Early detection of autism spectrum disorder (ASD) is a major objective of clinical developmental research, given ASD’s lifelong impact and the established association between early intervention and improved outcomes (Estes, Munson, et al., Reference Estes, Munson, Rogers, Greenson, Winter and Dawson2015; Fuller and Kaiser, Reference Fuller and Kaiser2020; Shi et al., Reference Shi, Wu, Dai, Zeng, Luo, Cai, Wan and Jing2021). Prospective infant sibling studies have shown that consolidation of core ASD symptoms of social communication impairment and restricted and repetitive behaviors and interests (RRBs) is often preceded by diverse signs of atypical development, such as motor and attention deficits, which are not unique to ASD (Elsabbagh & Johnson, Reference Elsabbagh and Johnson2016; Sacrey et al., Reference Sacrey, Bennett and Zwaigenbaum2015). Assessments indexing initial manifestations of core ASD symptom domains could clarify how diverse pre-diagnostic features converge on ASD, which is vital for a more complete clinical science of its ontogeny and characterization of early ASD risk.
Social motivation, defined as the disposition to orient to social stimuli, to seek, want, and like social contact, and to maintain social interactions (Chevallier et al., Reference Chevallier, Kohls, Troiani, Brodkin and Schultz2012), has been implicated in the development and presentation of core autistic features. Low social motivation is hypothesized to contribute to social communication deficits and may also reinforce non-social, object-oriented fixations associated with RRBs. Like ASD, social motivation is heritable (Marrus et al., Reference Marrus, Grant, Harris-Olenak, Albright, Bolster, Haber, Jacob, Zhang, Heath, Agrawal, Constantino, Elison and Glowinski2020; Sung et al., Reference Sung, Dawson, Munson, Estes, Schellenberg and Wijsman2005), and quantitative differences in social motivation track with familial ASD liability (Uljarević et al., Reference Uljarević, Frazier, Jo, Phillips, Billingham, Cooper and Hardan2021). Brain systems underpinning social motivation include components of social and reward neurocircuitry (Chevallier et al., Reference Chevallier, Kohls, Troiani, Brodkin and Schultz2012), both of which have shown differences in ASD (Clements et al., Reference Clements, Zoltowski, Yankowitz, Yerys, Schultz and Herrington2018; Sato & Uono, Reference Sato and Uono2019), including during early childhood (Abrams et al., Reference Abrams, Lynch, Cheng, Phillips, Supekar, Ryali, Uddin and Menon2013; Guo et al., Reference Guo, Duan, Suckling, Wang, Kang, Chen, Biswal, Cao, He, Xiao, Huang, Wang, Han, Fan, Guo, Zhao, Wu and Chen2021). Through its alignment with ASD’s defining characteristics, and hypothesized relationship to ASD outcomes and its underlying biology, social motivation represents a candidate quantitative marker for both ASD susceptibility and diagnosis. Currently, however, the field lacks dimensional measures of social motivation in infants, a prerequisite for characterizing its role in the emergence of ASD, with ramifications for advancing early risk assessment and targeted intervention.
In typical development, behavioral expressions of social motivation are observed from early infancy. For example, newborns direct attention to social stimuli such as human faces (Rosa Salva et al., Reference Rosa Salva, Farroni, Regolin, Vallortigara and Johnson2011) and biological versus scrambled motion (Simion et al., Reference Simion, Regolin and Bulf2008). Predictable social smiling with exposure to faces and interactions (Emde & Harmon, Reference Emde and Harmon1972), present by three months of age, as well as subsequent smiling with early communicative gestures (Messinger & Fogel, Reference Messinger and Fogel1998) both provide face-valid evidence for infants’ liking of social experiences. Within the first year, infants also spontaneously demonstrate the ability to initiate social contact with caregivers, novel adults, and other infants (Hay et al., Reference Hay, Nash and Pedersen1983; Ross & Goldman, Reference Ross and Goldman1977; Schaffer & Emerson, Reference Schaffer and Emerson1964). Typical behaviors during this period, such as interactive play (Markova, Reference Markova2018), reciprocal babbling (Albert et al., Reference Albert, Schwade and Goldstein2018), and bids to re-engage an interactive partner (Tremblay-Leveau & Nadel, Reference Tremblay-Leveau and Nadel1996) foster ongoing interaction and relationships. By affording a repertoire of social experiences, social motivation is hypothesized to stimulate social learning, while disrupted social motivation is hypothesized to constrain development of social communication, culminating in ASD (Chevallier et al., Reference Chevallier, Kohls, Troiani, Brodkin and Schultz2012).
Consistent with this hypothesis, literature on pre-diagnostic signs of ASD provides evidence of reduced social motivation before core social communication symptoms. Retrospective approaches using blinded coding of home videos found that within the first year, infants later diagnosed with ASD showed lower social initiative, looking at others, and response to interpersonal overtures compared to typically developing infants and infants with global delays (Palomo et al., Reference Palomo, Belinchón and Ozonoff2006; Saint-Georges et al., Reference Saint-Georges, Cassel, Cohen, Chetouani, Laznik, Maestro and Muratori2010). In prospective studies, decreased social function among children with later ASD has been most consistently observed after the first year (Sacrey et al., Reference Sacrey, Bennett and Zwaigenbaum2015), although reduction in specific behaviors reflecting aspects of social motivation, such as social smiling (Ozonoff et al., Reference Ozonoff, Iosif, Baguio, Cook, Hill, Hutman, Rogers, Rozga, Sangha, Sigman, Steinfeld and Young2010), response to name (Miller et al., Reference Miller, Iosif, Hill, Young, Schwichtenberg and Ozonoff2017), back-and-forth vocalizations (Sacrey et al., Reference Sacrey, Zwaigenbaum, Bryson, Brian, Smith, Roberts, Szatmari, Vaillancourt, Roncadin and Garon2021), and fixation on social versus non-social stimuli (Jones & Klin, Reference Jones and Klin2013) have been reported earlier. The diversity in quality and context of social motivation-related behaviors suggests that a broad construct encompassing naturalistic social orienting, seeking, liking, wanting, and maintaining could capture sufficient dimensional variation to quantify social motivation as a potential indicator of ASD susceptibility in infancy.
