INTRODUCTION
Pseudomonas aeruginosa is the most commonly identified opportunistic pathogen associated with pool-acquired bather disease [Reference Mena, Gerba and Whitacre1]. To better understand why this microorganism poses this protracted problem we recently appraised P. aeruginosa pool risk management. Much is known about the wider ecology of P. aeruginosa [Reference Rice2]. However, in contrast to ingested and inhaled pathogens, neither the preceding exposure conditions, the dermal route of infection, nor infectious doses, appeared well-defined. The goal of this follow-up review is to explore these knowledge gaps through a survey of data and theory on: (i) dose response, i.e. bodily responses arising from different microbial doses [Reference Julien3]; (ii) folliculitis and acute otitis externa (AOE) aetiology; and (iii) ecological processes preceding infection.
The review commences with an introduction to P. aeruginosa pool disease attributes, research drivers and knowledge gaps, and microbial dose-response theory. Next folliculitis and AOE aetiology relating to pools and experimental disease induction are reviewed, including uncertainties, notably whether P. aeruginosa might be autochthonous and whether folliculitis should be viewed as multiple localized infections rather than a systemic infection. Biophysical steps whereby P. aeruginosa migrates from the pool void to the epidermis are reviewed in the third section. How to progress dose-response theory bearing in mind aetiology and ecology is then analysed. Finally, using all information we discuss management implications, propose a conceptual framework and identify priority research.
Pool infections and P. aeruginosa disease
Pool-acquired P. aeruginosa infection most commonly involves folliculitis, a rash then lesions around hairs, which progress from discrete follicular pruritic papules to erythematous papulopustules within a day [Reference Jacobson4]. Although usually localized and rapidly resolved, folliculitis can persist for several months [Reference Maniatis5]. P. aeruginosa is also associated with AOE, colloquially ‘swimmer's ear’, an infection or inflammation of the external ear canal in rare cases progressing to necrotizing otitis externa [Reference Beers and Abramo6]. Although other pathogens are associated with folliculitis and AOE, P. aeruginosa is viewed as the most common agent [Reference Beers and Abramo6].
Knowledge gaps and the need to quantify dermal dose response
Defining risk factors and dose-response mechanisms operating in pools is central to quantitative microbial risk assessment (QMRA)-based management. However, characterization of dermal and aural dose response, and exposure processes preceding infection, have been neglected. Rather, dose-response information on P. aeruginosa primarily pertains to ingestion, inhalation and subdermal inoculation [Reference Rice2]. This gap is also evident in reviews by Mena & Gerba [Reference Mena, Gerba and Whitacre1] and Barna & Kádár [Reference Barna and Kádár7] of P. aeruginosa and pool infection, respectively. The only dermal dose response reported is a folliculitis minimum infective dose (MID) of 1000 c.f.u./ml [Reference Price and Ahearn8].
To improve understanding and management of pool infection there is the need, first, for more sophisticated dose-response conceptualization implied by the Key Events Dose-Response Framework (KEDRF) proposal [Reference Julien3, Reference Magnússon9]. Although folliculitis and AOE have been extensively documented since the 1980s [Reference Jacobson4, Reference Ratnam10], many past studies were relatively unsystematic, e.g. statistically, by modern experimental standards. Therefore second, many knowledge gaps remain to be addressed, e.g. rarity of head immersion data accompanying AOE reports (an exception is [Reference Schets, Schijven and de Roda Husman11]) and genomic studies, poor correspondence between outbreak frequency and water quality [Reference Mena, Gerba and Whitacre1, Reference Price and Ahearn8, Reference Ratnam10]. Finally there are diverse risk unknowns such as the safety of hydrotherapy pools for an ageing population (see also online Supplementary material).
Our review focuses on the first elements of the KEDRF framework (fig. 2 in [Reference Julien3]) which splits the dose-response process into: (i) intake/exposure; (ii) biological interaction/process; (iii) interaction/process (transport/distribution/excretion); (iv)interaction/process (metabolism); (v) target issue interaction; and (vi) ultimate effect: to support data evaluation, focus research, strengthen decision-making and advance dose-response assessment. Specifically, we explore KEDRF steps (i)–(iii), which correspond to the gaps we identified in dermal dose-response theory. These delineate infection from the better characterized illness induction processes, steps (iv)–(vi) [e.g. Reference Kaper, Nataro and Mobley12, Reference Sattentau13].
Dose-response theory and the epidermis
Modern dose-response characterization used in QMRA [Reference Teunis and Havelaar14–Reference Teunis, Ogden and Strachan16] reflects the introduction of the ‘single-hit’ exponential model of infection, i.e. a single viable propagule may cause systemic infection, with a probability reflecting exposure dose and infection site availability [ Table 1, equation (6)]. Algorithm coefficients are typically derived from human exposure and foodborne disease outbreak studies where pathogen density estimates and attack rate data are concurrently available. This paradigm's origin is credited to Furomoto & Mickey's [Reference Furumoto and Mickey17, Reference Furumoto and Mickey18] analysis of tobacco leaf mosaic virus (TMV) infection. Deviations from the exponential model have been addressed by introducing beta-Poisson, hypergeometric and other algorithm forms which allow better curve fitting [Reference Teunis and Havelaar14–Reference Teunis, Ogden and Strachan16]. These can incorporate secondary variance sources including between-strain variance and inoculum dispersion [Reference Teunis, Ogden and Strachan16].
