CVD is the main cause of death in Europe( Reference Townsend, Wilson and Bhatnagar 1 ). In addition to well-established risk factors such as hypercholesterolaemia, hypertension, diabetes and smoking( Reference D’Agostino, Vasan and Pencina 2 ), both fasting and postprandial hypertriacylglycerolaemia are associated with an increased risk of developing CVD. Whether this association is causal is still debated( Reference O’Keefe and Bell 3 – Reference Di Angelantonio, Sarwar and Perry 6 ). Dietary fat is mainly composed of TAG, which after absorption is incorporated in chylomicrons (CM) and consequently transported to the circulation( Reference Ramasamy 7 ). CM and its remnants, along with VLDL from the liver, cause the elevated TAG concentration in the postprandial phase( Reference Nakajima, Nakano and Tokita 8 ). The magnitude of the postprandial response is determined by several lifestyle factors such as diet, exercise and smoking. Among dietary factors, fat quantity is the main determinant of the postprandial lipidaemic response, but fat quality, i.e. fatty acids of different chain lengths and degrees of saturation, and additional intake of protein, dietary fibre and alcohol may also affect the response( Reference Bravo, Napolitano and Botham 9 ). High levels of VLDL and CM remnants may promote atherosclerosis directly, by entering the intima and by attracting inflammatory cells to the intima, but also indirectly by causing a more atherogenic lipoprotein profile in the circulation( Reference Bravo, Napolitano and Botham 9 ).
Patients with familial hypercholesterolaemia (FH) are characterised by elevated cholesterol levels, resulting in an increased risk of developing early-onset CVD( Reference Mundal, Sarancic and Ose 10 ). FH is mainly caused by a mutation in the gene coding for the LDL receptor( Reference Brautbar, Leary and Rasmussen 11 ). Subsequently, the deficiency in the LDL-receptor function may affect the metabolism of TAG-rich lipoproteins, including VLDL and CM( Reference Chan and Watts 12 , Reference Kolovou, Kostakou and Anagnostopoulou 13 ). Some have reported an increased postprandial TAG response in FH subjects when compared with normolipidaemic controls( Reference Kolovou, Anagnostopoulou and Pilatis 14 ), whereas others have not( Reference Rubinsztein, Cohen and Berger 15 ). Furthermore, studies have shown increased postprandial levels of CM or increased production of VLDL in subjects with FH compared with controls( Reference Cummings, Watts and Umpleby 16 – Reference Mamo, Smith and Yu 19 ).
Proprotein convertase subtilisin/kexin type 9 (PCSK9) affects the metabolism of LDL; however, it has also been suggested to affect the metabolism of TAG-rich lipoproteins( Reference Druce, Abujrad and Ooi 20 ). Circulating PCSK9 has been shown to be positively correlated with plasma TAG, VLDL particle concentration, intermediate-density lipoprotein particle concentration( Reference Kwakernaak, Lambert and Dullaart 21 ) and CM particle concentration( Reference Chan, Wong and Pang 22 ). Moreover, Bjermo et al.( Reference Bjermo, Iggman and Kullberg 23 ) recently showed that intake of PUFA compared with SFA reduced PCSK9 levels, and hypothesised that down-regulation of PCSK9 could be a novel mechanism behind the cholesterol-lowering effects of PUFA.
Whether the content of SFA and PUFA in the diet could differently affect the postprandial TAG response is still not clarified( Reference Monfort-Pires, Delgado-Lista and Gomez-Delgado 24 ), and it is scarcely explored in subjects with FH. In the present study, the aim was to investigate the postprandial response of TAG and lipid sub-classes after consumption of high-fat meals with different fat quality in subjects with FH compared with healthy normolipidaemic controls.
Methods
Subjects
Subjects with genetically verified FH and healthy normolipidaemic controls aged 18–30 years were recruited from March to May 2016. FH subjects were recruited from the out-patient Lipid Clinic, Oslo University Hospital, Norway, and the patient organisation, FH Norge, Norway, by mail invitations with a subsequent telephone request. Control subjects were primarily recruited by email invitations and advertisements by posters at the University of Oslo, Norway. Screening interviews were performed by telephone, and the interventions were performed at the University of Oslo, Norway.