Although comprehensive, quantitative infant assessments of social motivation have not yet been developed, recent parent-report instruments (Marrus et al., Reference Marrus, Grant, Harris-Olenak, Albright, Bolster, Haber, Jacob, Zhang, Heath, Agrawal, Constantino, Elison and Glowinski2020; Phillips et al., Reference Phillips, Uljarević, Schuck, Schapp, Solomon, Salzman, Allerhand, Libove, Frazier and Hardan2019), and eye-tracking measures (Vernetti et al., Reference Vernetti, Senju, Charman, Johnson and Gliga2018) support the ability to measure social motivation in toddlerhood. Parent-report instruments assessing clinically informative behaviors across day-to-day contexts have shown lower social motivation scores in children with ASD (Phillips et al., Reference Phillips, Uljarević, Schuck, Schapp, Solomon, Salzman, Allerhand, Libove, Frazier and Hardan2019) and associations between social motivation and quantitative ASD traits (Marrus et al., Reference Marrus, Grant, Harris-Olenak, Albright, Bolster, Haber, Jacob, Zhang, Heath, Agrawal, Constantino, Elison and Glowinski2020). These findings suggest that a parent-report measure for infants may provide a translatable approach to index individual differences in social motivation and enhance ASD screening and characterization of ASD’s emergence during development.
To determine whether measurable differences in infant social motivation precede ASD diagnosis, as well as differentiate toddlers with and without ASD, we leveraged existing parent-report data from the Infant Brain Imaging Study (IBIS) (Estes et al., 2015). This longitudinal, multisite study of infant siblings at high and low familial risk for ASD afforded a continuum of typical and atypical social development, while enriching for social deficits. The prospective family study design allowed investigation of relationships between infant social motivation and later ASD, as well as between social motivation and inherited ASD liability stratified according to familial risk in conjunction with presence or absence of an ASD diagnosis. As an initial test of a clinically feasible approach to index social motivation in infancy, we reviewed items on a series of parent-report measures querying aspects of social behavior consistent with displays of social motivation, including measures of temperament, adaptive function, social development, and communication in infants at ages 6, 12, and 24 months. We incorporated those items with strong face validity for social motivation into a composite social motivation index (SMI) for each study time point. Following examination of the SMI’s psychometric properties, we tested the hypothesis that lower social motivation could distinguish children with ASD both prior to and at the time of diagnosis. We additionally tested the hypothesis that lower social motivation scores would be observed with greater levels of familial ASD liability.
Method
Participants
The IBIS is a prospective, multisite study of infant siblings at high and low familial risk of ASD, with assessments at 6, 12, and 24 months (Estes et al., 2015). High-risk (HR) infants have an older biological full-sibling with ASD, whereas comparison low-risk (LR) infant siblings have no first-degree relatives with ASD or intellectual disability. For these analyses, 597 participants had appropriate data for generating a score on the SMI (n 6 = 374, n 12 = 318, n 24 = 396). The LORIS data management platform (Das et al., Reference Das, Glatard, MacIntyre, Madjar, Rogers, Rousseau, Rioux, MacFarlane, Mohades, Gnanasekaran, Makowski, Kostopoulos, Adalat, Khalili-Mahani, Niso, Moreau and Evans2016) served as the behavioral, clinical, and imaging hub for data collection, curation, preparation for analysis, and archiving. See supporting information for exclusion criteria.
At 24 months, an experienced psychologist or psychiatrist reviewed behavioral testing, including the Autism Diagnostic Observation Schedule (ADOS, Lord et al., Reference Lord, Rutter, DiLavore and Risi2000), to determine a clinical best estimate diagnosis of either autistic disorder or pervasive developmental disorder not otherwise specified using the DSM-IV-TR checklist (American Psychiatric Association, 2000). All families provided informed consent in accordance with each site’s Institutional Review Board.
Derivation of a SMI
Data from the IBIS study battery, collected prospectively when infants were ages 6, 12, and 24 months, were reviewed for items querying social behavior in parent-report measures, as these provide clinically relevant and scalable assessment of individual differences in infancy, with ratings representing real-world behavior across time and contexts. Items were selected from validated developmental instruments, specifically, the Vineland Adaptive Behavior Scales, an adaptive function measure (Sparrow et al., Reference Sparrow, Balla and Cicchetti2005); the Infant Behavior Questionnaire-Revised, a temperament measure (Gartstein & Rothbart, Reference Gartstein and Rothbart2003); the Macarthur-Bates Communicative Development Inventory, a language measure (Fenson et al., Reference Fenson, Marchman, Thal, Dale, Reznick and Bates2006); and the First Year Inventory, a screener for ASD risk (Watson et al., Reference Watson, Baranek, Crais, Reznick, Dykstra and Perryman2007). See supporting information for details on study instruments.