Table 1. Selected algorithms, which support dose-response quantification
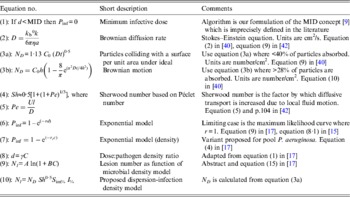
a, Particle radius; A and B, algorithm constants; C, density of pathogen; C 0, density of pathogen at time 0; γ, constant describing the relative infectivity; d, pathogen dose; D, Brownian diffusion rate; h, depth of fluid above surface; I %, infection efficiency; k b, Boltzmann constant; l, characteristic length of a particle; MID, minimum infectious dose; N, number of lesions; N D , number of particles absorbed under ideal Brownian diffusion assuming 100% adherence; N l , lesions per unit area; η, viscosity; Pe, Péclet number; P inf, probability of infection; r, constant; r C , constant; S im%, per cent surface adherence + migration efficiency; Sh, Sherwood number; t, time; U, velocity from flow regimen.
‘Single-hit’ theory has proved a great advance over the MID concept [Reference Magnússon9] [Table 1, equation (1)] especially for inhalation and ingestion of water, food and aerosols. Unlike MIDs, algorithms are grounded in probability theory and experimental data, and support infection ecology and water and food-safety investigations. However, skin infection dose-response algorithms are few: Tamrakar & Haas's [Reference Tamrakar and Haas19, Reference Tamrakar20] application of dose-response theory to inoculation by scratches and bites, and Rose & Haas's [Reference Rose and Haas21] equations for hand washing; neither of which considers whole body exposure. Nevertheless, these studies do incorporate several mechanistic factors, e.g. exposure time (t), skin interfaces, bacterial growth dynamics, and the latter [Reference Rose and Haas21] endeavours to ground dose-response theory in pathogen–host biophysics.
AETIOLOGY AND DOSE RESPONSE
Water quality and infection likelihood
Folliculitis incidents and bathing water quality data (Table 2) point to high variance in the P. aeruginosa density associated with outbreaks (0·01–107 c.f.u./ml) [Reference Mena, Gerba and Whitacre1, Reference Ratnam10]. Survey data also suggests that levels could reach 103 c.f.u./ml without outbreaks, consistent with Price & Ahearn's [Reference Price and Ahearn8] proposed threshold. These discrepancies make defining hazardous levels difficult and may reflect unknown disease induction factors. The value of outbreak reports is also limited by: (i) little concurrent reporting of P. aeruginosa densities [Reference Mena, Gerba and Whitacre1, Reference Ratnam10]; (ii) data being seldom systematic and statistically robust; (iii) context information paucity; (iv) delays between infection and water sampling; (v) analytical technology limitations; and (vi) limited strain virulence or genotyping data.
Table 2. P. aeruginosa densities reported in folliculitis pool outbreaks and contamination survey data
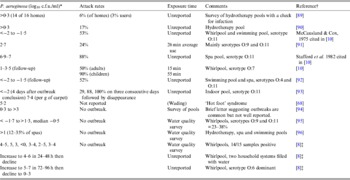
* For most outbreaks counts were taken several days later.
† Another seven references detailed attack rate and timing but not microorganism density.
‡ All commercial and disinfected by chlorine but poorly maintained. Bather load 1–6/h.
P. aeruginosa O:11 is suggested as the main serotype of concern in pools; however, other biotypes also cause infection [Reference Maniatis5, Reference Highsmith22] and whether O:11 prevalence reflects virulence or environmental abundance is unclear. Subtyping of virulent isolates appears inconclusive [Reference Ratnam10, Reference Highsmith22] and systematic [Reference Teunis, Ogden and Strachan16] molecular typing has not been applied to P. aeruginosa bathing outbreaks.
Information on AOE is similarly deficient. Hajjartabar [Reference Hajjartabar23] reported an infection rate of 79% when average pool densities were 13–18 P. aeruginosa/100 ml while Van Asperen et al. [Reference Van24] reported infection odds of 15·5% for natural waters with 1–17 c.f.u. (median = 2) P. aeruginosa/100 ml. However, others [Reference Jones25, Reference Prieto26] report no strong correlation between ear infections and P. aeruginosa (medians <10, maximum reported 103/100 ml, n > 500) despite it being the dominant pathogen [Reference Beers and Abramo6]. Critically, spa and pool surveys typically lack head immersion data and it is unclear whether simple water contact promotes infection [Reference Hojyo-Tomoka, Marples and Kligman27].