Exclusion criteria were as follows: FH subjects with FH mutation in genes not coding for LDL-receptor (data collected from the patient’s medical journal at Oslo University Hospital), self-reported BMI <18·5 or >30·0 kg/m2, C-reactive protein >10 mg/l, TAG >4 mmol/l, any co-morbidities, use of medication other than lipid-lowering or contraceptive pills, hormonal treatment, pregnancy or lactation, allergy or intolerance for gluten or egg, tobacco smoking, large alcohol consumption (>40 g daily), weight change ±5 % of body weight within the past 3 months, blood donation within the past 2 months or unwillingness to stop using medications or supplements affecting the lipid metabolism the last 4 weeks before both intervention days. All FH subjects were treated with lipid-lowering medications but agreed to discontinue the treatment the last 4 weeks before the 1st test day and in the period between the 1st and 2nd test day.
Study design
The study was a randomised controlled double-blind cross-over study. Subjects with FH (without current lipid-lowering treatment) and normolipidaemic controls ingested two meals with different fat quality in a randomised order with a wash-out period of 3–5 weeks between the meals. The two meals had a similar appearance and were marked A or B to ensure blinding of participants and care providers. Each meal consisted of a high-fat muffin with the same amount of energy, carbohydrates, protein and total fat, but with different fatty acid composition, as shown in Table 1. The fatty acid composition of the meals was analysed by Eurofins. Each meal was 150 g and contained about 60 g of fat (70 E%). One of the meals was rich in SFA from palm oil and coconut oil and the other was rich in PUFA from sunflower oil and rapeseed oil (AAK Sweden AB). The amount of MUFA was similar in the two meals. The meal was consumed within 15 min. Venous blood samples were taken at baseline (fasting) and at 2, 4 and 6 h after consumption of the meal.
Table 1 Nutritional values and fatty acid composition in the two meals

E%, energy percentage.
The subjects were advised to keep their habitual diet and physical activity level during the study period. Before each test meal, they had to be fasting for 12 h. They were also asked not to consume alcohol or conduct strenuous exercise within the last 24 h before the test meal, nor to consume high-fat foods the evening before (the last 14 h). A list of low-fat foods that could be consumed the evening before the test days was given to the participants. They all reported to have followed the guidelines regarding food intake and physical activity the last 24 h before the interventions. During the test day, the subjects engaged in minimum physical activity and were not allowed to eat or drink anything but water (maximum 1 litre). After the last blood sample, the subjects were offered a meal.
Anthropometry, blood pressure and diet
Waist and hip circumferences were measured according to the World Health Organization guidelines( 25 ). Body composition was assessed by dual-energy X-ray absorptiometry (GE Lunar iDXA, Software: EnCore version 16). Blood pressure was measured in the participants’ non-dominant arm in a sitting position after 15 min of rest, by using a Dinamap Carescape v100 (GE Medical System). Diet was assessed by a fourteen-page semi-quantitative FFQ including 270 food items, designed to capture the habitual food intake of Norwegian adults in the preceding year, as described elsewhere( Reference Carlsen, Lillegaard and Karlsen 26 ). Average daily intake of energy and different nutrients from the FFQ was computed using the food database KBSAE-14 and KBS software system (KBS version 7.3, 2017) developed at the Department of Nutrition, University of Oslo, Norway.
Routine measures
Serum was obtained from silica gel tubes (Becton Dickenson Vacutainer Systems) and kept at room temperature for 30–60 min until centrifugation (1500 g , 15 min). Whole-blood samples were collected in EDTA tubes (Becton Dickenson Vacutainer Systems) and kept at room temperature until analysis. Standard blood chemistry and lipid variables were measured in serum and whole blood using routine laboratory methods at an accredited medical laboratory (Fürst Medical Laboratory).
ELISA
Serum was divided into aliquots and frozen at −80°C for analysis of apoB48 and PCSK9. ApoB48 concentration, which reflects the amount of CM, was determined by the Human ApoB48 ELISA kit (catalogue no.: RSHAKHB48R; producer: BioVendor Research and Diagnostic Products, distributor: BioVendor Laboratorni Medicina as., intra variation: 3·5 %, inter variation: 2·8–8·6 %). PCSK9 concentration was determined by the Human Proprotein Convertase 9/PCSK9 Immunoassay ELISA kit (catalogue no.: DPC900; producer: R&D Systems, Inc., distributor: R&D Systems Europe, Ltd, intra variation: 4·8–27·7 %, inter variation: 4·6–27·9 %).
Lipoprotein sub-classes
Plasma was obtained from EDTA tubes (Becton Dickenson Vacutainer Systems), kept on ice immediately and centrifuged within 15 min at 2000 g for 15 min at 4°C. Plasma was divided into aliquots and frozen at −80°C for analysis of lipoprotein sub-classes. Lipoprotein sub-classes were analysed by using a metabolomics platform (Nightingale’s Biomarker Analysis Platform). By this method, the concentration and composition of fourteen different lipoprotein sub-classes can be determined (six VLDL, one intermediate-density lipoprotein, three LDL, four HDL)( Reference Würtz, Kangas and Soininen 27 ).