Candidate social motivation items were selected (NM) based on face validity for Chevallier et al.’s definition of social motivation (Reference Chevallier, Kohls, Troiani, Brodkin and Schultz2012) as the disposition to orient to social stimuli, to seek social contact, to want and like social experiences, and to work to maintain social interactions. Items were chosen to index social motivation in aggregate across its key elements as manifested via a series of common, observable infant behaviors that included directing attention to others (orienting), initiating interaction (seeking), positive affect in social contexts (liking or wanting), or responses likely to sustain engagement (maintaining). Social motivation items were distinguished from items probing emotion recognition, instrumental requests, or physical play. To maximize variation in the representation of social motivation during early development, unique items from available study measures at each time point were included in separate 6-, 12-, and 24-month social motivation indices. Five items were reverse-coded so that for all items, higher scores indicated greater social motivation, and items and were weighted equally by re-scaling scores to range from 0 to 1 (Cohen et al., Reference Cohen, Cohen, Aiken and West1999). Duplicate item content was not present across instruments with the SMI for a given age.
In a preliminary review of candidate items, Cronbach’s alpha, a well-established measure of internal reliability which quantifies the inter-relatedness across items (Cronbach, Reference Cronbach1951), was calculated separately for each full series of SMI items identified at the 6, 12, and 24-month time points and again for cases when each item was singly removed. Items whose removal resulted in a higher alpha, indicating improved inter-relatedness among remaining items, were eliminated. As an additional validity check, a consensus of authors (JNC, KB, and LM) with expertise in ASD and/or infant development confirmed that remaining items showed good face validity for Chevallier and colleagues’ definition of social motivation.
Next, finalized items (Table 1) were summed into separate 6-, 12-, and 24-month composites. To facilitate cross-age comparisons, these scores were standardized to T-scores with a mean = 50 and standard deviation = 10. T-scores were calculated as equal to the Z-score * 10 + 50, where the Z-score = (mean score-raw score)/(standard deviation). Analyzed participants (n 6 = 374, n 12 = 318, n 24 = 396) had complete item-level scores, excepting 38 whose 12-month scores were normalized to account for “not applicable” ratings on two items regarding social behavior while being held during feeding. Scores for one participant with notably low ratings across age were winsorized to T-score = 5. All analyses involved the same finalized 6-, 12-, and 24-month SMI item sets.
Table 1. Social motivation index item content

Note. Correspondence of concepts in social motivation items to Chevallier et al.’s definition (2012).
Statistical Analyses
Participant characteristics
Student’s t-tests and chi-square tests evaluated group differences in continuous and categorical data, respectively. Effect sizes for continuous measures were Cohen’s d, where d ≥ 0.8 is large, d ≥ 0.5 and < 0.8 is medium, and d ≤ 0.2 is small. Effect sizes for comparisons of proportions were odds ratios or Cramer’s V, where V ≥ 0.5 is large, 0.2 < V < 0.5 is medium, and V ≤ 0.2 is small (Cohen, Reference Cohen1988).
Measurement properties of the SMI
Cronbach’s alpha was calculated to index the inter-relatedness of SMI item sets at each age and across age. Values of alpha > 0.7 are considered acceptable, >0.8 are considered good, and >0.9 are considered excellent (Nunnally, Reference Nunnally1978). We applied exploratory factor analysis to test the hypothesis that items across 6, 12, and 24 months loaded onto a single factor, using principal axis factoring and an oblimin rotation in SPSS. To allow for a sufficient ratio of participant number to items, items were pooled into 10 parcels, an established approach in measurement modeling (Stucky et al., Reference Stucky, Gottfredson, Panter, Cooper, Camic, Long, Panter, Rindskopf and Sher2012), consisting of composites of items with balanced distribution by instrument type and age of administration. We followed the EFA with a confirmatory factor analysis (CFA) to determine fit indices for a unitary factor structure (see supplement for details).
Spearman’s correlations indexed rank-order cross-age stability and Pearson’s correlations were computed for convergent and divergent validity. Zou’s confidence interval method for the difference in correlations (Zou, Reference Zou2007), which accounts for dependency between correlations, was used to compare correlations for variables involving primary tests of convergent and divergent validity.
To evaluate convergent validity, we utilized measures assessing social communicative performance, since social communication is theorized to be influenced by social motivation (Chevallier et al., Reference Chevallier, Kohls, Troiani, Brodkin and Schultz2012; Su et al., Reference Su, Rogers, Estes and Yoder2021) and since separate measures of infant social motivation were not present in IBIS. We anticipated correlations consistent with partially overlapping relationships of the SMI and these metrics. The Autism Observation Scale for Infants (AOSI), a direct assessment of ASD features (Zwaigenbaum et al., Reference Zwaigenbaum, Bryson, Brian, Smith, Sacrey, Armstrong, Roberts, Szatmari, Garon, Vaillancourt and Roncadin2021, see supplement), or the ADOS were used as primary metrics to evaluate convergent validity for the SMI given the theorized relationship between decreased social motivation and increased signs of ASD. Secondary analyses of convergent validity included the Mullen Receptive (RL) and Expressive Language (EL) scales, which encompass aspects of communication.
For testing of divergent validity, we predicted lower correlations than those observed for testing of convergent validity. For a primary analysis of divergent validity, we selected the Mullen Fine Motor (FM) scale, as this measure entails a distinct domain of function that may be demonstrated independently from social interaction. In secondary analyses of divergent validity, we also obtained correlations for the Mullen Gross Motor (GM) and Visual Reception (VR) scales, two functions also not contingent on social interaction. These two latter behaviors were included since they have demonstrated associations with subsequent ASD outcome in infancy (Estes et al, 2015), and their relationships to ASD outcome were compared with those for social motivation as part of our analyses.
Relationship of social motivation to ASD diagnosis
To evaluate relationships between social motivation and ASD, binary logistic regressions were first performed for base models with diagnosis as the dependent variable and social motivation T-score as the independent variable. Full models included covariates of sex and cognition based on the Early Learning Composite (ELC) from the Mullen Scales of Early Learning (Mullen, Reference Mullen1995). The Hosmer-Lemeshow test indicated adequate fit (p > .05) for all models. Variance estimates were Nagelkerke’s pseudo-R2.