Experimentally induced skin infections
Verifying Koch's postulates for P. aeruginosa folliculitis has proved difficult despite the diverse characteristic symptoms reported in the literature (e.g. [Reference Maniatis5]). Forearm skin infection experiments [Reference Hojyo-Tomoka, Marples and Kligman27] have shown that skin occlusion and super-saturation (plastic film ± saturated dressing) induced blooms of P. aeruginosa, at the expense of the normally dominant Gram-positive skin bacteria [Reference Grice and Segre28], yet no folliculitis ensued. Leyden et al. [Reference Leyden, Stewart and Kligman29] inoculated occluded and saturated skin with 13 different strains of P. aeruginosa, including six pool outbreak and four serotype O:11 representatives. Clingfilm-covered dry intact skin (106 c.f.u/cm2), remarkably yielded only 0% and 5% clinical response in subjects after 2 and 7 days, respectively, despite undiminished P. aeruginosa numbers. Under super-hydration, however, 68% of volunteers developed a clinical response after 7 days. Contrary to the serotype O:11 high-risk hypothesis [Reference Highsmith22] there was no clear difference in virulence between strains eliciting papules, pustules or surrounding erythema (50–90% of volunteers). In further experiments [Reference Leyden, Stewart and Kligman29], forearms (n = 112) were scarified, inoculated with 103–109 P. aeruginosa, dried and covered with semi-occlusive dressings. No clinical reactions were seen with ⩽105 c.f.u. (n = 30), although 60% of treated areas did react to a dose of 107 c.f.u. Folliculitis-like pustules were rarely induced, although oedema and erythema, resembling AOE symptoms [Reference Beers and Abramo6], were induced. Consistent with these observations, folliculitis is more severe on bathing suit-occluded skin [Reference Ratnam10]. Although folliculitis appears more common on the torso and thighs [Reference Gustafson30], Maniatis et al. [Reference Maniatis5] reported folliculitis on the arms of 5/13 patients indicating forearms are a valid experimental system. Leyden et al. [Reference Leyden, Stewart and Kligman29] concluded: (i) >104 P. aeruginosa/cm2 are required for dermal infection, (ii) super-hydration is essential with healthy individuals, (iii) pool isolates are not especially more pathogenic; and (iv) the ‘epidemic skin infection’ pool model may be too simplistic in view of the ubiquity of Pseudomonas (and) cuts and abrasions. These results suggest that infection is influenced by exposure duration and super-hydration as well as dose. Subsequent work [Reference Rice31] has not resolved whether strains vary in virulence.
Autochthonous or exogenous pathogens?
As well as coming from contaminated water and soil [Reference Maniatis5, Reference Lyczak, Cannon and Pier32], P. aeruginosa could be a minor component of the normal ear microbiota whose growth is promoted by skin environment changes [Reference Hojyo-Tomoka, Marples and Kligman27–Reference Leyden, Stewart and Kligman29, Reference Lyczak, Cannon and Pier32]. Alternatively, autochthonous P. aeruginosa [Reference Cogen, Nizet and Gallo33] may be transferred from elsewhere on the body. P. aeruginosa appears to be common around fingernails, leading in extreme cases to ‘green nails’ [Reference Mena, Gerba and Whitacre1, Reference Jacobson4, Reference Leyden, Stewart and Kligman29]. Although Gram-negative bacteria are normally minor components of the skin microbiota, ear canal community structure is unstable. High moisture promotes their growth [Reference Hojyo-Tomoka, Marples and Kligman27–Reference Leyden, Stewart and Kligman29] and P. aeruginosa have been isolated from 3% to 8% of healthy bather and non-bather ears [Reference Jones25]. These and other observations (see Supplementary material), raise the question of how often do AOE and folliculitis, reflect blooms of endogenous bacteria?
A conceptual challenge for folliculitis dose-response algorithms
In the ‘single-hit’ infection model, the endpoint dose is a single pathogen inducing systemic disease. Folliculitis, however, appears localized [Reference Jacobson4] rather than systemic, and generally resolves without major therapy. This suggests folliculitis involves multiple independent infections analogous to those produced by Trichobilharzia (‘swimmer's itch’) [Reference Schets34] and epiphyte pathogens prior to systemic spread [e.g. Reference Furumoto and Mickey18, Reference Hattermann and Ries35]. If so, dose-response algorithms should incorporate a relationship between water quality and numbers of infected follicles.
That folliculitis pustule density could correlate with water quality and exposure time raises the question of what lesion densities correspond to clinical folliculitis? As we found no such data we estimated lesion density from outbreak report photographs which arguably represent what is clinically considered folliculitis (for calculations see Supplementary material). Minimum and geometric means were 200 and 1000 follicular lesions/m2, respectively. This indicated only a small proportion of follicles (120000–300000/m2) [Reference Otberg36, Reference Poet and McDougal37] are typically infected.
THE ECOLOGY OF INTIMATE CONTACT
Traversing ‘the void’
To induce infection of follicles and abrasions, exogenous dermal pathogens must: (i) cross the pool ‘void’ to the skin; (ii) Intimately, but not irreversibly, interact with the skin interface; and (iii) migrate to final invasion sites; or (iv) collide with them directly from the void. Sattentau [Reference Sattentau13] explored how gut pathogens could avoid the ‘void’ (intestinal lumen) and subsequent excretion through re-infection while Spagnuolo et al. [Reference Spagnuolo, DiRita and Kirschner38] modelled how Vibrio cholerae traversed the intestinal lumen. With this ‘void’ concept in mind, we reviewed how bacteria traversed the pool ‘ocean’ to bathers' skin and ears. No comprehensive analysis was identified, but germane studies were found on: (i) microbial diffusion and motility; (ii) plankton interaction; (iii) (plant leaf) pathogen infection; (iv) biofilm rheology and life-cycle theory.
