26·7 %, TAG:TL 19·5–20·0 %, means, P>0·05 between the four time points, Friedman test); this finding is probably an important condition for the strong correlations between fatty acids of plasma TL and lipid fractions. These strong correlations leave the required group size as the main determinant of the decision on which lipid domain to measure for checking the biochemical effects of fatty acid supplementation studies.
Based on fatty acid levels, responses (week 8 minus week 0) and variations observed in this study, sample size determinations for unpaired t tests (using BIAS for Windows, version 8.1, Epsilon-Verlag, Hochheim-Darmstadt, Germany) indicate that for a minimal GLA increase of 20 % from basal values caused by FSO/EPO supplementation to be detected as significantly different compared to placebo changes (assumed mean change 0 %) at α = 5 % and β = 20 %, about 62, 63, 104 and 172 volunteers per group are needed when determining GLA in plasma TL, CE, PL and TAG, respectively. For a DGLA increase of 20 % to be detected as significantly different from placebo changes, about 14, 11, 15, and 41 volunteers per group are needed when determining DGLA in plasma TL, CE, PL and TAG, respectively. For a DHA increase of 20 % to be detected as significantly different compared with placebo changes, about 28, 31, 27, and 158 volunteers per group are needed when determining DHA in plasma TL, CE, PL and TAG, respectively. Thus, plasma TL and CE seem to be most suitable to detect between-group differences in GLA changes, while plasma TL, CE, and PL might be similarly suitable compartments to detect differences in plasma DGLA or DHA changes.
To sum up, plasma TL as biomarkers for n-6 and n-3 fatty acid status have the following positive attributes: the tested n-6 and n-3 fatty acids (GLA, DGLA, ARA, and DHA) are present in adequate amounts in plasma TL and the fatty acid composition responds to increasing EFA–LCPUFA intakes and is correlated with fatty acid levels in plasma PL, CE and TAG. In addition, plasma TL offer, compared to plasma PL and TAG, a higher statistical power to detect significant and clinically meaningful differences in GLA, DGLA and DHA changes compared with a placebo group. Statistical power of plasma CE analysis is similar to plasma TL analysis. Finally, the analyses of plasma TL fatty acids are less demanding than lipid fraction analyses, and the required plasma volume is smaller. A disadvantage is that the fatty acid composition in plasma TL is influenced by the fatty acid composition of the last meal and the scheduling of the blood sampling relative to the last meal. Consequently, blood should be sampled in the fasting state. This may be problematic in pregnant women and is not acceptable in neonates. For these subjects, plasma CE might be the best choice to investigate GLA, DGLA and DHA changes.
In conclusion, the fatty acid supplement is well tolerated and appears safe. FSO/EPO intake resulted in the anticipated increase of plasma GLA, DGLA, and DHA levels without impairing the ARA status. These data provide a basis for testing FSO/EPO not only in respect to biochemical changes in pregnant women and their neonates, but also to evaluate its effects on maternal mood, cognition, and selective attention, as well as on visual and cognitive development of the infants, early markers of allergy risk and prevention of obesity, insulin resistance, hypertriacylglycerolaemia or other chronic diseases in later life.
Acknowledgements
We thank EFAMOL Ltd (Brackenholme, Selby, North Yorkshire, UK) for providing the FSO/EPO and placebo capsules and for financial support. Thanks to Jeannette Franke and Dr Hiromichi Shoji for taking blood samples. We acknowledge the excellent technical assistance of Monika Rachl and the team of Anna-Maria Prause. Special thanks to Dr Reto Muggli and Dr Peter Clough for their helpful advice. Most importantly, we would like to thank our subjects for their commitment to this study. J. G. recruited the subjects and collected, analysed, and interpreted the data. She was also the primary writer of the manuscript. H. D., G. H., and B. K. were involved in the design of the study, data interpretation, and writing the manuscript. B. K. is the recipient of a Freedom to Discover Award of the Bristol Myers Squibb Foundation, New York, NY, USA. None of the other authors had any conflict of interest.