Parallel base models with independent variables of Mullen GM function and VR were also run to informally compare the relative effects observed across for these domains versus social motivation. These behaviors were selected for comparison because they have previously been identified as infant behavioral markers of ASD risk by age 6 months (Estes et al., 2015). A third series of models also evaluated whether additive contributions for ASD outcome were present for social motivation, GM, VR, and pre-diagnostic ASD-related behaviors measured by the AOSI.
Development of social motivation relative to familial ASD liability
Hierarchical linear modeling (HLM), an advanced regression technique accounting for shared variance in nested data, was applied to investigate the longitudinal relationships between familial liability for ASD and social motivation, controlling for sex. For the familial liability variable, children were classified into three risk-diagnostic groups denoting increasing levels of familial background for ASD (Marrus et al., Reference Marrus, Hall, Paterson, Elison, Wolff, Swanson, Parish-Morris, Eggebrecht, Pruett, Hazlett, Zwaigenbaum, Dager, Estes, Schultz, Botteron, Piven and Constantino2018): LR− (low-risk children without ASD), HR− (high-risk children without ASD), and HR+ (high-risk children with ASD). SMI scores were hypothesized to be lowest for the HR+ group, given that decreased social motivation is observed in ASD. Scores for the HR− group were hypothesized to fall between the HR+ and LR− groups given prior evidence supporting the heritable nature of ASD symptoms and social motivation (Marrus et al., Reference Marrus, Grant, Harris-Olenak, Albright, Bolster, Haber, Jacob, Zhang, Heath, Agrawal, Constantino, Elison and Glowinski2020). Categorical variables were dummy coded, with sex = male and familial liability = HR− siblings as reference groups. Age was centered at 6 months to test for early-arising group differences in social motivation. Longitudinal observations (6, 12, and 24 months) were nested within individuals, allowing intercepts to be modeled separately for each individual (i.e., as random effects). Interaction terms for risk-diagnostic variables with age evaluated behavioral course as a function of familial liability. Conditional R2 described variance accounted for by effects within and between time points.
Relative decline in social motivation in HR children with versus without ASD
Chi-square testing evaluated whether the presence or absence of a strong individual-level decline in social motivation distinguished HR children with versus without ASD. Here, findings were parameterized in terms of a categorically strong decline in social motivation, rather than continuous scores. This was due to our hypothesis that a large decrease in social motivation, but not necessarily a range of less substantial declines, particularly for above-average SMI scores, would be more consistently associated with a clinically significant reduction in social function and increased likelihood of ASD diagnosis. Strong decline was defined as a 15 point drop in SMI T-score, since this increment of 1.5 standard deviations is commensurate with developmental delay (Marrus et al., Reference Marrus, Hall, Paterson, Elison, Wolff, Swanson, Parish-Morris, Eggebrecht, Pruett, Hazlett, Zwaigenbaum, Dager, Estes, Schultz, Botteron, Piven and Constantino2018) and thus a difference constituting clinical impact. Differences in SMI T-scores were calculated between the first year of life and 24 months, using the higher available SMI T-score at 6 or 12 months.
In addition to primary analyses using subjects with available SMI scores, models testing relationships between social motivation and ASD diagnosis were run following multiple imputation (Enders, Reference Enders2017; Rubin, Reference Rubin1996). This procedure replaced missing SMI scores with predicted values based on available data, as missing data may bias reported relationships (see supplemental methods for details).
Results
Participant characteristics
SMI T-scores were generated for low-risk (LR) and HR participants at 6-, 12-, and 24-month visits (Table 2). Across visits, participants did not significantly differ in proportions by sex, ASD diagnosis, race, or ethnicity, or in mean general cognition on the ELC (see supplement for statistics). At the 12-month time point, a higher proportion of HR versus LR infants was observed in comparison to proportions at 6 and 24 months (Table 2, χ2(2) = 10.64, p = .005; V = 0.01).
Table 2. Participant characteristics
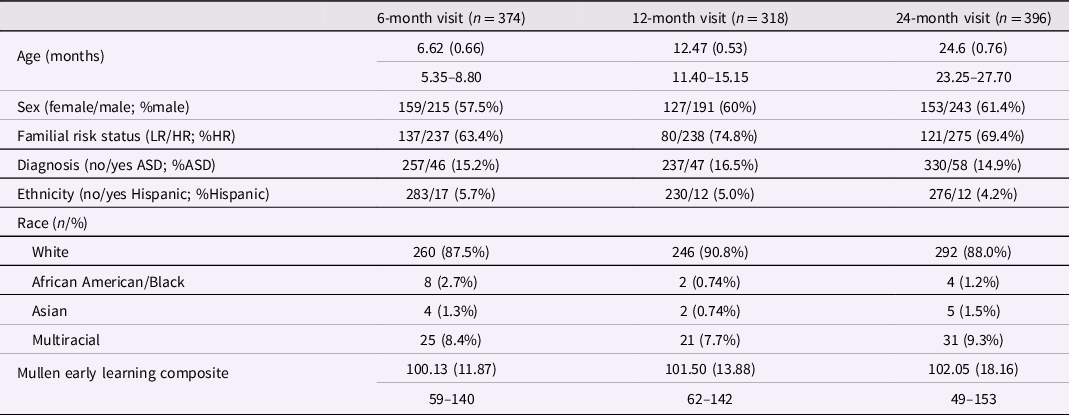
Note. Participants include infants at high (HR) and low familial risk (LR) of autism spectrum disorder (ASD). Three LR children met criteria for ASD. Ranges presented for continuous variables.