Movement of P. aeruginosa towards the skin appears driven by a combination of Brownian diffusion (D) and ‘superdiffusive Levy processes' [Reference Bartumeus39], e.g. settling, turbulence and flagella swimming. We use the term ‘dispersion’ to denote these combined processes and distinguish them from Brownian diffusion alone.
Using the Stokes–Einstein equation [Table 1, equation (2)] Valentine & Allison [Reference Valentine and Allison40, Reference Allison and Valentine41] developed two formulae relating diffusion to surface attachment [Table 1, equations (3a) and (3b)] for poxviruses and 0·69 μm latex particles. Where attachment approached 100%, experiment matched Brownian theory within ± 20% (tables 1 and 2 in [Reference Valentine and Allison40]). Surprisingly, agitation had little effect on adsorption kinetics, with dispersion being dominated by Brownian diffusion. Diffusion also dominates viral and bacterial interactions in large water volumes [Reference Murray and Jackson42]. The limited effect of mixing (table 2 in [Reference Valentine and Allison40]) appears to be explained by shear and Sherwood numbers [Table 1, equations (4) and (5)], which quantify increases in transport over Brownian diffusion. Dispersion is calculated by multiplying diffusion by the Sherwood number. For bacteria and viruses, Sherwood numbers are small (about 1 and 1·5 for sinking and swimming bacteria, respectively) [Reference Murray and Jackson42].
Some studies [Reference Mueller43–Reference Kim45] suggest that bacterial motility increases dispersion velocities by 100-fold (∼2 × 10−13 m2/s under ideal Brownian diffusion vs. 1–2 × 10−9 m2/s, noting particle velocity increases as the square root of dispersion). These rates may reflect experimental system design and scale. Dispersion coefficients of Wei et al. [Reference Wei44] and Kim [Reference Kim45] were measured in chemotaxis experiments with stable (e.g. capillary) gradients while Mueller's [Reference Mueller43] dispersion rates were measured in a chamber tens of micrometres in depth, comparable to bacterial free run distances, where the randomizing effects of tumbling and Brownian motion [Reference Ping46] and shear forces were likely suppressed. Conversely, larger scale experiments report substantially lower dispersion. Pseudomonad infection experiments [Reference Hattermann and Ries35, Reference Panopoulos and Schroth47] suggest dispersion increases of only 2 × to 10 × . Liu et al. [Reference Liu, Ford and Smith48] also reported intermediate dispersion enhancement with porous media. Separately, Bartumeus et al. [Reference Bartumeus39] concluded from predator–prey modelling that ‘Lévy motion’ does not lead to significantly higher encounter rates than Brownian strategies except for scarce, small, and slow target scenarios, the opposite of the bathing situation. Murray & Jackson [Reference Murray and Jackson42] similarly concluded that although bacterial motion can be rapid over short time scales, dispersion is diffusion dominated over large scales. We provisionally concluded that bacterio-plankton scenarios [Reference Murray and Jackson42] most likely describe the pool situation, and Brownian diffusion [Table 1, equations (2), (3a), (3b)], with modest Sherwood number corrections, provides a first-cut mechanistic description of bacterial dispersion towards bathers' skin, recognizing that other dispersion and diffusion formulations need incorporation, e.g. [Reference Mueller43].
Epidermal deposition and adhesion
Biofilm models view surface interaction as a two-stage process of reversible then ‘irreversible’, biofilm-mediated, adherence [Reference Katsikogianni and Missirlis49]. Biofilm micro-colonies then evolve mediated by quorum sensing, the regulation of gene expression in response to fluctuations in cell-population density [Reference Miller and Bassler50–Reference Charlton52]. Given the need to reach follicles or abrasions to initiate infection, P. aeruginosa must either contact such sites directly or adhere sufficiently reversibly for horizontal dispersion, but not irreversibly or so weakly they are scoured back to the ‘void’.
Adherence is a well-documented virulence factor and potential control point [Reference Kaper, Nataro and Mobley12, Reference Krachler, Ham and Orth53] involving many surface attachment-mediating factors including pili, fimbriae, flagella and capsular material [Reference Lyczak, Cannon and Pier32, Reference Krachler, Ham and Orth53] whose action is likely mediated by motility, colloids and other ‘stickiness’ factors [Reference Liu, Ford and Smith48, Reference Jacobs54].
Reversible adherence involves weak electrostatic and hydrophobic interactions [Reference Katsikogianni and Missirlis49] whose formulation is termed the extended Derjaguin, Landau, Verwey and Overbeek (DLVO) theory. It describes how particles closely approaching a surface reach a free-energy minimum where binding is loose [Reference Jacobs54]. Factors controlling subsequent interaction are diverse and include physical/rheological (Van der Waals, opposite charge attraction, free energy of surface, surface tension, hydrophobicity, surface roughness, topography), chemical (hydrogen liaison, ionic pair and triplet formation, inter-particulate bridges), biochemical (cellular surface dehydration, membrane fusion) and biological (competition, flagella function, surface coatings, e.g. mucin) [Reference Katsikogianni and Missirlis49, Reference Liu and Tay55].