Ethics
The study was approved by the Regional Committees for Medical and Health Research Ethics (REK 2015/2392/REK sør-øst B) and conducted according to the principles of the Declaration of Helsinki. All subjects provided written informed consent. The study was registered at www.ClinicalTrials.gov with the registration no. NCT02729857.
Statistics
The participants were randomised and the randomisation list was created using Excel random list generator. Data are presented as medians and interquartile range: 25th–75th percentiles when not otherwise specified. A Mann–Whitney U test or a χ 2 test was used to test differences between FH subjects and healthy controls at baseline. AUC( Reference Matthews, Altman and Campbell 28 ) and incremental AUC (AUC adjusted for baseline values as described elsewhere, iAUC( Reference Carstensen, Thomsen and Hermansen 29 )) were calculated on all repeated variables by using the trapezoid method. AUC and iAUC were analysed by a linear mixed model for repeated measures to study the effect of the meals within each group and between groups. Meal, group, period (which meal the subjects received first) and their interactions were included in the model. When no significant interaction was found between meal and group (i.e. when group effects did not differ between meals and vice versa), P values are presented from testing differences between meals and between groups. In the case of significant interaction between group and meal, P values are presented from analysis stratified by group and meal. Period and interactions with period were not significant and therefore not presented. The residuals were examined to check model assumptions. Spearman’s rank correlation coefficient, r, was estimated for circulating PCSK9 and lipids (AUC and iAUC) stratified by group with both meals combined. P values <0·05 were considered significant. The statistical analyses were performed with SPSS version 20.0.
Results
Baseline characteristics
We included thirteen subjects with FH and eighteen healthy normolipidaemic controls. Flow chart of the participants is shown in Fig. 1. Four control subjects dropped out after the 1st test day and were therefore not included in the statistical analysis. Reasons for dropping out were lack of time, fear of multiple blood samples and mild headache during the post-prandial period. Baseline characteristics of the participants are shown in Table 2. The groups were similar with regard to age, sex, anthropometric measures and most biomarkers. As expected, FH subjects had significantly higher baseline levels of total cholesterol, LDL-cholesterol and apoB, but also higher diastolic blood pressure compared with control subjects (Table 2). Furthermore, the two groups did not differ with regard to dietary intake (Table 3). All FH subjects had LDL receptor missense mutations, except one that had a mutation that disrupts normal splicing of the LDL receptor (Table 2). On average, the FH subjects had used lipid-lowering treatment for 10 years, but they discontinued the medication 4 weeks before to the 1st test day and during the study.
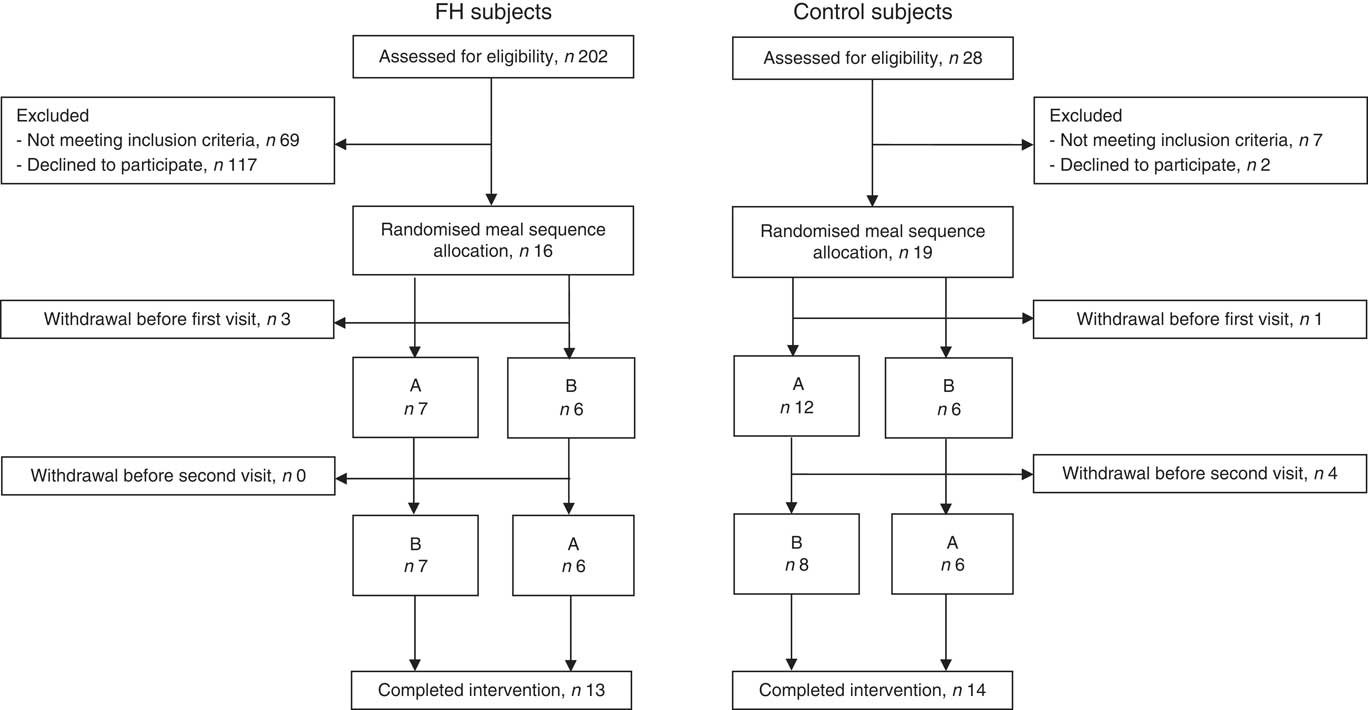
Fig. 1 Flow chart of the participants. FH, familial hypercholesterolaemia.