Measurement properties of the SMI
At all ages, SMI T-scores displayed a continuous, unimodal distribution with slight negative skew (skew6 = -0.82, standard error (SE) = 0.13; skew12 = −1.63, SE = 0.14; skew24 = −0.41, SE = 0.12) (Figure 1a–c). Items showed good internal reliability at each age (α6 = .75, α12 = .89, α24 = .81) and when pooled across age (α6-12-24 = .87). An exploratory factor analysis using item parcels comprised of items from all three ages was consistent with a single factor accounting for 42% of the variance based on the inflection point of the scree plot and an eigenvalue >1. Item parcels exhibited moderate to high loadings onto this factor, where loadings ranged between 0.55 and 0.79 (see supplement for details). Fit indices obtained by CFA of a unitary social motivation factor were consistent with acceptable to good fit: CFI = 0.95, TFI = 0.93, RMSEA = 0.08, and SRMR = 0.43.

Figure 1. Social Motivation Score Distributions at 6, 12, and 24 Months Social Motivation Index T-score distributions (A-C) among all low- and high-risk participants. Bars (D-F) categorize participants by diagnosis. Gray and black arrows indicate group means for children without and with ASD.
Composite SMI scores, in turn, showed good temporal stability across age: ρ = 0.58 (p < .001, 95% confidence interval (CI) [0.47, 0.67], n = 194) from 6–12 months, ρ = 0.40 (p < .001, 95% CI [0.27, 0.51], n = 214) from 12–24 months, and ρ = 0.22 (p = .001, 95% CI [0.09, 0.35], n = 210) from 6 to 24 months. These cross-age correlations were of comparable or greater magnitude to those for the Mullen ELC, a standardized measure of infant cognitive development: ρ = 0.27 (p < .001, 95% CI [0.18, 0.36]) from 6 to 12 months, ρ = 0.43 (p < .001, 95% CI [0.35, 0.50]) from 12–24 months, and ρ = 0.17 (p < .001, 95% CI [0.08, 0.27]) from 6 to 24 months. At 24 months, females scored higher than males, including when analyzing children without ASD to account for male predominance of cases (t(327) = 2.62, p = .009; d = 0.29).
In a primary test of convergent validity, SMI scores were negatively correlated (in the expected direction) with concurrent total scores on the AOSI: r AOSI-6 = −.33 (p < .001, n = 361) and r AOSI-12 = −0.38 (p < .001, n = 306), and 24-month ADOS calibrated severity score: r ADOS-24 = −0.47 (p < .001, n = 373). We secondarily evaluated convergent relationships between the SMI and Mullen Expressive (EL) and Receptive (RL) Language scales. The SMI showed mostly moderate magnitude correlations with these measures (r EL-6 = .26, r EL-12 = .31, and r EL-24 = .30; r RL-6 = .36, r RL-12 = .24, and r RL-24 = .39, all p’s < .001). Correlations across the AOSI and language measures were consistent with partially overlapping variance with the SMI, with shared variance (R2) up to 14.4% from 6 to 12 months and 22.1% at 24 months.
Conversely, a primary test of divergent validity with the Mullen FM scale showed weaker associations between concurrent SMI and FM scores than SMI and AOSI/ADOS scores at all ages (r FM-6 = .18, r FM-12 = .17, and r FM-24 = .22, p’s < .01, n > 316, see supplement for tests of significant differences from AOSI/ADOS correlations). In secondary tests of divergent validity, similarly low correlations were observed for Mullen GM scale [r GM-6 = .21 (p < .001), r GM-12 = .11 (p = .052) and r GM-24 = .22 (p < .001)] and VR scale [r VR-6 = .18, r VR-12 = .16, and r VR-24 = .25 (p’s < .01)].
Relationship of social motivation to ASD diagnosis
Given the hypothesis that early deficits in social motivation contribute to later ASD, we examined in LR and HR infants whether children diagnosed with ASD at 24 months exhibited lower SMI scores at 6 and 12 months. At both ages, later-diagnosed infants displayed lower scores [means and standard deviations: SMI6-noASD: 50.63 (9.54) versus SMI6-ASD: 44.01 (11.27); SMI12-noASD: 50.95 (8.74) versus SMI12-ASD: 44.24 (11.25)]. These differences represented moderate effect sizes (d 6 = 0.67; d 12 = 0.73, p’s < .001; Table S1) comparable to differences observed for the AOSI (d 6 = 0.37; d 12 = 0.57, p’s < .05).
At 24 months, lower SMI scores in children with versus without ASD [SMI24-ASD: 38.18 (10.34) versus SMI24-noASD: 51.87 (8.39)] constituted a large effect size (d = 1.57, p < .001), with a larger downward shift and greater separation in SMI score distributions for children with versus without ASD (Figure 1d–f). Effect sizes (Table S1) and distributions (Fig S1) did not substantively change after removing children with significant cognitive delay, i.e., ELC < 70 (Marrus et al., Reference Marrus, Hall, Paterson, Elison, Wolff, Swanson, Parish-Morris, Eggebrecht, Pruett, Hazlett, Zwaigenbaum, Dager, Estes, Schultz, Botteron, Piven and Constantino2018), from analysis.
Using binary logistic regression, we next evaluated the magnitude of the relationship between social motivation at each age and ASD diagnosis in LR and HR infants (Table 3). At all ages, base models without covariates were significant. Six- and 12-month SMI scores respectively explained 8.8% and 9.9% of the variance in ASD diagnosis. Concurrent 24-month SMI scores explained 39% of the variance in ASD diagnosis.