Fletcher's [Reference Fletcher56] experiments indicate, consistent with Langmuir isotherm theory, that the number of bacteria attached is a function of time and cell density, and can exceed 5 × 1010/m2. Adherence rates vary with surface, bacteria and hydrodynamic conditions. With P. aeruginosa, adherence of 5–50 × 106 cells/m2 per minute has been observed at liquid densities of ∼106/ml [Reference Mueller57]. Sand experiments suggest the reversible adherence free energy is low, up to 15 mJ/m2 [Reference Jacobs54]. By contrast, biofilm adhesive strength can be 100–1000 mJ/m2 where flow is 0·6–1·6 m/s [Reference Chen, Zhang and Bott58]. Bacteria, including motile cells, can then orient themselves horizontally and accumulate at interfaces even in the absence of chemotaxis [Reference Li, Tam and Tang59].
Horizontal dispersion
Dispersion of P. aeruginosa horizontal to the epidermis likely involves diffusion and motility. Four types of surface motility: walking, crawling, swimming and spinning, can propel bacteria at velocities of 10–100 μm/s [Reference Conrad60]. Follicle entry could be facilitated by spinning motility and induced changes in the viscosity of blocking polymers [Reference Celli61]. The possession of a single flagellum by P. aeruginosa may influence its dispersion. Monotrichous bacteria cannot tumble, but Brownian diffusion may substitute [Reference Li, Tam and Tang59]. Moreover, a polar flagellum could reverse direction, improving the potential to explore a structured surface and avoiding excessive circular motility [Reference Xie62]. Conceptually, chemotactic attraction to nutrients escaping follicles could facilitate infection. P. aeruginosa's genome has 46 genes potentially involved in chemotactic responses toward amino acids, inorganic phosphate, phospholipids and fatty acids [Reference Kato63] and it can respond strongly to <100 μ m of attractant [Reference Armitage and Evans64].
Irreversible adherence would self-evidently initially immobilize bacteria and inhibit infection. However, after biofilm formation propagules could subsequently ‘creep’ to infection sites [Reference Stoodley65].
Ultrastructure and infection
Skin and ear canal anatomy and physiology will likely influence interface interactions, infection ecology, and folliculitis and AOE dose responses. Three distinct structures provide different microbial habitat and infection points, the epidermis proper, sweat glands, and hair follicles, including shafts and sebaceous glands. Discounting micro-topography, the area of bather skin available for colonization totals 1–2 m2 [Reference Poet and McDougal37]. Nano-particle delivery studies suggest follicles are favoured infection points [Reference Wosicka and Cal66]. Toll et al. [Reference Toll67] showed that 0·75–1·5 μm particles migrate from ‘stripped’ skin into skin follicles during drying. The centrality of follicles is supported by ‘hot hand/foot’ syndrome's rarity and distinct aetiology [Reference Fiorillo68].
Macroscopically, folliculitis is most common on the lower trunk and thighs even though hair follicle density is 10–25 times lower than on the forehead and scalp (∼12–30 vs. 300/cm2). This difference cannot be explained solely by degree of immersion, as torso and thigh follicle densities are comparable to arms and calves [Reference Gustafson30, Reference Otberg36, Reference Poet and McDougal37] but reportedly less commonly infected. Other factors could include epidermis thickness, hair morphology [Reference Otberg36, Reference Poet and McDougal37], varying microbial communities [Reference Grice and Segre28], occluding surface debris [Reference Toll67] and bathing suit material [Reference Ratnam10].
Insights into AOE can be gained from similarly considering the structure of the ear canal. The volume and surface area of each adult outer ear canal are about 1 ml and 6 cm2, respectively [Reference Maroonroge, Emanuel, Letowski and Rash69]. Ear canal volume indicates exogenous P. aeruginosa densities of concern >50 c.f.u./100 ml depending on water retention and colonization rates, consistent with infectious doses [Reference Hajjartabar23, Reference Van24], and represents ∼0·1% of the total skin available for infection (12 cm2).
Adherence and follicle invasion efficiency
Many factors likely reduce the efficiency of skin, follicle and ear colonization such as dissolved protein, and adherence is typically much less than 100% (e.g. 7–33% in [Reference Allison and Valentine41]). In addition there is desorption probability (3–84% depending on motility and substrate for P. aeruginosa and P. fluorescens [Reference Mueller43]), competition and interaction with normal microbiota [Reference Grice and Segre28, Reference Cogen, Nizet and Gallo33] and follicle blockage (<25–87% [Reference Toll67]). However, in vivo, obstruction could be overcome if P. aeruginosa can ‘drill down’ through mucin [Reference Conrad60, Reference Celli61]. Separately, infection could be constrained by migration distance. Arms, torso and thighs/upper legs have median follicle densities of 17–32 cm−2 and orifice diameters of 80–130 μm [Reference Otberg36], indicating a median of 700–1000 μm from contact to a follicle's boundary (for estimation see Supplementary material).
Hydrodynamic scouring and infection
Conceptually, surface hydrodynamic forces (shear, turbulence) could dislodge bacteria prior to infection. A likely critical point is where water movement parallel to the epidermis exceeds the critical velocity where flow changes from laminar to turbulent, associated with a rapid increase in Reynolds number [Reference Stoodley65]. Hydraulics theory indicates that loose particles in the 2–200 μm range are mobilized at flows of 0·1–0·4 m/s [Reference Miller, McCave and Komar70] while biofilm induction is inhibited by water velocity increases of 1·4–2·7 m/s [Reference McCoy71].