Table 2 Subject characteristics at baseline* (Medians and interquartile ranges (IQR); percentages; numbers)

FH, familial hypercholesterolaemia; hs-CRP, high-sensitivity C-reactive protein; ASAT, aspartate amino transferase; ALAT, alanine amino transferase; TSH, thyroid-stimulating hormone; Lp(a), lipoprotein(a).
* P values from Mann–Whitney U test or χ 2 test. Blood samples are fasting.
Table 3 Daily dietary intake among the participants* (Medians and interquartile ranges (IQR))

FH, familial hypercholesterolaemia; E%, energy percentage.
* P values from Mann–Whitney U test. Dietary intake is measured by a FFQ.
Postprandial response in lipid parameters
There was no significant difference in the postprandial response (neither AUC nor iAUC) in the level of TAG, apoB48, HDL-cholesterol or glucose between the different meals (SFA or PUFA) or between the groups, as shown in the online Supplementary Table S1 (0·08≤P≤0·96). Interestingly, the TAG concentration peaked at 2 h after intake of the PUFA meal, whereas at 4 h after intake of the SFA meal in both groups (Fig. 2). This was also reflected in the level of apoB48 in FH subjects (Fig. 2). The distinct TAG peaks were also apparent in the individual TAG and apoB48 curves, even though large individual differences were observed (online Supplementary Fig. S1). Compared with controls, subjects with FH had significantly higher AUC for PCSK9, total cholesterol and LDL-cholesterol (P≤0·02), but not when adjusted for baseline (0·40≤P≤0·95, iAUC) (Fig. 2 and online Supplementary Table S1). We found no significant effect of meals for any of these variables (0·61≤P≤0·91, AUC; 0·13≤P≤ 0·99, iAUC).
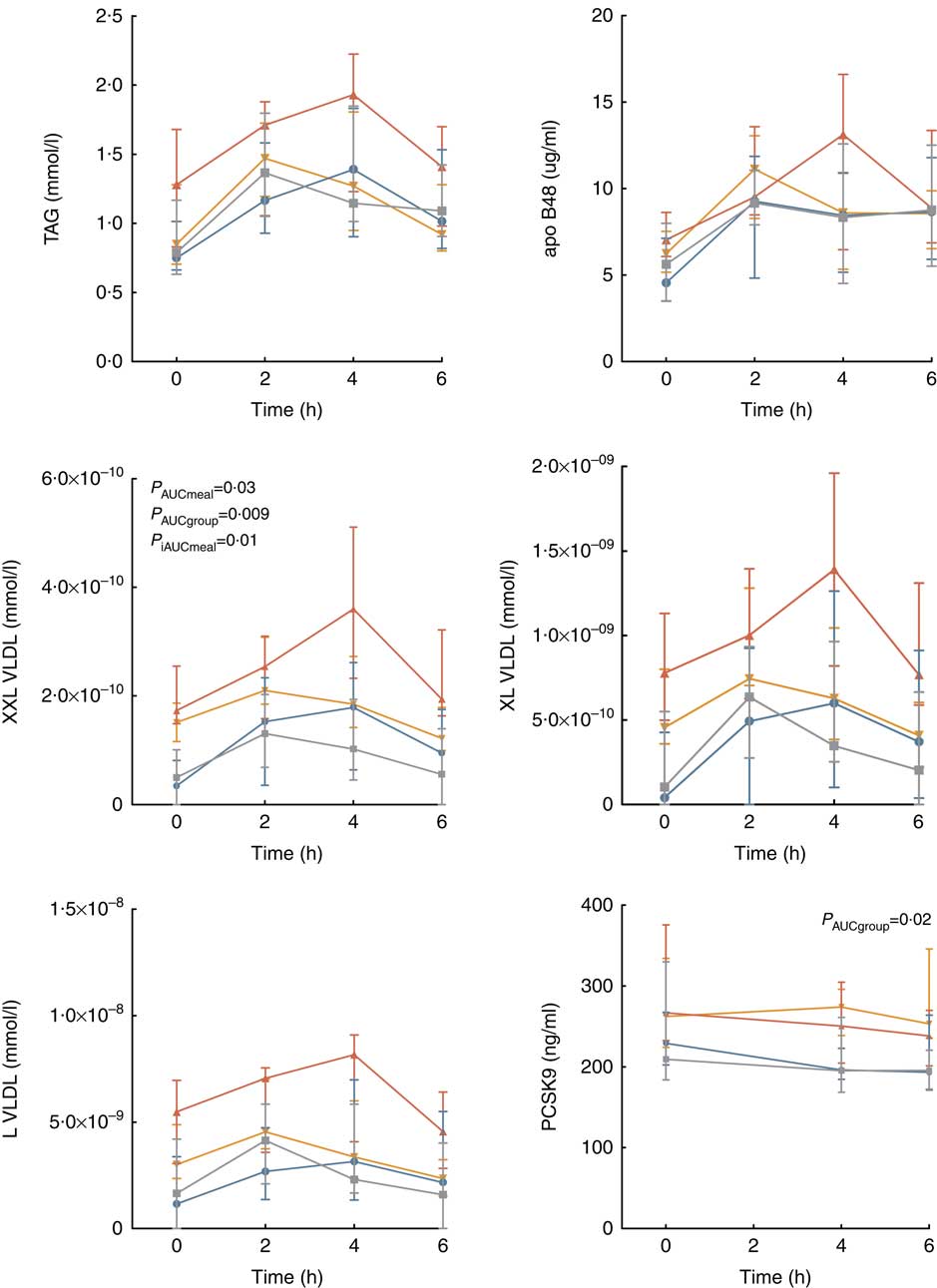