Table 3. Relationship of social motivation index scores to ASD

Note. Binary logistic regression models test the relationship of social motivation (SM) to autism spectrum disorder (ASD; No = 0, Yes = 1) in children at low and high familial risk. Base models examine sole contributions of SM. Full models first account for sex (Female = 0, Male = 1) and cognition (ELC = Early Learning Composite). Exponentiated β coefficients reported for SM, sex, and ELC index the relationship between independent variables and ASD outcome, which is calculated as the natural log of the odds for diagnosis (odds = probability of ASD/probability of no ASD). For sex, a categorical variable, βs indicate the male to female odds ratio of ASD; for continuous variables, β < 1 indicates that higher values correspond with lower odds of ASD. 95% confidence intervals in parentheses. *p < 0.05; **p < 0.01; ***p < 0.001.
In full models (Table 3), where SMI scores were entered after accounting for effects of sex and cognition (using the concurrent ELC score), SMI scores remained significant predictors of ASD outcome at all ages. At 6 months, sex, but not cognition, was significantly related to ASD diagnosis, while at 12 and 24 months, sex, cognition, and social motivation contributed additively to ASD outcome. Similar findings were observed when confining analyses to HR infants (Table S2) and when accounting for missing data using multiple imputation (Table S3).
In the same sample, findings for social motivation were qualitatively compared to analogous base models for GM function and VR (Table S4). These behaviors offered an informal standard of comparison given that, in IBIS, both showed lower 6-month scores in HR+ (HR with ASD) versus LR- (low-risk without ASD) groups (Estes et al., 2015) while showing low correlations with the SMI, as detailed above. At all ages, GM and VR T-scores explained numerically less variance in ASD outcome than social motivation (≤4% at 6 and 12 months, ≤23% at 24 months).
A joint model including social motivation, GM, and VR, and ASD-related behaviors on the AOSI or ADOS, revealed that at age 6 months, only social motivation significantly predicted ASD outcome. Small additive contributions to ASD outcome were present for the SMI and AOSI at 12 months and additive contributions of all behaviors occurred at 24 months (Table S5).
Development of social motivation relative to familial ASD liability
Using HLM, we next tested whether social motivation across ages 6, 12, and 24 months varied as a function of familial ASD liability stratified by risk-diagnostic groups (i.e., LR-, HR-, and HR+). HR- siblings served as the reference group for comparison of LR− to HR− (low versus medium liability) and HR+ to HR− (high versus medium liability). A base model covaried for age and sex, followed by a model including liability group, and a full model including interaction terms of age with liability group (Table S6).
The full model exhibited the lowest Akaike information criterion, supporting the best fit, and the highest conditional R2 of 47% (Table S6). Main effects of familial liability – indicative of risk-diagnostic group differences at 6 months (i.e., center of age) – were observed. Specifically, 6-month SMI T-scores were significantly greater for LR− versus HR− children (B = 2.79, p < .01; d = 0.18) and for HR− versus HR+ children (B = −4.19, p < .01; d = −0.21), consistent with an association of decreasing social motivation with increasing familial ASD liability (Figure 2a). In contrast, analogous models for GM and VR (Figure 2b, c; Table S8) showed lower 6-month T-scores in HR- versus LR- groups only (GM: B = 3.01, p < .05, d = 0.16; VR: B = 2.96, p < .01, d = 0.17).

Figure 2. Social Motivation, Gross Motor Function, and Visual Reception Across Age Trajectories of social motivation (A), gross motor function (B), and visual reception (C) are shown for risk-diagnostic groups (LR−, HR−, HR+) based on hierarchical linear models incorporating sex, age (centered at 6 months), ASD familial liability, and interaction of liability with age. In order of increasing familial liability: LR− = low-risk negative, HR− = high-risk negative, HR+ = high-risk positive.
Familial liability by age interactions for social motivation were significant for the comparison of HR− and HR + groups (B = −0.50, p < .001, d = −0.38), reflecting declining SMI T-scores between 6 and 24 months in the HR+ group. This finding was also observed for GM and VR (Figure 2a–c, Table S6). Additional models testing interactions with sex failed to show significant interactions and resulted in worse model fit. Comparable findings were observed for datasets generated following multiple imputation (Table S7).
Relative decline in social motivation in HR children with versus without ASD
Given the finding of group-level decline in SMI T-scores for HR+ children, we conducted an exploratory analysis to evaluate the extent to which individual-level relative decline differentiated HR children with versus without ASD (n HR+ = 42; n HR− = 151). HR children were classified by presence or absence of a strong decline in SMI T-scores (≥15 points) between the first year and 24 months (Fig S2). Strong decline occurred in 31% HR+ versus 4% HR-negative children (χ2(1) = 26.95, p < .001), corresponding to an odds ratio = 10.83, 95% CI [3.81, 30.84] for ASD in HR children. A similar odds ratio was observed in analyses using multiple imputation (odds ratio = 9.85, 95% CI [3.38, 28.67]).
Among HR+ children, those with versus without a strong decline displayed lower 24-month SMI T-scores (SMIdecline = 30.67 ± 7.32; SMIother = 41.82 ± 10.96; t(40) = 3.34, p < .01, d = 1.11) but no significant difference in 24-month ADOS or ELC scores (Table S6). The declining group’s low 24-month SMI scores contrasted with unremarkable mean SMI T-scores (53.70 ± 6.31; sample mean = 50) observed in the first year of life.