Conversely, agitation reportedly has a moderate impact on adherence [Reference Valentine and Allison40, Reference Stanley72]. Moreover, the skin has a complex topography of primary and secondary furrows 5–100 μm in depth [Reference Vargiolu, Zahouani and Agache73] which could protect bacteria from scouring [Reference Scheuerman, Camper and Hamilton74]. Where follicle/abrasion invasion does not immediately occur, infection could be facilitated long term by furrow accumulation [Reference Wosicka and Cal66, Reference Vargiolu, Zahouani and Agache73, Reference Frank75, p. 339]. Removal by superficial (e.g. towel) drying after pool exit is probably limited unless abrasion is vigorous [Reference Frank75, p. 351] while air drying may draw surface bacteria deep into the epidermis [Reference Toll67]. Overall, shear driven remobilization of P. aeruginosa seems plausible but its extent remains to be assessed.
Epidermal hydrodynamics considerations also raise the question of whether bulk water velocity influences adhesion. Filter turnover times of 2–6 h [Reference Judd and Bullock76, Reference Goeres77] indicate that even pool inlet to outlet distances of 2 m, should generate bulk flow >100 μm/s exceeding P. aeruginosa velocities [Reference Armitage and Evans64]. Body movement arising from buoyancy changes (>1000 μm/s) and swimming (0·5–2 m/s), also exceed flagella enhanced dispersion. Flagella-enhanced dispersion may be more important where water is occluded (ear canal, under bathing garments). For ears interesting questions include how well bacteria adhere and are flushed during bathing? Dispersion theory should provide estimates. Following adherence bacteria would be difficult to dislodge due to surface topography [Reference Scheuerman, Camper and Hamilton74] and the rapid establishment [Reference Stanley72] and resilience of biofilms [Reference Stoodley65, Reference McCoy71].
Quorum sensing
Attachment of P. aeruginosa could also depend on between strain differences and virulence factor regulation via quorum sensing. The genetic diversity of P. aeruginosa and its implications are now better understood and >16 major serotypes are distinguishable [Reference Highsmith22]. Sequenced strains have genomes of ∼6 Mbp, 10% of which may regulate virulence [Reference Stover78]. This large genome could also aid P. aeruginosa-colonizing diverse habitats, organs and organisms including nematodes, insects and plants [Reference Ramos79]. One key regulatory system involves the production and secretion of small, diffusible ‘signals’, called acylated homoserine lactones (AHLs). When signals accumulate, in high concentrations, P. aeruginosa respond by inducing virulence genes, such as elastase. In swimming pools, quorum-sensing-regulated genes would likely be inactive due to signal dilution in water but could still accumulate in biofilms. In the confined space of an epidermal follicle or ear canal, biofilms and signals might also accumulate, activating the quorum-sensing system and further facilitating signal accumulation and infection [Reference Charlton52].
Time dependency of infection induction
Although exposure time is not included in traditional ‘single-hit’ dose-response formulation, there is increasing interest in this variable [Reference Rose and Haas21, Reference Huang and Haas80] and several reasons why exposure time likely affects folliculitis and AOE dose response. Pool ‘voids’ of tens to hundreds of centimetres would require many minutes to traverse for P. aeruginosa swimming at 2–4 mm/min [Reference Murray and Jackson42, Reference Armitage and Evans64]. Valentine & Allison (fig. 1 in [Reference Valentine and Allison40]) showed surface attachment increases markedly over a timescale of minutes as does plant leaf infection [Reference Hattermann and Ries35, Reference Panopoulos and Schroth47]. Time will be required for bacteria to adhere [Reference Fletcher56], migrate to infection points and express virulence factors, especially if a biofilm is required. Skin super-hydration leads to marked changes in ultrastructure again over a period of minutes to hours [Reference Warner, Stone and Boissy81], potentially influencing pustule or rash development. Finally, folliculitis frequency and severity reportedly increases with exposure time [Reference Hudson82].
TOWARDS DOSE-RESPONSE MODELS FOR P. aeruginosa POOL INFECTIONS
Applicability of ‘single-hit’ theory
With aetiology and ecology in mind we first assessed the applicability of conventional QMRA algorithms to bathing risk models. Furumoto & Mickey [Reference Furumoto and Mickey17, Reference Furumoto and Mickey18] developed their ‘single-hit’ hypothesis using plant leaves dipped into suspensions of TMV, an experimental system analogous to the pool+P. aeruginosa+bather skin scenarios. In addition to the single-hit formulation, they derived two other relevant algorithms. First, they described a variant of the exponential model [Table 1, equation (6)], relating microorganism density, rather than dose, to infection probability (cf. equations (1) and (9) in [Reference Furumoto and Mickey17]). In their model, ‘dose’ a, was defined by the pathogen density V and a correction factor c [γ in our equation (8)]. Coefficient r and ‘dose’ d in the exponential ‘single-hit’ model are replaced by pathogen density C and a probability coefficient analogous to r we suggest be designated r C [Table 1, equation (7)].