Fig. 2 Postprandial response in TAG, apoB48, the three largest VLDL sub-classes and proprotein convertase subtilisin/kexin type 9. Values are medians and interquartile ranges. P values from analysis of total and incremental AUC (AUC and iAUC) in a linear mixed model for repeated measures. PCSK9, proprotein convertase subtilisin/kexin type 9; FH, familial hypercholesterolaemia; P
meal, P value SFA v. PUFA meal; P
group, P value FH v. controls. ![]() , FH, SFA;
, FH, SFA;
![]() , FH, PUFA;
, FH, PUFA;
![]() , controls, SFA;
, controls, SFA;
![]() , controls, PUFA.
, controls, PUFA.
Similar to the TAG response, the level of the three largest VLDL sub-classes (L, XL and XXL) peaked later after intake of the SFA meal compared with the PUFA meal in both groups (Fig. 2). The postprandial responses in the three smallest VLDL, all LDL and HDL sub-classes are shown in the online Supplementary Fig. S2 and Table S2. Subjects with FH had significantly higher levels (AUC) than controls for the largest (XXL VLDL P=0·009) and the two smallest (S and XS VLDL P≤0·001) VLDL sub-classes, as well as for the intermediate-density lipoprotein (P<0·001), all LDL sub-classes (P<0·001) and the largest HDL sub-class (XL HDL P=0·002). Meal was not significant for any of these variables (0·10≤P≤0·96, AUC), except for XXL VLDL, which was higher after SFA than after PUFA (P=0·03, AUC) (Fig. 2).When adjusting for baseline (iAUC), differences between the meals were found for some of the lipid sub-classes (online Supplementary Table S2).
Several differences were seen in the TAG concentration of the different lipoprotein sub-classes between meals and groups (online Supplementary Table S2). The findings were similar as for the total lipoprotein concentration for all VLDL sub-classes (AUC and iAUC); however, a difference was not found for S VLDL between groups after the PUFA meal (P=0·66, AUC), nor for XS VLDL between meals among FH subjects (P=0·17, iAUC).