Discussion
Here we demonstrate that individual differences in social motivation are quantifiable during infancy by parental report, an accessible assessment modality for clinical practice. The SMI showed temporal stability during a rapid developmental stage and as hypothesized, differentiated children with versus without ASD at 6, 12, and 24 months, an effect not attributable to cognition. Consistent with prior reports of heritability of social motivation, increasing levels of familial ASD liability (i.e., LR− < HR− < HR+) corresponded to decreasing levels of social motivation at 6 months, prior to ASD diagnosis. At the group level, SMI T-scores declined from infancy through toddlerhood in HR+ infants, whereas at the individual level, strong decline occurred in a subgroup of these children, illustrating heterogeneous developmental pathways in ASD. These findings collectively support a role for social motivation as a biobehavioral marker of ASD susceptibility and diagnosis, for which both lower levels in infancy and strong decline in toddlerhood are associated with development of ASD.
Social motivation is quantifiable during infancy
The SMI displayed a continuous, unimodal distribution across children with and without ASD, as well as good internal consistency and cross-age stability. Items in the SMI, when pooled across age, showed moderate to high loadings on a single factor, consistent with representation of interrelated aspects of social motivation from infancy through toddlerhood. Moderate correlations of the SMI with the AOSI and ADOS, assessments of ASD-related symptoms, as well as communication skills on MSEL language measures, were consistent with convergent validity, while the finding of <25% overlapping variance between these metrics and the SMI supported its ability to measure a unique social construct. Lower correlations with metrics of distinct motor and VR abilities, in turn, demonstrated divergent validity. These features support evidence for the SMI’s quantification of a dimensional, trait-like construct.
In terms of participant characteristics associated with differences in social motivation, higher 24-month SMI scores in females versus males and lower scores in ASD-affected toddlers corroborated findings at older ages (Phillips et al., Reference Phillips, Uljarević, Schuck, Schapp, Solomon, Salzman, Allerhand, Libove, Frazier and Hardan2019) and supported downward extension in the measurement of this construct. Pre-diagnostic and concurrent associations of lower SMI scores with ASD persisted when accounting for cognition, confirming specificity of these relationships to social motivation. In models evaluating joint behavioral contributions of social motivation with GM, VR, and ASD-related features on the AOSI, social motivation showed the earliest relationship to ASD outcome at 6 months, whereas additive contributions of other behaviors and social motivation were found at 12 and 24 months. These findings suggest that the SMI, in quantifying a specific behavioral dimension hypothesized to underlie development of ASD, offers value-added for tracking the emergence of ASD, while contrasting with prior literature emphasizing that ASD-related alterations in social behavior arise during the second year (Sacrey et al., Reference Sacrey, Bennett and Zwaigenbaum2015). From the standpoint of clinical practice, a parent-report SMI, in comparison to semi-structured assessments such as the AOSI, would offer the advantage of obviating the need for trained assessors and additional scheduling, which could expedite evaluation for ASD and referral for intervention.
Six-month social motivation corresponds with familial ASD liability
Lower 6-month SMI scores distinguished groups according to increasing familial ASD liability. First, when considering family history of ASD among children without a diagnosis, 6-month-olds with a family history had lower scores than 6-month-olds without a family history (HR− < LR−). Next, when considering ASD diagnosis among children with a family history, 6-month-olds with subsequent ASD had lower scores than children without ASD (HR+ < HR−). This stepwise relationship of LR− < HR− < HR+ is consistent with previously reported heritability of social motivation (Marrus et al., Reference Marrus, Grant, Harris-Olenak, Albright, Bolster, Haber, Jacob, Zhang, Heath, Agrawal, Constantino, Elison and Glowinski2020; Sung et al., Reference Sung, Dawson, Munson, Estes, Schellenberg and Wijsman2005) and the potential for developmentally-sensitive genetic factors that influence vulnerability and/or resiliency to ASD (Elison, Reference Elison2020).
In contrast to social motivation, 6-month GM and VR, which are not aspects of core ASD features, differentiated HR infants only by presence or absence of a family history of ASD and not by subsequent diagnosis. At all three ages, GM and VR also exhibited nominally lower associations with ASD outcome (Table S3). Collectively, these findings support the specificity of social motivation as an infant behavioral marker of ASD recurrence and familial genetic risk, underscoring the utility of tracking precursors of core features to elucidate origins of ASD.
Heterogeneity in emergence of social motivation deficits in ASD
HR infants with either lower SMI T-scores or a decline in SMI T-scores were more likely to develop ASD. The longitudinal decline from infancy through toddlerhood (Figure 2) paralleled widening cross-sectional ASD group differences in social motivation first observed at 6 months and demonstrated incremental development of core ASD symptoms. Individual courses, in turn, revealed variable social motivation profiles across age (Fig S2), with a subgroup of 31% of HR+ infants exhibiting moderate SMI T-scores followed by a strong decline, reminiscent of reports of regression in ASD. This decline was associated with 10-fold higher odds of ASD among HR children, a notable elevation for a population with 20% baseline recurrence (Ozonoff et al., Reference Ozonoff, Young, Carter, Messinger, Yirmiya, Zwaigenbaum, Bryson, Carver, Constantino, Dobkins, Hutman, Iverson, Landa, Rogers, Sigman and Stone2011). Thus, surveillance of social motivation from early infancy may be especially informative for risk assessment in children with a family history of ASD.