The second algorithm [equation (15) in [Reference Furumoto and Mickey17], reproduced as equation (9) in Table 1], relates lesions per unit area to pathogen density using an infectivity-dilution function. Two coefficients (A and B) are quantified by least squares fitting. The form of this equation indicates lesion numbers should be proportional to pathogen density and equation (9) (Table 1) might be used to relate folliculitis lesion density to P. aeruginosa density where lesion and count data are obtained simultaneously. These algorithms could also apply to Trichobilharzia (‘swimmer's itch’) [Reference Schets34].
Single-hit theory caveats
Consideration of folliculitis and AOE aetiology and ecology suggested qualifications should be attached to empirical applications of ‘single-hit’ theory. Equations (6) and (7) in Table 1 estimate systemic infection probability and seem applicable to AOE as this disease involves P. aeruginosa multiplication potentially from a single locus. Folliculitis papules, however, generally appear localized, although systemic disease is possible [Reference Jacobson4]. Another limitation of Furumoto & Mickey's [Reference Furumoto and Mickey17] algorithms is that none incorporate exposure time, probably because plant defence mechanisms were reactivated (<2 min) following inhibition by leaf abrasion, and lesion density rapidly plateaued. This contrasts with pool infections where exposure time seems important and raises a tangential question of how exposure time influences gut infections? Spagnuolo et al. [Reference Spagnuolo, DiRita and Kirschner38] considered this issue across multi-second scales, although not the hour scales over which exposure occurs during digestion. An interim solution may be to use Furumoto & Mickey's implied assumption that exposure time variance is relatively small compared to other factors and might be discounted. Discounting is supported by the plateauing of lesion density observed with plant pathogenic pseudomonads (fig. 5 in [Reference Hattermann and Ries35], fig. 2 in [Reference Panopoulos and Schroth47]). A different uncertainty is how far P. aeruginosa should be viewed as an exogenous pathogen, perhaps more adapted for another eukaryotic host (cf. [Reference Richards83]) or an endogenous skin inhabitant out of control [Reference Hojyo-Tomoka, Marples and Kligman27, Reference Cogen, Nizet and Gallo33].
‘Third-generation’ mechanistic modelling of folliculitis
The information reviewed suggested that folliculitis lesion density might be estimable by combining: (i) ‘void’ crossing; (ii) surface adsorption/interaction; and (iii) follicle invasion likelihoods. Sufficient theory appears available to start framing follicle invasion and surface adherence in terms of attachment rates while dispersion equations appear to describe the ‘void’ crossing. In this conceptual model, folliculitis lesion density should be estimable as the product of dispersion, the probability of P. aeruginosa interacting with the skin boundary layer, and the probability of a follicle being invaded.
We propose equation (10) (Table 1) as the first step in mechanistic model development, recognizing that other migration and attachment formulations exist [Reference Mueller43, Reference Fletcher56, Reference Mueller57] in addition to that of Valentine & Allison [Reference Valentine and Allison40]. Equation (10) describes the number of lesions per unit area of skin N l as the product of (i) the number of particles absorbed under ideal Brownian diffusion assuming 100% adherence N D (using equation (9) in [Reference Valentine and Allison40]); (ii) the Sherwood number Sh 0·5; (iii) the per cent surface adherence + migration efficiency S im%; and (iv) the infection efficiency I %. N D is derived from the Stokes–Einstein–Brownian diffusion theory and assumes <40% removal of suspended particles [Reference Valentine and Allison40]. Sh 0·5, the increase in transport over diffusion [Reference Murray and Jackson42] is square-root-transformed because the diffusion coefficient is similarly transformed in calculating N D [Reference Valentine and Allison40]. S im% and I % could be estimated experimentally using existing procedures, e.g. [Reference Hattermann and Ries35, Reference Panopoulos and Schroth47].
Third-generation models have significance beyond folliculitis. ‘Second-generation’ ‘single-hit’ dose-response algorithms have proved a major advance over ‘first-generation’ MIDs [Reference Magnússon9]. However, they are still grounded in empirical curve fitting (e.g. [Reference Teunis, Ogden and Strachan16]), and do not provide full insight into infection processes. Further, model validation and coefficient estimation depends on rare, seldom comprehensive, outbreak data and some human and animal feeding/dosing studies [Reference Tamrakar and Haas19]. By comparison ‘third-generation’ mechanistic models could address these constraints and provide predictions and checks for comparison with second-generation algorithms, disease aetiology and epidemiology. The only other prototype mechanistic models we are aware of are the schemes of Rose & Haas [Reference Rose and Haas21] and Spagnuolo et al. [Reference Spagnuolo, DiRita and Kirschner38].
Exogenously induced AOE
AOE is reportedly associated with ear infection when P. aeruginosa occurs at densities of 10–100 c.f.u./100 ml [Reference Hajjartabar23, Reference Van24] whereas folliculitis appears associated with >105 c.f.u./100 ml [Reference Price and Ahearn8]. Despite this, folliculitis outbreaks can occur with little or no concurrent AOE, e.g. [Reference Gustafson30]. We suggest this indicates that the role of exogenous vs. endogenous swimming pool P. aeruginosa in AOE needs attention.