Correlations between PCSK9 and lipids
For both control and FH subjects, no significant correlation was found between circulating PCSK9 and TAG, total cholesterol, LDL-cholesterol and apoB48 (−0·34≤r≤0·36, AUC and iAUC, online Supplementary Table S3). In controls, a significant correlation was found between PCSK9 and VLDL particle concentration (r 0·51, P=0·006, iAUC) and between PCSK9 and IDL particle concentration (r 0·44, P=0·02, AUC).
Discussion
In the present study, there was no significant difference in the postprandial response (neither AUC nor iAUC) of TAG or apoB48 elicited by the different meals or between the two different groups. However, we found that the TAG concentrations peaked at 2 h after intake of the PUFA meal, and at 4 h after intake of the SFA meal in both groups. This may reflect differences in the postprandial lipid metabolism after intake of fatty acids with different chain lengths and degrees of saturation.
Differences between the meals
In both FH and normolipidaemic subjects, we found no significant difference in TAG AUC after intake of the SFA-rich meal compared with the PUFA-rich meal. FH subjects are characterised by reduced number of functional LDL receptors( Reference Mundal, Sarancic and Ose 10 ). Previous studies have suggested that long-term intake of SFA may lead to down-regulation of the LDL receptor( Reference Mustad, Ellsworth and Cooper 30 ), thus possibly affecting the metabolism of TAG-rich lipoproteins( Reference Chan and Watts 12 , Reference Kolovou, Kostakou and Anagnostopoulou 13 ). The novel finding in this study is that FH subjects had a similar TAG response to meals with different fat quality. Our data are in accordance with a systematic review and meta-analysis summarising the effect elicited by meals of different fat qualities among different populations 6 h after the meal( Reference Monfort-Pires, Delgado-Lista and Gomez-Delgado 24 ). In the meta-analysis, however, the TAG AUC was significantly higher 8 h after intake of SFA compared with PUFA( Reference Monfort-Pires, Delgado-Lista and Gomez-Delgado 24 ). In this study, we only followed the subjects 6 h postprandially, and thus the postprandial period in the present study may have been too short to reveal differences in AUC. Interestingly, we found a delayed peak in TAG after intake of the SFA-enriched meal compared with the PUFA-enriched meal. This difference in TAG peak is supported by previous findings( Reference Dekker, Wright and Mazurak 31 , Reference Masson and Mensink 32 ). Moreover, different TAG peaks have also been shown between different SFA, such as lauric, myristic, palmitic and stearic acid, in which intake of palmitic acid, the major fatty acid in the present SFA meal, also resulted in a TAG peak at 4 h( Reference Karupaiah, Tan and Chinna 33 ). The postprandial differences in the TAG response following intake of SFA v. PUFA may be mediated through stimulation of fat absorption( Reference Jones, Stolinski and Smith 34 – Reference Tholstrup, Sandstrom and Bysted 36 ), CM secretion( Reference van Greevenbroek, van Meer and Erkelens 37 – Reference Sakr, Attia and Haourigui 39 ), VLDL secretion( Reference Sundaram and Yao 40 ) and lipoprotein lipase activity( Reference Botham, Avella and Cantafora 41 , Reference Weintraub, Zechner and Brown 42 ).
Some studies have indicated that PUFA are absorbed more efficiently than SFA( Reference Jones, Stolinski and Smith 34 – Reference Tholstrup, Sandstrom and Bysted 36 ). This may be because of the lower melting point of PUFA compared with long-chain SFA( Reference McKimmie, Easter and Weinberg 35 , Reference Berry, Miller and Sanders 43 ), or different ability of SFA and PUFA to form lipid droplets in the intestine( Reference Armand, Borel and Pasquier 44 ). Furthermore, the fatty acids may be taken up by both passive and active transport( Reference Schwenk, Holloway and Luiken 45 ), and thus it is possible that the active transporters may promote selective uptake of different fatty acids. PUFA have been shown to be more potent in stimulating apoB48 secretion than SFA in vitro, probably through stimulating the microsomal TAG transfer protein activity( Reference van Greevenbroek, van Meer and Erkelens 37 ). Moreover, it has been shown that unsaturated fatty acids result in a larger number or size of CM particles when compared with SFA( Reference Williams, Bateman and Jackson 38 , Reference Sakr, Attia and Haourigui 39 ), thus affecting the postprandial TAG response.