Clinical implications of variation in social motivation in ASD
As hypothesized, group-level SMI scores were lower in ASD-diagnosed toddlers, although their downward-shifted score distribution partially overlapped the distribution for toddlers without ASD. This observation resembles findings for school-aged children with ASD (Neuhaus et al., Reference Neuhaus, Bernier and Webb2021) and suggests that categorically low levels of social motivation are not necessary for all cases of ASD, as expected under a stringent interpretation of the social motivation hypothesis (Chevallier et al., Reference Chevallier, Kohls, Troiani, Brodkin and Schultz2012). Thus, toddlers with ASD likely exhibit individualized profiles of relative strengths and weaknesses in aspects of core symptom domains, which could correspond to differences in the balance of the drive versus ability to engage in social communication with others. This notion is consistent with accounts of variability in social motivation and its expression among individuals with ASD (Jaswal & Akhtar, 2018). In addition, our findings of additive relationships between social motivation, cognitive domains, and ASD symptom features to ASD outcome (Table 3; Table S4) align with models in which cumulative inherited liability implicating multiple developmental domains underlies ASD’s ontogeny (Constantino et al., Reference Constantino, Charman and Jones2021; Elsabbagh & Johnson, Reference Elsabbagh and Johnson2016). Quantitative developmental assessment across an array of ASD phenotypes relevant to both core symptoms and co-occurring features may thus improve screening, clinical subtyping, and more personalized intervention from an early stage in development.
Strengths and Limitations
As a composite derived from pre-existing data, the SMI incorporated items queried at specific ages. This approach maximized our ability to detect clinically relevant variation in social motivation while accounting for infants’ expanding behavioral repertoire during development. However, this strategy precluded item-level continuity and uniform representations of social motivation across age by the composite scores, and some relevant items were not assessed at all time points. In an extension of this work, we have developed an a priori SMI with balanced representation of elements of social motivation and analogous items across developmental stages. Together with more advanced psychometric approaches in large longitudinal samples, such efforts will further clarification of social motivation’s behavioral architecture in early childhood.
SMI items were systematically vetted according to the established conceptualization of social motivation as a disposition to engage with others. However, the disposition to socially engage may by necessity entail some demonstration of social ability. The SMI, in prioritizing parental impressions of cumulative experiences with their child, was designed to provide an overarching, real-world representation of social motivation rather than to explicitly separate social motivation from social skill. Our findings of partially overlapping variance between the SMI and direct metrics involving social communication skills, as well as additive contributions of the SMI and AOSI to ASD outcome, suggest that the SMI does capture unique variation relative to measures of social skills. This differentiation is noteworthy given strong intercorrelations for aspects of social behavior (Frazier et al., Reference Frazier, Ratliff, Gruber, Zhang, Law and Constantino2014; Marrus et al., Reference Marrus, Grant, Harris-Olenak, Albright, Bolster, Haber, Jacob, Zhang, Heath, Agrawal, Constantino, Elison and Glowinski2020) and the theorized contribution of social motivation to the development of social communication abilities (Chevallier et al., Reference Chevallier, Kohls, Troiani, Brodkin and Schultz2012; Su et al., Reference Su, Rogers, Estes and Yoder2021). An important future direction to disambiguate social motivation from social skill includes integrating informant-based measures of real-world behavior with quantitative task-based measurements, such as eye-tracking, that can objectively index behavior in a controlled context designed to parse elements of social motivation (e.g., Vernetti et al., Reference Vernetti, Senju, Charman, Johnson and Gliga2018). Applying social motivation metrics to populations with evidence for dissociation between social motivation and social cognition, such as children with William’s Syndrome (Klein-Tasman et al., Reference Klein-Tasman, Li-Barber and Magargee2010), represents another approach to advance quantification of this construct.
Finally, we note that although the IBIS sample afforded a large dataset, replication to evaluate the consistency of our findings is warranted. Future studies would benefit from more diverse samples due to ASD’s known heterogeneity, the sample’s small number of ASD-affected females (consistent with ASD’s 4:1 sex ratio), and the multiplex nature of the sample’s HR+ cases, whose development might not generalize across all forms of ASD.
Conclusion
To our knowledge, this work describes the first example of a quantitative, dimensional approach to index social motivation, a heritable aspect of core ASD features, in infancy. The ability of parent report to detect associations between infant social motivation and later ASD highlights a translatable opportunity for novel social motivation measures to advance early detection, individualize intervention, and resolve developmental mechanisms of ASD.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0954579422001006
Acknowledgments
The Infant Brain Imaging Study (IBIS) Network is an NIH-funded Autism Center of Excellence project and consists of a consortium of 8 universities in the US and Canada. Clinical Sites: University of North Carolina: J. Piven (IBIS Network PI), H.C. Hazlett, C. Chappell; University of Washington: S. Dager, A. Estes, D. Shaw; Washington University: K. Botteron, R. McKinstry, J. Constantino, J.Pruett; Children’s Hospital of Philadelphia: R. Schultz, J. Pandey; University of Alberta: L. Zwaigenbaum; University of Minnesota: J. Elison, J. Wolff; Data Coordinating Center: Montreal Neurological Institute: A.C. Evans, D.L. Collins, G.B. Pike, V. Fonov, S. Das, L. MacIntyre; Image Processing Core: University of Utah: G. Gerig; University of North Carolina: M. Styner; Statistical Analysis Core: University of North Carolina: H. Gu.
We thank the children and families for their participation and dedication to this longitudinal study. We acknowledge Santiago Torres Gomez, PhD, of the Montreal Neurological Institute for constructive feedback on the manuscript.
Funding statement
This study was supported by National Institutes of Health Autism Center of Excellence R01 grant (National Institute of Child Health and Human Development, #HD055741 to J.P.), Autism Speaks (#6020 to J.P.), the Simons Foundation (grant number #140209 to J.P.), the National Institute of Mental Health (K08 MH112891 to N.M.) and as well as U54 Intellectual and Developmental Disabilities Research Centers HD079124 to the University of North Carolina (J.P.); HD087011 to Washington University (J.N.C.); HD86984 to the Children’s Hospital of Philadelphia (R.T.S.); and HD083091 to the University of Washington.
Conflicts of interest
Dr Constantino receives royalties from Western Psychological Services for the creation of the Social Responsiveness Scale. The remaining authors have no conflicts of interest to disclose.