Resolving this puzzle will likely require a mechanistic analysis of dose response leading to understanding of how infection, skin saturation and ear canal microbial ecology interact [Reference Hojyo-Tomoka, Marples and Kligman27–Reference Leyden, Stewart and Kligman29, Reference Frank84]. Improved understanding is also needed of interactions between hydration, bacterial strains, mechanical disturbance (cotton tips, fingernails, hair clips) and cerumen (ear wax), the mixture of ceruminous and pilosebaceous glands secretions, epithelium squames, dust and other foreign debris [Reference Hanger and Mulley85]. Cerumen had been thought to protect against infection, but disinfection capacity is now disputed [Reference Campos86] and wet, impacted cerumen is associated with ear disease [Reference Hanger and Mulley85]. Further, cerumen function could be modified by ear cleaning, excessive water exposure, acid neutralization by swimming pool alkalinity. AOE likelihood could be increased by ear canal epithelium disruptions, e.g. maceration, high humidity, psoriasis, eczema, hearing aids, exostoses, and genetic predisposition associated with blood type A [Reference Steuer87].
A FRAMEWORK FOR EXOGENOUS FOLLICULITIS AND AOE
Pool management and dose-response algorithms
Our review raises many questions pertaining to pool management such as: whether the epidemiology, infection ecology and biophysics pictures are consistent; are current water quality guides satisfactory, overconservative or too lax; and can dose-response models account for the folliculitis vs. AOE contrast? Exposure duration appears central to infection severity and likelihood. Diffusion/dispersion theory promises estimates of the minimum water quality posing folliculitis and exogenous AOE risk. Conversely the aetiologies of folliculitis and AOE are not well grounded, and P. aeruginosa management is currently based on empirical hygiene principles rather than quantitative satisfaction of Koch's postulates.
Presently these pool management questions and propositions should be viewed as provisional. To resolve them and confirm or disconfirm major pool infection causes, we consider the next step should be infection modelling and experiments. Such work could also provide a reality check on this review's conclusions and the proposed models. As an aid we now propose a conceptual framework for exploring folliculitis and AOE.
Conceptual model
Steps (i)–(iii) of the KEDRF scheme (fig. 2 in [Reference Julien3]) propose targeted research of intimate processes leading to infection initiation. We reviewed the analogous ‘key events’ during P. aeruginosa bathing infection. Motile, non-motile and aggregated bacteria disperse through pool ‘voids’ (Fig. 1, process 1) driven by bulk water movement, swimming and agitation (Fig. 1, process 2). A few approach the epidermis (Fig. 1, processes 3a, 3b) where dispersion leads to reversible attachment (Fig. 1, processes 4a, 4b). Where shear forces are high (Re > ∼1000 ≈ 1 m/s) bacteria detach and return to the ‘void’ or bind irreversibly and potentially multiply (Fig. 1, processes 5a, 5b). Some cells disperse horizontally and infect follicles and abrasions (Fig. 1, processes 6a, 6b), or infect from the ‘void’. Infection is promoted by prolonged epidermal water retention, low shear (ear canals, occluded skin) and drying and possibly microbiome changes. Abrasion (scratching, clearing ear canals) (Fig. 1, process 1a) directly inoculates pathogens (Fig. 1, process 4b).
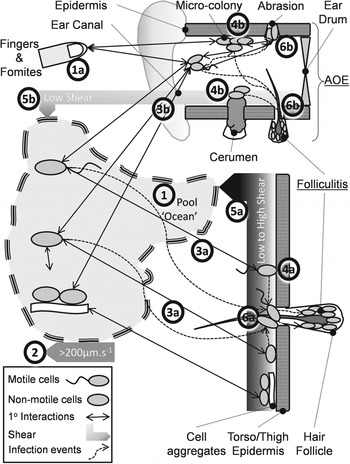
Fig. 1. Conceptual model of the pathways preceding folliculitis and acute otitis externa (AOE) by exogenous P. aeruginosa.
Next steps
Guidelines on managing P. aeruginosa in pools (p. 45 in [88]) suggest that cause-and-effect relationships are well understood. Our analysis suggests otherwise. To resolve matters we recommend the following research based on the framework in Figure 1 and themes in Figure 2:
-
(1) Develop and test mechanistic and empirical dose-response model options for opportunistic pathogens using constructed pool environments as study models.
-
(2) Basic research into: (i) bacterial biodiversity and distribution in the recreational water environment; (ii) interactions in skin and ear model systems covering motility, skin adherence and saturation, hydrodynamics, blooms, chemotaxis, biofilms and quorum sensing; (iii) (limited) human experiments [Reference Leyden, Stewart and Kligman29]; (iv) AOE aetiology and the risk posed by contaminated water.
-
(3) Pool and bathing management research covering: (i) pool and filter ecology; (ii) impacts of bather load and occurrence of individuals shedding P. aeruginosa into the pool environment.
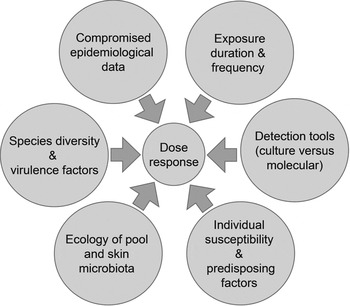
Fig. 2. Constraints associated with developing a dermal dose-response model for P. aeruginosa.
SUPPLEMENTARY MATERIAL
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0950268813002690.
DECLARATION OF INTEREST
None.