VLDL particles are major contributors to the elevated TAG concentration in the postprandial phase( Reference Nakajima, Nakano and Tokita 8 ). The availability of apoB100 and TAG in the liver is the most important regulator of VLDL synthesis; however, fatty acid quality of the diet may further affect the synthesis( Reference Sundaram and Yao 40 ), possibly through stimulation of microsomal TAG transfer protein activity( Reference Bennett, Billett and Salter 46 ). Studies comparing the intake of SFA v. PUFA on VLDL secretion are inconsistent( Reference Bennett, Billett and Salter 46 , Reference Lopez-Soldado, Avella and Botham 47 ). Furthermore, TAG is hydrolysed by lipoprotein lipase, which is tightly regulated by several apo and angiopoietin-like proteins( Reference Kersten 48 ). In vitro studies have shown that the lipolysis of PUFA is more efficient than the lipolysis of SFA( Reference Botham, Avella and Cantafora 41 , Reference Weintraub, Zechner and Brown 42 ), supporting our data with an earlier TAG peak after intake of the PUFA meal than after the SFA meal. Moreover, it may be speculated that after intake of SFA the TAG-rich lipoproteins may contain more apoCIII and apoE than after intake of PUFA, thus reducing lipoprotein lipase activity and prolonging the microsomal TAG transfer protein TAG response( Reference Jackson, Wolstencroft and Bateman 49 ).
The clinical impact of the difference in TAG response should be further clarified. There may be a biological advantage in prioritising the uptake of essential fatty acids before other fatty acids. The delayed microsomal TAG transfer protein TAG response after intake of an SFA-rich meal compared with a PUFA-rich meal may further adversely affect the vasculature or cause a more atherogenic lipoprotein profile in the circulation( Reference Bravo, Napolitano and Botham 9 ). Furthermore, we found some differences in lipoprotein sub-classes between the meals, but these differences were rather small and may not be clinically relevant.
Differences between the groups
In the present study, we found that the microsomal TAG transfer protein TAG and apoB48 response to high-fat meals seem to be similar in young and normal-weight subjects with and without FH, suggesting that reduced LDL receptor activity does not influence the post-prandial metabolism of TAG-rich lipoproteins. Conflicting data have been reported previously, with some studies finding no difference( Reference Rubinsztein, Cohen and Berger 15 , Reference Eriksson, Angelin and Henriksson 50 , Reference Watts, Barrett and Marais 51 ), whereas other studies finding a difference in the postprandial TAG response or in the catabolism of CM remnants between subjects with FH compared with normolipidaemic controls( Reference Kolovou, Anagnostopoulou and Pilatis 14 , Reference Mamo, Smith and Yu 19 ). Furthermore, we found an increased level of PCSK9 (AUC) in subjects with FH compared with controls. Statins have been shown to increase the level of PCSK9( Reference Dubuc, Chamberland and Wassef 52 ), and the level of PCSK9 has been shown to be positively associated with LDL-cholesterol( Reference Lambert, Sjouke and Choque 53 ). Similar to the study by Kwakernaak et al., we found a significant correlation between PCSK9 and VLDL and intermediate-density lipoprotein particle concentration( Reference Kwakernaak, Lambert and Dullaart 21 ), indicating that PCSK9 also affects the metabolism of TAG-rich lipoproteins.
In the present study, we found significantly higher levels (AUC) of several lipoprotein sub-classes in FH subjects compared with normolipidaemic controls; however, when adjusting for baseline values (iAUC), the responses were not significantly different between the groups. The differences between groups (AUC) in VLDL sub-classes were similar for both total concentration and TAG content, which is in accordance with the fact that TAG are the most abundant compounds of VLDL. However, the total TAG level in the circulation was not different between the groups. The increased levels of VLDL may partly be due to an increased production rate of apoB100 in subjects with FH( Reference Cummings, Watts and Umpleby 16 , Reference Millar, Maugeais and Ikewaki 17 , Reference Tremblay, Lamarche and Ruel 54 ). In addition to the well-established effect of elevated LDL, enhanced VLDL levels may also increase the risk of developing CVD in subjects with FH, as it has been shown that lipoproteins <70–75 nm may enter the endothelial wall and promote atherogenesis( Reference Hyson, Rutledge and Berglund 55 , Reference Nordestgaard and Zilversmit 56 ).
In the present study, subjects with FH had significantly higher level of the largest HDL sub-class than controls. These particles contain more TAG than the smaller particles. Christensen et al.( Reference Christensen, Ulven and Retterstol 57 ) also found higher level of the largest HDL sub-class in children with FH compared with controls, particularly in non-statin-treated subjects. This may be related to an enhanced cholesteryl ester transfer protein activity in subjects with FH( Reference Ooi, Barrett and Watts 58 ). TAG-enriched HDL has been found to elicit a reduced anti-inflammatory and anti-oxidative activity( Reference Ottestad, Halvorsen and Balstad 59 ). This may result in increased systemic oxidative stress in FH subjects( Reference Ottestad, Halvorsen and Balstad 59 ). An altered lipid composition of HDL particles in subjects with FH may further result in an impaired cholesterol efflux( Reference Ganjali, Momtazi and Banach 60 ). Furthermore, Frenais et al. showed an increased production and catabolism of apoA1 in subjects with FH compared with normolipidaemic controls( Reference Frenais, Ouguerram and Maugeais 61 ).
A strength in the present study was the inclusion of two homogeneous groups with similar age, sex and BMI. The subjects were young and had no known co-morbidities complicating the interpretation of the findings. Moreover, the controlled and randomised design of the study also represents a major strength. However, the study also has some limitations. By chance, the FH subjects had higher baseline TAG at the SFA visit than at the PUFA visit, despite following the study protocol (including standardisation of food intake the last 14 h before the intervention) and the study randomisation. This may imply that the diet and physical activity the day(s) before the intervention may have had an impact on the fasting TAG values( Reference Williams 62 , Reference Petitt and Cureton 63 ). A limitation of the study is that blood samples were only collected for 6 h after the meals( Reference Monfort-Pires, Delgado-Lista and Gomez-Delgado 24 ), whereas including collection of 8-h samples may have generated a different result. Another limitation is that PCSK9 measurement at 2 h after intake of meal was not performed. We mainly included FH patients with LDL receptor missense mutations, and can therefore not exclude the possibility that the effect may differ between FH subjects with different LDL receptor mutations. When evaluating such a large number of variables, the concern with multiple comparisons must be kept in mind. Finally, the study has rather few participants; however, the sample size was determined on the basis of previous studies( Reference Chan and Watts 12 , Reference Kolovou, Anagnostopoulou and Pilatis 14 , Reference Dane-Stewart, Watts and Mamo 18 , Reference Eriksson, Angelin and Henriksson 50 ).
Conclusions
In the present study, young FH subjects had similar post-prandial TAG and apoB48 response as healthy age- and sex-matched controls. Serum TAG peaked later after intake of SFA compared with PUFA in both groups. This may reflect differences in the postprandial metabolism of fatty acids with different chain lengths and degrees of saturation. The clinical impact of these findings, as well as the additive effect of subsequent fatty rich meals, should be further clarified.
Acknowledgements
The authors thank Mills AS (Pb. 4644 Sofienberg, 0506 Oslo, Norway) for providing edible oil for the muffins. The authors thank Anne Randi Enget, Ingunn Jermstad and Navida Akhter Sheikh (Department of Nutrition, University of Oslo, Norway) for assisting with the blood collection, Navida Akhter Sheikh for assisting with the laboratory analysis and Anne Marte Wetting Johansen (Department of Nutrition, University of Oslo, Norway) for analysing the FFQ. Finally, the authors thank the participants, without whom this study would not have been possible.
The study was supported by the University of Oslo, Norway, the National Advisory Unit on Familial Hypercholesterolemia, Oslo University Hospital, Norway, The Throne Holst Foundation for Nutrition Research and Mills AS (Pb. 4644 Sofienberg, 0506 Oslo, Norway). Mills AS was involved in the design of the study, but K. B. H. and S. M. U. had the final responsibility of the design. Mills AS was involved in conducting the trial and provided oils for the trial. None of the employees at Mills AS were involved in the statistical analysis.
The research was designed (project conception, development of overall research plan and study oversight) by L. K. L. Ø., P. H., M. P. B., S. M. U. and K. B. H. The research was conducted (hands-on conduct of the experiments and data collection) by L. K. L. Ø., P. H., G. F. and I. N. The data were analysed by L. K. L. Ø. and M. B. V. The paper was written by L. K. L. Ø., P. H., M. P. B., I. N., M. B. V., S. M. U. and K. B. H., and all authors critically reviewed the paper. K. B. H., L. K. L. Ø. and S. M. U. hold primary responsibility for the content.
During the past 5 years, M. P. B. reports grants and personal fees from Amgen, grants and personal fees from Sanofi, personal fees from MSD and personal fees from Aegerion, none of which are related to the content of this manuscript. During the past 5 years, S. M. U. has received research grants from Mills AS, TINE SA and Olympic Seafood, none of which are related to the content of this manuscript. During the past 5 years, K. B. H. has received research grants or honoraria from Mills AS, TINE SA, Olympic Seafood, Amgen, Sanofi and Pronova, none of which are related to the content of this manuscript. L. L. and M. G. B. are employed by Mills AS. None of the other authors has any conflicts of interest to declare.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114518000673







